
Implication of sacubitril/valsartan on N-terminal pro B-type natriuretic peptide levels in hypertensive patients
Introduction
In healthy cohorts, even a slight increase in plasma N-terminal-pro B-type natriuretic peptide (NT-pro BNP) level is associated with incremental cardiovascular incidence (1-3).
Sacubitril/valsartan, one of the angiotensin receptor-neprilysin inhibitors, decreases mortality and morbidity in patients with chronic heart failure with reduced left ventricular ejection fraction via stimulating the synthesis of natriuretic peptide families and suppressing the renin-angiotensin-aldosterone system (4). As a surrogate marker, sacubitril/valsartan therapy decreases plasma NT-pro BNP levels (5).
Sacubitril/valsartan has recently been reimbursed also for hypertension in Japan (6). However, its impact on the plasma NT-pro BNP levels in this cohort remains unknown (7). We hypothesized that sacubitril/valsartan might decrease plasma NT-pro BNP levels in the hypertensive patients who do not have heart failure. A decrease in plasma NT-pro BNP levels might prevent future cardiovascular events among this cohort.
In this study, we investigated the changes in plasma NT-pro BNP levels in hypertensive patients during sacubitril/valsartan therapy by comparing with those of the pre-treatment period (without sacubitril/valsartan). We present the following article in accordance with the MDAR reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-483/rc).
Methods
Patient selection
Consecutive patients who received sacubitril/valsartan to treat their hypertension on a de novo basis at clinically stable condition and continued it for three months between September 2021 and January 2022 were considered to be included in this retrospective study. As a primary concern, a change in plasma NT-pro BNP levels during 3-month sacubitril/valsartan therapy was compared with a change in plasma NT-pro BNP levels during the prior 3-month without sacubitril/valsartan.
Patients who had a history of heart failure or current active heart failure were excluded. Patients with any cardiac abnormalities were excluded. Those with missing data or lost follow-up were excluded. Those in whom sacubitril/valsartan was terminated within three months were also excluded.
The present study was approved by the Institutional Review Board of University of Toyama (No. R2015154, April 11, 2016). Written informed consents were obtained from all participants. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the ethical standard of the responsible committee on human experimentation.
Study protocol
All data were retrospectively collected from the medical record charts. Changes in clinical data, including NT-pro BNP levels, during 3 months sacubitril/valsartan treatment (on-treatment period), were compared with those during pre-treatment 3-month without sacubitril/valsartan (pre-treatment period).
Administration of sacubitril/valsartan
Sacubitril/valsartan was considered to be administered for those with hypertension according to the manufacturer-recommended indication. Sacubitril/valsartan was initiated via a conversion from renin-angiotensin system inhibitors. The initial dose of sacubitril/valsartan was 100 or 200 mg per day, which was adjusted according to the patient’s symptoms and blood pressure. The final decision to administer sacubitril/valsartan and its dose were made by the attending physicians.
Clinical data
Baseline characteristics at the time of sacubitril/valsartan initiation, including demographics, comorbidity, and medication data, were collected. Blood pressures and pulse rates data were obtained at three months prior to the initiation of sacubitril/valsartan, baseline, and three months following the initiation of sacubitril/valsartan. Drug-related adverse events data including symptomatic hypotension during sacubitril/valsartan therapy were surveyed.
Statistical analyses
As a primary concern, changes in plasma NT-pro BNP levels during the on-treatment period was compared with those during the pre-treatment period using Mann-Whitney U test.
All continuous variables were expressed as median and interquartile irrespective of their distributions given a small sample size. All categorical variables were expressed as numbers and percentages. Trends in continuous variables (i.e., three months before, baseline, and three months later) were assessed using Friedman test and ad-hoc Wilcoxon signed-rank test.
Statistics were performed using SPSS Statistics 24 (SPSS Inc., Armonk, IL, USA). Two-sided P values <0.05 were considered statistically significant.
Results
Baseline characteristics
From 51 patients, 5 patients with missing data and 13 patients with heart failure comorbidity were excluded. Finally, 33 patients were included. All patients had hypertension. Median age was 73 [64, 77] years old and 27 (82%) were men (Table 1). Median systolic blood pressure was 138 [134, 149] mmHg and median pulse rate was 69 [63, 76] bpm. Plasma NT-pro BNP level on median was 207 [107, 386] pg/mL.
