
The status and risk factors for anxiety/depression in patients with atrophic chronic gastritis: a cross-sectional study
Introduction
Atrophic chronic gastritis (ACG) is an inflammatory lesion of the gastric mucosa caused by various aetiologies. Multiple damages to the gastric mucosal epithelium cause spontaneous repair after damage and eventually lead to irreversible atrophy or even disappearance of the gastric glands (1). The global incidence rates of ACG range from 0 to 11% annually (2). ACG can progress to gastric cancer. It is estimated that the risk of progression of ACG to gastric adenocarcinoma ranges from 0.1% to 0.3% per year, which may be higher after considering the severity and extent of atrophic, intestinal metaplasia, and other factors (3). Globally, gastric cancer has the third leading incidence and third highest mortality of all types of cancers in China. In 2020, 482,300 new gastric cancer cases and 374,000 deaths occurred in China (4). Risk factors for ACG, such as Helicobacter pylori (Hp), diet and environment, and autoimmunity, are well known. It has been recognized that psychosocial factors have an important influence on both the onset and the exacerbations of functional gastrointestinal disorders and on health care seeking, illness behavior, and therapeutic outcome (5). Studies have shown that mood disorders dominated by anxiety and depression are important risk factors for many gastrointestinal diseases (6), and individuals with ACG have a significantly higher risk of experiencing psychological distress (7). The incidence of anxiety in patients with ACG was reported to be 42.51% (8). In recent years, it has been shown that ACG patients are commonly accompanied by psychological distress (9) and often required anxiolytic drug treatment (10). However, there are no studies to specifically elucidated the association between anxiety or depression and ACG progression until now.
Previous studies have shown that anxiety and depression can affect gastrointestinal function and contribute to digestive diseases (11,12). One study suggests that modulation of the brain-gut axis could be a treatment in gastrointestinal disease (11). Brain-gut peptides secreted by the brain-gut axis modulate hormone signalling pathways to modify gastrointestinal function (13). Thus, anxiety and depression are likely to contribute to the progression of ACG by similar mechanisms. However, the risk factors for anxiety and depression in ACG patients are not yet fully understood. As anxiety and depression play an increasingly important role in gastrointestinal disorders, there is a strong need to understand the risk factors for anxiety and depression in ACG patients. We present the following article in accordance with the STROBE and TRIPOD reporting checklists (available at https://apm.amegroups.com/article/view/10.21037/apm-22-730/rc).
Methods
Patients
In total, 154 patients with ACG were invited. All patients had at least one outpatient clinic visit to Guangzhou First People’s Hospital from July 2021 to May 2022. Finally, 118 patients with ACG were included. All patients received gastroscopy and were diagnosed after pathological biopsy in our hospital or other tertiary hospitals within one year. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Guangzhou First People’s Hospital Institutional Review Board (approval No. K-2021-203-01). All patients signed informed consent.
Inclusion and exclusion criteria
The inclusion criteria were patients who: (I) were 25–75 years old; (II) received gastroscopy and were diagnosed after pathological biopsy in our hospital or other tertiary hospitals according to the diagnosis criteria of the Chinese Consensus Opinion on Chronic Gastritis (2017, Shanghai) (1); and (III) had a disease duration within 1 year.
The exclusion criteria were patients with: (I) a previous history of benign tumours; (II) a previous history of other chronic diseases that seriously affect survival, including malignancy, acute myocardial infarction in the past 2 years, and severe hypertension; (III) a previous history of psychological disorder disease; (IV) a previous history of antidepressant or antianxiety medication; (V) concurrent acute or severe digestive system diseases, including acute gastritis, pancreatitis, and acute hepatitis; and (VI) pregnancy or lactation.
