
Analysis of the causes of failed placement of nasoenteric tube under DSA guidance and treatment strategies for successful re-catheterization
Introduction
For patients with advanced malignant tumors or after digestive tract reconstruction, enteral nutrition is crucial for clinical antitumor treatment (1,2), not only because of convenience and efficacy, but also reducing the probability of enterogenous infection and further improving the stress and immune function of patients (3-5). Patients with advanced malignant tumors can have widely dispersed metastases and different degrees of organ invasion, often resulting in an intestinal obstruction that is difficult to pass with a gastroscopically guided nasoenteric tube. Patients who have undergone digestive tract reconstruction (6-8) have significantly decreased peristalsis and the utility of gastroenterostomy is also greatly reduced. In both groups, imaging-guided placement of the nasoenteric tube has obvious advantages, but there can still be failure. At present, the success rate of digital subtraction angiography (DSA) guided nasoenteric catheterization is still very high, but the probability of catheterization failure in some special populations is greatly increased, such as patients after multiple digestive tract reconstructions, patients with multiple digestive system metastases, patients with secondary anastomotic leakage, patients with incomplete obstruction, etc. For the above patients, if enteral nutrition cannot be given in time, subsequent clinical treatment cannot be carried out normally, and even critical life, we should actively optimize the strategy of successful catheterization to meet the needs of enteral nutrition in more patients with advanced cancer.
This study analyzed all cases of initial tube placement failure from February 2015 to July 2020, and explored the causes and influential factors. All cases of successful reintubation were analyzed for related strategies, such as preoperative preparation or intraoperative techniques, that improved the success rate of DSA-guided nasoenteric tube placement. We present the following article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-903/rc).
Methods
Case data
A total of 3810 clinical cases of nasoenteric tube placement guided by DSA between February 2015 and July 2020 were retrospectively analyzed, and a total of 94 cases of primary intubation failure were analyzed for the causes of failure. In each case, tube placement was attempted at least twice and was successful in 42 cases, which were further analyzed for the management strategies.
Inclusion criteria
(I) Failure of initial tube placement; (II) able to tolerate second or multiple tube placement attempts; (III) enteral nutrition established within 3 days of successful tube placement; (IV) no serious complications such as gastrointestinal bleeding and gastrointestinal perforation after tube placement.
Exclusion criteria
(I) Failure of ≥2 attempts at tube placement; (II) unable to cooperate with procedure; (III) gastroparesis diagnosed by relevant examination before operation; (IV) abnormal coagulation function; (V) gastrointestinal bleeding in the first week before placement; (VI) complete gastrointestinal obstruction; (VII) abnormal cardiopulmonary function.
Placement method
Preoperative gastrointestinal radiography, computed tomography and other relevant imaging data were routinely assessed to rule out serious complications such as gastrointestinal perforation and gastrointestinal obstruction. After a 6-h fast, the patients were placed in the supine position, and lidocaine mucilage was sprayed into the nasal cavity 10 min before the procedure for nasopharyngeal topical anesthesia. The catheter with a superslippery guide wire was inserted from one nostril into the digestive tract, and DSA was performed to determine correct placement (i.e., not in the fistula or bronchus), the postoperative digestive tract reconstruction, and the extent and degree of gastrointestinal stenosis before advancing to the distal gastrointestinal tract as the ideal position for the nasoenteric feeding tube (~10–20 cm into the jejunum under the Treitz ligament or the distal anastomosis).
Efficacy assessment
Failure of nasoenteric tube placement under DSA guidance for the first time was defined as a case of initial placement failure, we collected and counted the causes of initial catheterization failure through preoperative related preparation, preoperative gastrointestinal tract initial situation of patients, intraoperative gastrointestinal imaging data of patients and intraoperative related operating procedures, and included inadequate preoperative preparation, uncooperative patient or sudden serious complications, prolonged placement time due to inappropriate operation or imperfect technique, and intraoperative accidents requiring immediate termination of the operation; after failure of initial intubation, there were 83 cases of secondary intubation, 11 of multiple attempts, and a total of 42 cases of successful reintubation with the tube reaching the ideal site, and the patient able to tolerate the procedure. Because this article only involves count statistics, does not involve the relevant statistical methods and statistical software.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of the Fourth Hospital of Hebei Medical University and Hebei Tumor Hospital (No. 2021KY182). Informed consent was taken from all the patients.