Table 1
| Characteristics | N=33 |
|---|---|
| Demographics | |
| Age, years | 73 [64, 77] |
| Men | 27 (82%) |
| Body mass index, kg/m2 | 21.6 (20.3, 22.8) |
| Initial dose of sacubitril/valsartan | |
| 100 mg/day | 27 (82%) |
| 200 mg/day | 6 (18%) |
| Anti-hypertension agents | |
| Calcium channel blocker | 11 (33%) |
| Diuretics | 6 (18%) |
| Beta-blocker | 28 (85%) |
| Mineralocorticoid receptor antagonist | 10 (30%) |
| ACE inhibitor or ARB | 33 (100%) |
| Three or more agents | 19 (58%) |
| Comorbidity | |
| Hypertension | 33 (100%) |
| Ischemic heart disease | 17 (52%) |
| Diabetes mellitus | 4 (12%) |
| Dyslipidemia | 23 (70%) |
| Hemodynamics | |
| Systolic blood pressure, mmHg | 138 [134, 149] |
| Diastolic blood pressure, mmHg | 73 [62, 87] |
| Pulse rate, bpm | 69 [63, 76] |
| Plasma NT-pro BNP, pg/mL | 207 [107, 386] |
Continuous variables are presented as median and interquartile. Categorical variables are presented as numbers and percentages. ACE, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor II blocker; NT-pro BNP, N-terminal-pro B-type natriuretic peptide.
All patients had received angiotensin-converting enzyme inhibitors or angiotensin receptor II blockers before the initiation of sacubitril/valsartan and they were converted to sacubitril/valsartan at the timing of baseline. Most of them (82%) initiated sacubitril/valsartan at 100 mg/day and others (18%) at 200 mg/day. Approximately half of them [19 (58%)] had received 3 or more anti-hypertension agents.
All patients continued sacubitril/valsartan for three months without any drug-related complications such as symptomatic hypotension. Other anti-hypertension agents were also continued during the on-treatment period without any dose adjustments.
Trends in clinical variables including plasma NT-pro BNP levels
During 3-month pre-treatment period, systolic blood pressure tended to decrease from 145 [138, 154] to 138 [134, 149] mmHg (P=0.091; Figure 1A), whereas plasma NT-pro BNP levels remained statistically unchanged from 204 [132, 412] pg/mL to 207 [107, 386] pg/mL (P=0.84; Figure 1B).

During 3-month on-treatment period, systolic blood pressure decreased significantly from 138 [134, 149] to 130 [120, 136] mmHg (P<0.001; Figure 1A). Plasma NT-pro BNP levels also decreased significantly from 207 [107, 386] to 118 [64, 355] pg/mL (P=0.001; Figure 1B).
During the pre-treatment period without sacubitril/valsartan, plasma BNP levels remained unchanged from 38 [34, 46] to 56 [54, 78] pg/mL (P=0.67). During the 3-months sacubitril/valsartan treatment, plasma BNP levels increased significantly up to 76 [66, 95] pg/mL (P=0.027).
Changes in clinical variables including plasma NT-pro BNP levels
The amount of decrease in systolic blood pressure was significantly greater during the on-treatment period than the pre-treatment period {−10 [−19, −6] versus −4 [−11, 0] mmHg, P=0.006; Figure 2A}.
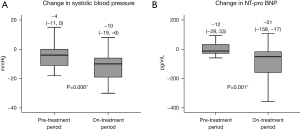
The amount of decrease in plasma NT-pro BNP levels were significantly greater during the on-treatment period compared with the pre-treatment period {−51 [−158, −17] versus −12 [−28, 33] pg/mL, P=0.001; Figure 2B}.
Discussion
In this study, we investigated the trends in NT-pro BNP levels following the initiation of sacubitril/valsartan therapy in hypertensive patients. During the pre-treatment period without sacubitril/valsartan, blood pressure tended to decrease whereas plasma NT-pro BNP levels remained unchanged. During the 3-month on-treatment period with sacubitril/valsartan, both blood pressure and plasma NT-pro BNP levels decreased significantly.