Demographics, variables, and scoring
Thirty-two variables were analysed, including gender, age, body mass index (BMI), marital status, educational level, work situation, monthly income, sleep, number of family members, family history of tumours, weekly exercise time, dietary habits, smoking, drinking, and digestive symptoms. The two scales used in this study were the Generalized Anxiety Disorder Scale-7 (GAD-7) and the Patient Health Questionnaire-9 (PHQ-9) (the authors have permission to use this instrument from the copyright holders). Both scales are quantitative assessment criteria recommended by the American Psychiatric Association in May 2013 and the Psychiatric Branch of the Chinese Medical Association on June 8, 2015. These two scales are easy for patients to fill out and have been validated in China (14,15) and around the world by numerous studies (16,17). The GAD-7 scale is used to screen and assess generalized anxiety symptoms and is a 7-item self-rating scale on a 4-point scale; the cut-off of 5 points corresponds to mild anxiety, 10 to moderate anxiety, and 15 to severe anxiety. The PHQ-9 scale is used to screen and assess depressive symptoms and is a 9-item self-rating scale on a 4-point scale; the cut-off of 5 points corresponds to mild depression, 10 to moderate depression, and 15 to severe depression.
Dietary habits were defined as follows: a high-salt diet (≥6 g/day), frequent consumption of smoked foods (≥1 time/week), frequent consumption of grilled foods (≥1 time/week), frequent consumption of spicy and stimulating foods (hot and spicy food, dehydration food) (≥1 time/day) (18,19), frequent consumption of fried foods (≥1 time/week), and lack of fresh vegetables and fruits (<200 g/day) (20). Sleep was defined as whether one was satisfied with the quality of one’s sleep. Smoking was defined as follows: never smoking, currently smoking (smoking continuously or cumulatively for 6 months), and quitting smoking (stopped smoking for 6 months or more). Drinking alcohol status was defined as non-drinking, occasional drinking (<1 time per week), and frequent drinking (≥3 times per week). Digestive system symptoms were requested to last lasting more than 3 months.
Statistical analysis
Statistical analysis was conducted using SPSS software version 26.0 (IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp.). The one-sample Kolmogorov-Smirnov test was used to test the normality of continuous variables, and the normally distributed measures were expressed as mean ± standard deviation. Comparisons between groups were carried out by t-test (two tails, unequal variance) and Mann-Whitney nonparametric test. Count data are expressed as the counts (percentages), and the measurement data are presented as the means ± standard deviation. Factors identified as being significant (P<0.05) by univariate analysis were subsequently entered into a multivariate logistic regression model. A stepwise approach was used for sifting through large numbers of potential independent variables. The ROC curve was used to verify the accuracy of the model.
Analysis of risk factors for anxiety and depression in ACG patients
In total, 118 ACG patients were assessed using the Anxiety Disorder Scale-7 (GAD-7) and PHQ-9. They were stratified according to the scoring. Risk factors were analysed for patients with ACG with anxiety, depression, both anxiety and depression, moderate to severe anxiety, and moderate to severe depression. Univariate and multivariate conditional logistic regression analyses were performed. Identifying the independent risk factors for anxiety and depression and establishing risk prediction models. The model was evaluated through the ROC curve and validated by verifying the sensitivity and calibration.
Results
Demographic data
Overall, 154 patients participated in the study (Figure 1); of these, 118 ACG patients finally met the inclusion criteria and were enrolled, including 52 females and 66 males. The mean age of the patients was 52.30±12.46 years. Middle-aged patients accounted for the majority of the ACG patients (52.6%). Marital status, education, work, and monthly income are shown in Table 1.
Table 1
| Variables | ACG patients (n=118) |
|---|---|
| Age, years | 52.30±12.46 |
| 25–44 | 26 (22.0) |
| 45–59 | 62 (52.6) |
| 60–77 | 30 (25.4) |
| Gender | |
| Male | 66 (55.9) |
| Female | 52 (44.1) |
| BMI, kg/m2 | 22.33±2.81 |
| <18.5 | 11 (9.3) |
| 18.5–23.9 | 75 (63.6) |
| 24–26.9 | 26 (22.1) |
| 27–29.9 | 5 (4.2) |
| ≥30 | 1 (0.8) |
| Marital | |
| Single | 11 (9.3) |
| Married | 102 (86.4) |
| Divorced | 3 (2.6) |
| Widowed | 2 (1.7) |
| Education | |
| Low | 33 (28.0) |
| Middle | 28 (23.7) |
| High | 57 (48.3) |
| Employment status | |
| Employed | 66 (56.0) |
| Unemployed | 52 (44.0) |
| Monthly income, | |
| ≥4,000 CNY | 67 (56.8) |
| <4,000 CNY | 51 (43.2) |
Values were presented as mean ± standard deviation or n (%). ACG, atrophic chronic gastritis; BMI, body mass index; CNY, Chinese Yuan; Low, elementary school and junior high school; Middle, high school, technical or vocational school; High, university or college.