Results
Causes of initial placement failure
Among the 94 patients who had initial placement failure, anastomotic stenosis or obstruction, overexpansion of the gastric lumen, pyloric stenosis or obstruction of the gastric sinus, stenosis or obstruction of the output collaterals, fistula lumen obscuring the normal digestive tract, and duodenal stenosis or obstruction were the main causes, accounting for 80% of all failed cases (Figure 1A-1F, Table 1).
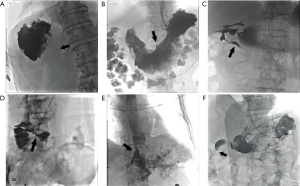
Table 1
| Failure reason | n | % |
|---|---|---|
| Anastomotic stenosis or obstruction | 19 | 20.2 |
| Hyperdilation of the gastric cavity | 16 | 17.0 |
| Pyloric stenosis or obstruction of the gastric sinus | 13 | 13.8 |
| Narrowing or obstruction of the output collaterals | 11 | 11.7 |
| Fistula lumen obscuring the normal digestive tract | 9 | 9.6 |
| Duodenal stenosis or obstruction | 7 | 7.4 |
| Localized luminal stenosis of the small intestine | 6 | 6.4 |
| Intraoperative complications | 6 | 6.4 |
| Unable to tolerate the operation | 4 | 4.3 |
| Stent narrowing | 1 | 1.1 |
| Misplacement of the guidewire into the abdominal cavity | 1 | 1.1 |
| Giant esophageal diverticulum | 1 | 1.1 |
| Total | 94 |
Strategies for successful reintubation
Management responses in the 42 cases of successful reintubation were: adequate negative pressure drainage, adjustment of the C-arm projection angle, use of cone-beam CT, balloon dilation of the stenotic segment, application of a stent, and combined gastroscopic placement (Figure 2A-2F, Table 2).
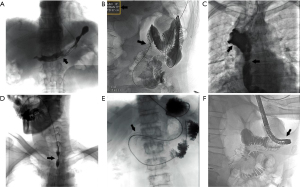
Table 2
| Strategy | n | % |
|---|---|---|
| Adequate negative pressure drainage | 9 | 21.4 |
| Adjust the C-arm projection angle | 7 | 16.7 |
| Using cone-beam CT | 5 | 11.9 |
| Balloon dilation of stenotic segment | 4 | 9.5 |
| Application brackets | 4 | 9.5 |
| Combined gastroscopic tube placement | 3 | 7.1 |
| Inject a large amount of gas | 2 | 4.8 |
| Use of a three-chamber feeding tube | 2 | 4.8 |
| Use of contrast catheters instead of nutrition tubes | 2 | 4.8 |
| Preoperative patient education | 2 | 4.8 |
| Combined application of intestinal obstruction catheter | 2 | 4.8 |
| Total | 42 |
Discussion
Nasoenteric nutrition is indispensable for patients with malignant digestive system tumors or who have undergone digestive tract reconstruction (9-11). Insertion of a nasoenteric feeding tube can not only maintain safe and efficient enteral nutrition (12,13), but can be also used for targeted gastrointestinal decompression, accurate fistula drainage, local radiotherapy of the digestive tract, etc. (14,15). However, because of their complex conditions, placement of the nasoenteric feeding tube by conventional endoscopy can be difficult (16-18). DSA-guided nasoenteric tube placement is relatively safe and reliable, serious complications are rare, and intraoperative imaging can identify the intestinal access after complex surgeries (19-21), as well as extensive abdominal metastasis and multiple intestinal strictures. By thorough analysis of cases of failed intubation (22-24), we can improve both preoperative preparation and intraoperative technique (25-27), thus improving the success rate and establishment of enteral nutrition in patients with malignant tumors.