NT-pro BNP and cardiovascular events
Similar to BNP, NT-pro BNP is stimulated to be synthesized via the share stress toward the ventricle and atrium (8). Thus, NT-pro BNP is a well-known biomarker that indicates the severity of ongoing heart failure (9). Furthermore, NT-pro BNP is considered to be an important biomarker to predict further cardiovascular events even in the healthy cohorts (1-3). In the residential healthy cohort, the NT-pro BNP levels were associated with an incremental relative risk of cardiovascular events, independently on other cardiovascular risk factors, even among their normal range (i.e., below a cutoff of heart failure: <400 pg/mL). In other words, even though the level is within normal limit, a slight increase in NT-pro BNP level was associated with incremental cardiovascular risk among the healthy cohort.
Plasma NT-pro BNP (or BNP) level increases in several clinical situations. Patients with cardiac embolism had higher plasma BNP levels compared with other types of stroke, probably due to clinical or sub-clinical incremental intra-atrial pressure and thrombus formation in the left atrium (10). Patients with acute myocardial infarction had higher plasma BNP levels, probably due to acute cardiac ischemia (11). Detailed mechanism remains uncertain, but a slight increase in plasma NT-pro BNP level among those without baseline cardiovascular diseases might represent the sub-clinical progression of cardiovascular diseases that would clinically rise to the surface in the near future (2).
Sacubitril/valsartan and NT-pro BNP
The association between sacubitril/valsartan and NT-pro BNP in patients with chronic heart failure is well known (5), whereas their association among those with hypertension remains unknown (7). Sacubitril/valsartan has recently been reimbursed for hypertension in Japan. Most of the patients who were enrolled in phase II and phase III trials had mild to moderate de novo hypertension, and sacubitril/valsartan was administered alone without any other hypertensive drugs (12,13). In the actual clinical practice using several hypertensive drugs concomitantly, we found that sacubitril/valsartan therapy was associated with a decrease in blood pressure and plasma NT-pro BNP level.
Among those with chronic heart failure, sacubitril/valsartan facilitates cardiac unloading via vascular dilatation, suppression of sympathetic nerve activity, natriuresis, and amelioration of cardiac fibrosis, thereby decreasing plasma NT-pro BNP level (14). Patients achieving a greater reduction in plasma NT-pro BNP levels enjoy more reverse remodeling (15). Sacubitril/valsartan might decrease plasma NT-pro BNP levels even among those without heart failure, like our hypertensive cohort, via a similar mechanism in a subclinical manner. Consistently, plasma BNP levels increased following the initiation of sacubitril/valsartan in this study. Increased plasma BNP levels should have contributed to improve hypertension via natriuresis, vasodilation, and sympathetic nerve suppression.
Sacubitril/valsartan decreased blood pressure in our study as observed in the previous studies (16,17). Afterload reduction via blood pressure decrease has a potential to unload the left ventricle and decrease plasma NT-pro BNP level. However, in our study, an improvement in hypertension was not associated with a decrease in plasma NT-pro BNP level during the pre-treatment period (without sacubitril/valsartan). The impact of blood pressure decrease itself on the decrease in plasma NT-pro BNP level might be partial (18). Neurohormonal impact of sacubitril/valsartan should have additive effect in decreasing plasma NT-pro BNP levels in addition to its anti-hypertensive effect.
The plasma NT-pro BNP level is just a surrogate marker of future cardiovascular events. Further prospective studies are warranted to investigate the causality between the amelioration of plasma NT-pro BNP by sacubitril/valsartan therapy and the actual risk reduction in cardiovascular events.
Limitations
The sample size was small. Statistical non-significance in this study does not guarantee the similarity between the groups. We did not prepare a control group receiving placebo. Instead, we compared the outcomes during the on-treatment period with the pre-treatment period. There might have been some potential confounders. We investigated the plasma NT-pro BNP levels as a surrogate marker, and its impact on clinical outcomes such as cardiovascular events requires further studies. Given these limitations, we should be careful to interpret our findings and larger-scale studies are warranted to validate our findings.