The status of anxiety and depression in ACG patients
The patients’ mean scores on the GAD-7 scale and PHQ-9 scale were 4.33±4.96 and 3.71±5.19, respectively. The proportion of anxiety state in ACG patients was 36.4% (43/118). Among them, patients with moderate to severe anxiety accounted for 46.5% (20/43). The proportion of depression in ACG patients was 25.4% (30/118). Among them, patients with moderate to severe depression accounted for 40.0% (12/30). The proportion of both anxiety and depression states was 21.2% (25/118). Details are provided in Table 2.
Table 2
| Variables | ACG patients (n=118) |
|---|---|
| Score | |
| GAD-7 | 4.33±4.96 |
| PHQ-9 | 3.71±5.19 |
| Distribution | |
| Anxiety | 43 (36.4) |
| Mild | 23 (19.5) |
| Moderate | 15 (12.7) |
| Severe | 5 (14.2) |
| Depression | 30 (25.4) |
| Mild | 18 (15.3) |
| Moderate | 6 (5.1) |
| Severe | 6 (5.1) |
| Both anxiety and depression | 25 (21.2) |
| Without anxiety or depression | 70 (59.3) |
Values were presented as mean ± standard deviation or n (%). Mild: 5–10 points; Moderate: 11–15 points; Severe: 16–20 points. GAD-7, Generalized Anxiety Disorder Scale-7; PHQ-9, Patient Health Questionnaire-9; ACG, atrophic chronic gastritis.
Risk factors for anxiety and depression in ACG patients
Anxiety
Of the 118 ACG patients, 43 (36.4%) had anxiety. Univariate analysis showed that the following variables were associated with anxiety in ACG patients: age ≥60 years, poor sleep quality, fruit and vegetable deficiency, smoking, and belching. Multivariate logistic regression analysis showed that poor sleep quality [odds ratio (OR) 4.32, 95% confidence interval (CI): 1.60–11.65, P=0.004] was independent risk factor for anxiety in ACG patients. Smoking (OR 0.15, 95% CI: 0.03–0.68, P=0.014) and weekly exercise time (OR 0.89, 95% CI: 0.79–0.99, P=0.037) were independent protective factors in ACG patients. We conducted a further analysis of patients with moderate to severe anxiety. The results showed that poor sleep quality (OR 9.51, 95% CI: 1.87–48.30, P=0.007) was independent risk factor but weekly exercise time (OR 0.785, 95% CI: 0.64–0.96, P=0.018) was independent protective factors in ACG patients.
Depression
Of the 118 ACG patients, 30 (25.4%) had depression. Univariate analysis showed that the following variables were associated with depression in ACG patients: poor sleep quality, high-salt diet, fruit and vegetable deficiency, family history of tumours, abdominal pain, and diarrhoea. Multivariate logistic regression analysis showed that poor sleep quality (OR 23.89, 95% CI: 4.05–141.05, P<0.001), a high-salt diet (OR 6.94, 95% CI: 1.86–25.96, P=0.004), family history of tumours (OR 6.10, 95% CI: 1.33–27.93, P=0.020), and abdominal pain (OR 4.44, 95% CI: 1.29–15.23, P=0.018) were independent risk factors for depression in ACG patients. We conducted a further analysis of patients with moderate to severe depression. The results showed that poor sleep quality (OR 20.42, 95% CI: 1.18–351.92, P=0.038) was an independent protective factor.
Both anxiety and depression
Of the 118 ACG patients, 25 (21.2%) had both anxiety and depression. Univariate analysis showed that the following variables were associated with depression in ACG patients: poor sleep quality, high-salt diet, fruit and vegetable deficiency, family history of tumours, abdominal pain, and nausea. Multivariate logistic regression analysis showed that poor sleep quality (OR 21.46, 95% CI: 3.37–136.80, P=0.001), a high-salt diet (OR 6.81, 95% CI: 1.87–25.09, P=0.004), a family history of tumours (OR 7.57, 95% CI: 1.54–37.35, P=0.013), and abdominal pain (OR 5.42, 95% CI: 1.49–19.74, P=0.010) were independent risk factors for depression in patients. Details are provided in Tables 3-8.