Management of anastomotic stenosis or obstruction
Anastomotic stricture or obstruction is the most common cause of intubation failure. In complex digestive tract reconstructions, there are many anastomoses and relatively dense distribution. Patients have poor postoperative peristalsis, which prevents or slows the transit of contrast agent and makes judgment of the output and input loops very difficult. If the patient has anatomical variations, or concurrent intestinal torsion, or combined postoperative anastomotic mucosal edema and adhesion, luminal stenosis is further aggravated, resulting in significantly increased difficulty of using the guide wire to smoothly open the anastomotic stoma to find the anastomotic pathway. Thus the management strategy is to improve gastrointestinal radiography before intubation to identify the anastomotic pathway, monitor the motility of the digestive tract and the degree of intestinal dilatation throughout, clearly show the flow direction and stasis of contrast agent, clearly determine the location of the input and output loops and the degree of peristalsis, clearly understand in advance the anatomical structure after digestive tract reconstruction, clarify the number of anastomotic stoma and anastomotic category to locate narrowed or obstructed segments, and thus ensure sufficient preparation for tube placement. Relief of anastomotic or bowel dilatation and edema, prevention of contrast agent accumulation, and stimulation of peristalsis are successful management strategies.
Management of pyloric and duodenal stenosis or obstruction of the gastric sinus
Lesions of the antral pylorus and duodenum are common factors in unsuccessful intubation. Tumors in the antral, pyloric, or duodenal region will not only cause stenosis or even obstruction, but also have a high probability of causing secondary dilatation of the gastric lumen. There is already an anatomical difficulty in passing the guide wire through the pylorus, so the addition of invasion and compression significantly increases the difficulty of intubation. Dilatation of the gastric lumen increases the uncertainty of the location of guide wire catheter within the huge lumen and without peristalsis of the gastric body it is difficult to accurately determine the outlet of the pylorus. The management strategy is to collaborate with clinical departments to improve the preoperative preparation of patients who are prone to have dilatation of the gastric lumen. Gastrointestinal decompression with improved drainage for more than 24 h, symptomatic treatment as early as possible for patients with different degrees of gastroparesis after surgery, and adjuvant therapy to protect the gastric mucosa and normalize the volume of the gastric lumen will significantly improve the success rate of intubation.
Management of stenosis of the small bowel lumen
Local small intestinal stenosis is mostly due to tumor invasion, a long course of disease, or extensive abdominal adhesions. Prolonged exploration with the guide wire for passage through the stenosed segment will be intolerable for the patient and result in tube placement failure (11). In the case of simple luminal stenosis, it is necessary to carefully examine the preoperative radiography findings to identify the particularity of the postoperative anatomy and the specific location of gastrointestinal stenoses, adjust the angle of the C-arm projection and the patient’s position during the procedure to achieve the ideal view for observing luminal stenosis. In the case of only the guide wire being able to pass through the excessively narrow lumen but not the nasoenteric tube, balloon dilation can widen the segment (12). For critically ill patients who cannot tolerate the balloon operation, the angiographic catheter can temporarily replace the feeding tube for short-term enteral nutrition, until the patient is fit enough to undergo nasoenteric tube placement.
Management of fistula lumen obscuring normal digestive tract
For patients with anastomotic leakage or tumor invading the lumen of the digestive tract, contrast agent fills the localized fistula cavity, making it difficult to distinguish the correct distal digestive tract. In addition, if the fistula is large or the digestive tract is adherent due to infection, the guide wire can mistakenly enter the fistula cavity many times, which not only delays self-repair of the fistula, but also increases the risk of anastomotic bleeding and other related risks (13,14). The management strategy is to reduce overlapping access and contrast agent stasis through intraoperative use of cone-beam CT and minimal contrast agent. The opacity of the guide wire catheter can quickly identify the specific location of the guide wire, improve intubation efficiency and also reduce the risk of infection and other related complications. Cone-beam CT can be performed in advance to identify the condition of the fistula, and then the catheter can be selected according to the specific shape of the fistula. If the fistula is the greatest obstacle to smooth entry of the small intestine, a digestive tract stent can be placed to close the fistula.
Management of intraoperative complications or patients’ intolerance of the procedure
During intubation, different degrees of complications will occur due to different tolerance and physical differences of patients. For example, stressed patients with low pain threshold can be guided in advance through preoperative education; nasal lubrication, local anesthesia and other related preparations can also be performed in advance. Improvement of coagulation and cardiopulmonary function and performing other related laboratory tests before intubation can prevent intraoperative gastrointestinal bleeding, organ failure and other serious complications. Body temperature should be monitored in patients with definite anastomotic leakage, appropriate medications given to patients with tracheal fistula before surgery, tracheal intubation to prevent intraoperative aspiration in patients with severe dyspnea, and for patients who cannot tolerate simultaneous nasoenteric and decompression tubes in the same nostril, a three-lumen feeding tube can be used. For serious intraoperative complications such as sudden vomiting, choking cough, decreased blood oxygen, etc., it is necessary to immediately terminate the operation.