Conclusions
Plasma NT-pro BNP levels decreased significantly following 3-month sacubitril/valsartan therapy in hypertensive patients without heart failure. Its clinical implication requires further long-term studies.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-483/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-483/dss
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-483/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The present study was approved by the Institutional Review Board of University of Toyama (No. R2015154, April 11, 2016). Written informed consents were obtained from all participants. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the ethical standard of the responsible committee on human experimentation.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Doi Y, Ninomiya T, Hata J, et al. N-terminal pro-brain natriuretic peptide and risk of cardiovascular events in a Japanese community: the Hisayama study. Arterioscler Thromb Vasc Biol 2011;31:2997-3003. [Crossref] [PubMed]
- Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med 2004;350:655-63. [Crossref] [PubMed]
- Kistorp C, Raymond I, Pedersen F, et al. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA 2005;293:1609-16. [Crossref] [PubMed]
- McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993-1004. [Crossref] [PubMed]
- Myhre PL, Vaduganathan M, Claggett B, et al. B-Type Natriuretic Peptide During Treatment With Sacubitril/Valsartan: The PARADIGM-HF Trial. J Am Coll Cardiol 2019;73:1264-72. [Crossref] [PubMed]
- Rakugi H, Kario K, Yamaguchi M, et al. Efficacy of sacubitril/valsartan versus olmesartan in Japanese patients with essential hypertension: a randomized, double-blind, multicenter study. Hypertens Res 2022;45:824-33. [Crossref] [PubMed]
- Williams B, Cockcroft JR, Kario K, et al. Effects of Sacubitril/Valsartan Versus Olmesartan on Central Hemodynamics in the Elderly With Systolic Hypertension: The PARAMETER Study. Hypertension 2017;69:411-20. [Crossref] [PubMed]
- Martinez-Rumayor A, Richards AM, Burnett JC, et al. Biology of the natriuretic peptides. Am J Cardiol 2008;101:3-8. [Crossref] [PubMed]
- Hartmann F, Packer M, Coats AJ, et al. Prognostic impact of plasma N-terminal pro-brain natriuretic peptide in severe chronic congestive heart failure: a substudy of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) trial. Circulation 2004;110:1780-6. [Crossref] [PubMed]
- Shibazaki K, Kimura K, Iguchi Y, et al. Plasma brain natriuretic peptide can be a biological marker to distinguish cardioembolic stroke from other stroke types in acute ischemic stroke. Intern Med 2009;48:259-64. [Crossref] [PubMed]
- Morita E, Yasue H, Yoshimura M, et al. Increased plasma levels of brain natriuretic peptide in patients with acute myocardial infarction. Circulation 1993;88:82-91. [Crossref] [PubMed]
- Ruilope LM, Dukat A, Böhm M, et al. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet 2010;375:1255-66. [Crossref] [PubMed]
- Kario K, Sun N, Chiang FT, et al. Efficacy and safety of LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor, in Asian patients with hypertension: a randomized, double-blind, placebo-controlled study. Hypertension 2014;63:698-705. [Crossref] [PubMed]
- Hubers SA, Brown NJ. Combined Angiotensin Receptor Antagonism and Neprilysin Inhibition. Circulation 2016;133:1115-24. [Crossref] [PubMed]
- Januzzi JL Jr, Prescott MF, Butler J, et al. Association of Change in N-Terminal Pro-B-Type Natriuretic Peptide Following Initiation of Sacubitril-Valsartan Treatment With Cardiac Structure and Function in Patients With Heart Failure With Reduced Ejection Fraction. JAMA 2019;322:1085-95. [Crossref] [PubMed]
- Wang JG, Yukisada K, Sibulo A Jr, et al. Efficacy and safety of sacubitril/valsartan (LCZ696) add-on to amlodipine in Asian patients with systolic hypertension uncontrolled with amlodipine monotherapy. J Hypertens 2017;35:877-85. [Crossref] [PubMed]
- Supasyndh O, Wang J, Hafeez K, et al. Efficacy and Safety of Sacubitril/Valsartan (LCZ696) Compared With Olmesartan in Elderly Asian Patients (≥65 Years) With Systolic Hypertension. Am J Hypertens 2017;30:1163-9. [Crossref] [PubMed]
- Nakatsu T, Shinohata R, Mashima K, et al. Use of plasma B-type natriuretic peptide level to identify asymptomatic hypertensive patients with abnormal diurnal blood pressure variation profiles: nondippers, extreme dippers, and risers. Hypertens Res 2007;30:651-8. [Crossref] [PubMed]