Table 3
| Variables | Anxiety | Depression | Both anxiety and depression | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No (n=75) | Yes (n=43) | OR | 95% CI | P value | No (n=88) | Yes (n=30) | OR | 95% CI | P value | No (n=93) | Yes (n=25) | OR | 95% CI | P value | |||
| Gender | |||||||||||||||||
| Female | 33 (44.0) | 19 (44.2) | 0.992 | 0.47–2.11 | 0.984 | 37 (42.0) | 15 (50.0) | 0.725 | 0.32–1.67 | 0.449 | 45 (45.2) | 10 (40.0) | 1.235 | 0.50–3.03 | 0.645 | ||
| Age, years | 53.61±12.61 | 50.00±11.99 | NA | NA | 0.091 | 52.52±12.01 | 51.63±13.90 | – | – | 0.809 | 53.10±11.98 | 49.32±13.95 | – | – | 0.175 | ||
| 25–44 | 14 (18.7) | 12 (27.9) | NA | NA | 0.613 | 17 (19.3) | 9 (30.0) | – | – | 0.406 | 17 (18.3) | 9 (36.0) | – | – | 0.145 | ||
| 45–59 | 37 (49.3) | 25 (58.1) | 0.788 | 0.31–1.98 | 0.357 | 49 (55.7) | 13 (43.3) | 0.501 | 0.18–1.38 | 0.181 | 50 (53.8) | 12 (48.0) | 0.453 | 0.16–1.26 | 0.130 | ||
| 60–77 | 24 (32.0) | 6 (14.0) | 0.292 | 0.09–0.95 | 0.041* | 22 (25.0) | 8 (26.7) | 0.687 | 0.22–2.16 | 0.520 | 26 (28.0) | 4 (16.0) | 0.291 | 0.08–1.10 | 0.068 | ||
| BMI, kg/m2 | 22.52±2.67 | 21.99±3.05 | NA | NA | 0.330 | 22.34±2.59 | 22.29±3.43 | – | – | 0.936 | 22.39±2.64 | 22.07±3.42 | – | – | 0.617 | ||
| BMI ≥24 | 21 (28.0) | 11(25.6) | 0.884 | 0.38–2.07 | 0.776 | 24 (27.3) | 8 (26.7) | 0.970 | 0.38–2.47 | 0.949 | 26 (28.0) | 6 (24.0) | 0.814 | 0.29–2.27 | 0.693 | ||
| Marital | |||||||||||||||||
| Married | 60 (80.0) | 37 (86.0) | 1.542 | 0.55–4.33 | 0.411 | 74 (84.1) | 23 (76.7) | 0.622 | 0.22–1.73 | 0.361 | 77 (82.8) | 20 (80.0) | 0.831 | 0.27–2.54 | 0.746 | ||
| Education | |||||||||||||||||
| High school education or more | 48 (64.0) | 26 (60.5) | 0.860 | 0.40–1.86 | 0.702 | 55 (62.5) | 19 (63.3) | 1.036 | 0.44–2.45 | 0.935 | 58 (62.4) | 16 (64.0) | 1.073 | 0.43–2.69 | 0.881 | ||
| Employment status | |||||||||||||||||
| Employed | 40 (53.3) | 25 (58.1) | 1.215 | 0.57–2.59 | 0.614 | 50 (56.8) | 15 (50.0) | 0.760 | 0.33–1.74 | 0.517 | 50 (53.8) | 15 (60.0) | 1.290 | 0.53–3.17 | 0.578 | ||
| Monthly income | |||||||||||||||||
| Monthly income ≥4,000 CNY | 40 (53.3) | 27 (62.8) | 1.477 | 0.69–3.18 | 0.319 | 50 (56.8) | 17 (56.7) | 0.994 | 0.43–2.29 | 0.988 | 52 (55.9) | 15 (60.0) | 1.183 | 0.48–2.91 | 0.714 | ||