Management of other uncommon causes
There are some relatively rare causes of failure that should be noted. Multiple small intestinal strictures are common with multiple metastases or extensive intestinal adhesions. Although there is no anatomical abnormality, the lumen is abnormally twisted, so gentle and cautious technique is required to avoid serious complications of gastrointestinal perforation by the guide wire. In the case of strictures with extensive invasion of the tumor lesion, when the guide wire attempts to pass through the obstructed segment or stricture, catheter exchange technique or double guide wire technique can be used according to the degree of stricture to improve the success rate. Sometimes after stent implantation in patients with digestive tract tumors, excessive stent lumen stenosis prevents passage of the nasoenteric tube after significant tumor progression, and reintubation can be performed after removing the stent through the conveyor to ensure the patient’s enteral nutrition. For patients with luminal stricture due to benign lesions, such as hiatal hernia, giant diverticulum etc., intubation can be performed as the firstline treatment of the primary disease.
In summary, DSA-guided nasoenteric feeding tube placement is a safe and efficient operation to satisfy the clinical demand of enteral nutrition technology. However, for individual patients with advanced malignant tumors and/or digestive tract reconstruction, due to their complex conditions sometimes intubation is not successful on the first attempt. Detailed preoperative preparation and correct intraoperative techniques will greatly improve the success rate of nasoenteric tube placement and subsequent enteral nutrition.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-903/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-903/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-903/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of the Fourth Hospital of Hebei Medical University and Hebei Tumor Hospital (No. 2021KY182). Informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roeland EJ, Bohlke K, Baracos VE, et al. Management of Cancer Cachexia: ASCO Guideline. J Clin Oncol 2020;38:2438-53. [Crossref] [PubMed]
- Gliwska E, Guzek D, Przekop Z, et al. Quality of Life of Cancer Patients Receiving Enteral Nutrition: A Systematic Review of Randomized Controlled Trials. Nutrients 2021;13:4551. [Crossref] [PubMed]
- Hadefi A, Arvanitakis M. How to Approach Long-term Enteral and Parenteral Nutrition. Gastroenterology 2021;161:1780-6. [Crossref] [PubMed]
- Andersen S, Staudacher H, Weber N, et al. Pilot study investigating the effect of enteral and parenteral nutrition on the gastrointestinal microbiome post-allogeneic transplantation. Br J Haematol 2020;188:570-81. [Crossref] [PubMed]
- Amano K, Maeda I, Ishiki H, et al. Effects of enteral nutrition and parenteral nutrition on survival in patients with advanced cancer cachexia: Analysis of a multicenter prospective cohort study. Clin Nutr 2021;40:1168-75. [Crossref] [PubMed]
- Ding H, Xu J, You J, et al. Effects of enteral nutrition support combined with enhanced recovery after surgery on the nutritional status, immune function, and prognosis of patients with esophageal cancer after Ivor-Lewis operation. J Thorac Dis 2020;12:7337-45. [Crossref] [PubMed]
- Bi Y, Zhu X, Yu Z, et al. Radioactive feeding tube in the palliation of esophageal malignant obstruction. Radiol Med 2020;125:544-50. [Crossref] [PubMed]
- Smith ZL, Dua KS. Patient selection in studies evaluating esophageal stents during neoadjuvant therapy. Gastrointest Endosc 2019;89:205-6. [Crossref] [PubMed]
- Cotogni P, Stragliotto S, Ossola M, et al. The Role of Nutritional Support for Cancer Patients in Palliative Care. Nutrients 2021;13:306. [Crossref] [PubMed]