| Poor sleep quality | 35 (46.7) | 35 (81.4) | 5.000 | 2.50–12.20 | <0.001*** | 42 (47.7) | 28 (93.3) | 15.333 | 3.44–68.39 | <0.001*** | 47 (50.5) | 23 (92.0) | 11.26 | 2.51–50.49 | 0.002** | ||
| The number of family members | 3.61±1.676 | 4.07±1.944 | – | – | 0.061 | 3.84±1.79 | 3.60±1.79 | – | – | 0.363 | 3.80±1.77 | 3.72±1.86 | – | – | 0.676 | ||
| The number of family members >3 | 30 (40.0) | 25 (58.1) | 2.089 | 0.97–4.46 | 0.059 | 41 (46.6) | 14 (46.7) | 1.003 | 0.44–2.30 | 0.994 | 43 (46.2) | 12 (48.0) | 1.073 | 0.44–2.60 | 0.875 | ||
| Exercise time per week, hours | 6.77±9.87 | 3.57±4.425 | – | – | 0.061 | 6.01±9.29 | 4.43±5.10 | – | – | 0.753 | 6.11±9.05 | 3.72±5.26 | – | – | 0.152 | ||
| Exercise time per week, hours >3.5 h | 39 (52.0) | 17 (39.5) | 0.604 | 0.28–1.29 | 0.193 | 43 (48.9) | 13 (43.3) | 0.800 | 0.35–1.84 | 0.601 | 48 (51.6) | 8 (32.0) | 0.441 | 0.173–1.122 | 0.086 | ||
| Dietary habits | |||||||||||||||||
| High-salt diet | 15 (20.0) | 12 (27.9) | 1.548 | 0.65–3.71 | 0.327 | 16 (18.2) | 11 (36.7) | 2.605 | 1.04–6.53 | 0.041* | 17 (18.3) | 10 (40.0) | 2.980 | 1.14–7.76 | 0.025* | ||
| Pickled food | 14 (18.7) | 9 (20.9) | 1.153 | 0.45–2.94 | 0.765 | 14 (15.9) | 9 (30.0) | 2.265 | 0.86–5.96 | 0.098 | 15 (16.1) | 8 (32.0) | 2.447 | 0.90–6.69 | 0.081 | ||
| Smoked food | 3 (4.0) | 1 (2.3) | 0.571 | 0.06–5.67 | 0.633 | 3 (3.4) | 1 (3.3) | 0.977 | 0.10–9.77 | 0.984 | 3 (3.2) | 1 (4.0) | 1.250 | 0.12–12.56 | 0.850 | ||
| Spicy food | 13 (17.3) | 6 (14.0) | 0.773 | 0.27–2.21 | 0.631 | 14 (15.9) | 5 (16.7) | 1.057 | 0.35–3.23 | 0.922 | 14 (15.1) | 5 (20.0) | 1.411 | 0.54–4.38 | 0.552 | ||
| Fruit and Vegetable deficiency | 8 (10.7) | 14 (32.6) | 4.043 | 1.53–10.69 | 0.005** | 11 (12.5) | 11 (36.7) | 4.053 | 4.53–10.74 | 0.005** | 12 (12.9) | 10 (40.0) | 4.500 | 1.65–12.28 | 0.003** | ||
| Broiling and Barbequing food | 4 (5.3) | 1 (2.3) | 0.423 | 0.05–3.91 | 0.448 | 4 (4.5) | 1 (3.3) | 0.724 | 0.08–6.75 | 0.777 | 4 (4.3) | 1 (4.0) | 0.927 | 0.10–8.68 | 0.947 | ||
| Fried food | 5 (6.7) | 4 (9.3) | 1.436 | 0.36–5.66 | 0.605 | 6 (6.8) | 3 (10.0) | 1.519 | 0.36–6.50 | 0.573 | 6 (6.5) | 3 (12.0) | 1.977 | 0.46–8.54 | 0.361 | ||
| Family history of tumors | 7 (9.3) | 10 (23.3) | 2.944 | 1.03–8.43 | 0.044 | 9 (10.2) | 8 (26.7) | 3.192 | 1.10–9.24 | 0.032* | 10 (10.8) | 7 (28.0) | 3.228 | 1.08–9.62 | 0.035* | ||