- Iturbide-Casas MA, Cámara-Martos F, Molina-Luque R, et al. Survival Analysis of Enterally Fed Patients: Prognosis and Mortality Risk According to Baseline Characteristics. JPEN J Parenter Enteral Nutr 2020;44:1057-65. [Crossref] [PubMed]
- Chow R, Bruera E, Arends J, et al. Enteral and parenteral nutrition in cancer patients, a comparison of complication rates: an updated systematic review and (cumulative) meta-analysis. Support Care Cancer 2020;28:979-1010. [Crossref] [PubMed]
- Willemsen ACH, Kok A, Baijens LWJ, et al. Development and external validation of a prediction model for tube feeding dependency for at least four weeks during chemoradiotherapy for head and neck cancer. Clin Nutr 2022;41:177-85. [Crossref] [PubMed]
- Quilliot D, Michot N, Germain L, et al. Feasibility, acceptability of enteral tube feeding and self-insertion of a nasogastric tube in the nutritional management of digestive cancers, impact on quality of life. Clin Nutr 2020;39:1785-92. [Crossref] [PubMed]
- Williams GF, White H, Sen M, et al. Patients' experience of enteral feeding following (chemo) radiotherapy for head and neck cancer: A qualitative study. Clin Nutr 2019;38:1382-9. [Crossref] [PubMed]
- Smeets BJ, Heesakkers FF, Huijbregts CP, et al. Antioxidative Amino Acids in Early Enteral Versus Parenteral Nutrition Following Major Rectal Surgery. Crit Care Med 2020;48:e990-1. [Crossref] [PubMed]
- Kita R, Miyata H, Sugimura K, et al. Clinical effect of enteral nutrition support during neoadjuvant chemotherapy on the preservation of skeletal muscle mass in patients with esophageal cancer. Clin Nutr 2021;40:4380-5. [Crossref] [PubMed]
- Gresham G, Placencio-Hickok VR, Lauzon M, et al. Feasibility and efficacy of enteral tube feeding on weight stability, lean body mass, and patient-reported outcomes in pancreatic cancer cachexia. J Cachexia Sarcopenia Muscle 2021;12:1959-68. [Crossref] [PubMed]
- Yu K, Zheng X, Wang G, et al. Immunonutrition vs Standard Nutrition for Cancer Patients: A Systematic Review and Meta-Analysis (Part 1). JPEN J Parenter Enteral Nutr 2020;44:742-67. [Crossref] [PubMed]
- Chapek MA, Martindale RG. Nutrition in cancer therapy: Overview for the cancer patient. JPEN J Parenter Enteral Nutr 2021;45:33-40. [Crossref] [PubMed]
- Ye X, Chang YC, Findlay M, et al. The effect of timing of enteral nutrition support on feeding outcomes and dysphagia in patients with head and neck cancer undergoing radiotherapy or chemoradiotherapy: A systematic review. Clin Nutr ESPEN 2021;44:96-104. [Crossref] [PubMed]
- Coke A, Gilbert M, Hill S, et al. Nasogastric Feeding Tube/Dobhoff Placement: A Multidisciplinary Approach to the Management of Malnutrition During Radiation Therapy in Patients With Head and Neck Cancer. Cureus 2022;14:e24905. [Crossref] [PubMed]
- Deepjyoti K, Bannoth S, Purkayastha J, et al. Nasojejunal Feeding Is Safe and Effective Alternative to Feeding Jejunostomy for Postoperative Enteral Nutrition in Gastric Cancer Patients. South Asian J Cancer 2020;9:70-3. [Crossref] [PubMed]
- Bozzetti F. Does nutrition support during chemotherapy increase long-term survival of cancer patients? Lessons from the past and future perspectives. Support Care Cancer 2021;29:7269-77. [Crossref] [PubMed]
- Nunes G, Fonseca J, Barata AT, et al. Nutritional Support of Cancer Patients without Oral Feeding: How to Select the Most Effective Technique? GE Port J Gastroenterol 2020;27:172-84. [Crossref] [PubMed]
- Itou C, Arai Y, Sone M, et al. Transgastric Feeding Tube Insertion into the Jejunum after Esophagectomy: Direct Puncture of the Gastric Conduit. J Vasc Interv Radiol 2021;32:1464-9. [Crossref] [PubMed]
- Kanie Y, Okamura A, Fujihara A, et al. Long-Term Insufficiency of Oral Intake after Esophagectomy: Who Needs Intense Nutritional Support after Esophagectomy? Ann Nutr Metab 2022;78:106-13. [Crossref] [PubMed]
- Liao M, Xia Z, Huang P, et al. Early enteral feeding on esophageal cancer patients after esophageal resection and reconstruction. Ann Palliat Med 2020;9:816-23. [Crossref] [PubMed]