| Family history of digestive system tumors | 4 (5.3) | 6 (14.0) | 2.878 | 0.76–10.84 | 0.118 | 6 (6.8) | 4 (13.3) | 2.103 | 0.55–8.03 | 0.277 | 6 (6.5) | 4 (16.0) | 2.762 | 0.72–10.67 | 0.141 | ||
| Smoking | |||||||||||||||||
| Never | 43 (57.3) | 36 (83.7) | NA | NA | 0.018* | 54 (61.4) | 25 (83.3) | – | – | 0.099 | 58 (62.4) | 21 (84.0) | – | – | 0.144 | ||
| Smoking | 17 (22.7) | 3 (7.0) | 0.211 | 0.06–0.78 | 0.019* | 18 (20.5) | 2 (6.7) | 0.240 | 0.05–1.11 | 0.069 | 18 (19.4) | 2 (8.0) | 0.307 | 0.07–1.44 | 0.134 | ||
| Ex | 15 (20.0) | 4 (9.3) | 0.319 | 0.10–1.04 | 0.059 | 16 (18.2) | 3 (10.0) | 0.405 | 0.11–1.52 | 0.180 | 17 (18.3) | 2 (8.0) | 0.325 | 0.07–1.53 | 0.155 | ||
| Alcohol | |||||||||||||||||
| No | 67 (92.4) | 42 (88.4) | NA | NA | 0.372 | 73 (83.0) | 24 (80.0) | – | – | 0.542 | 76 (81.7) | 21 (84.0) | – | – | 0.901 | ||
| Occasional | 5 (13.3) | 1 (9.3) | 0.319 | 0.04–2.83 | 0.305 | 9 (10.2) | 5 (16.7) | 1.690 | 0.52–5.54 | 0.386 | 11 (11.8) | 3 (12.0) | 0.987 | 0.25–3.87 | 0.985 | ||
| Frequent | 3 (8.0) | 0 (2.3) | NA | NA | 0.999 | 6 (6.8) | 1 (3.3) | 0.507 | 0.06–4.43 | 0.539 | 6 (6.5) | 1 (4.0) | 0.603 | 0.07–5.29 | 0.648 | ||
| Gastrointestinal symptoms | 49 (65.3) | 33 (76.7) | 1.75 | 0.75–4.11 | 0.198 | 57 (64.8) | 25 (83.3) | 2.719 | 0.95–7.81 | 0.063 | 62 (66.7) | 20 (80.0) | 2.000 | 0.69–5.83 | 0.204 | ||
| Bloating | 28 (37.3) | 13 (30.2) | 0.727 | 0.33–1.62 | 0.436 | 28 (31.8) | 13 (43.3) | 1.639 | 0.70–3.83 | 0.255 | 32 (32.4) | 9 (36.0) | 1.072 | 0.43–2.70 | 0.882 | ||
| Belching | 18 (24.0) | 21 (48.8) | 3.023 | 1.36–6.72 | 0.007** | 27 (30.7) | 12 (40.0) | 1.506 | 0.64–3.56 | 0.350 | 29 (31.2) | 10 (40.0) | 1.471 | 0.59–3.66 | 0.407 | ||
| Abdominal pain | 13 (17.3) | 14 (32.6) | 2.302 | 0.96–5.52 | 0.062 | 16 (18.2) | 11 (36.7) | 2.605 | 1.04–6.53 | 0.041* | 17 (18.3) | 10 (40.0) | 2.980 | 1.14–7.76 | 0.025* | ||
| Nausea | 4 (5.3) | 6 (14.0) | 2.878 | 0.76–10.84 | 0.118 | 5 (5.7) | 5 (16.7) | 3.320 | 0.89–12.40 | 0.074 | 5 (5.4) | 5 (20.0) | 4.400 | 1.16–16.66 | 0.029* | ||
| Acid reflux | 10 (13.3) | 4 (9.3) | 0.667 | 0.20–2.27 | 0.517 | 8 (9.1) | 6 (20.0) | 2.500 | 0.79–7.91 | 0.119 | 10 (10.8) | 4 (16.0) | 1.581 | 0.45–5.54 | 0.474 | ||
| Diarrhea | 7 (9.3) | 8 (18.6) | 2.220 | 0.74–6.62 | 0.153 | 7 (8.0) | 8 (26.7) | 4.208 | 1.38–12.88 | 0.012* | 9 (9.7) | 6 (24.0) | 2.947 | 0.94–9.28 | 0.065 | ||
Values were presented as mean ± standard deviation or n (%). For these tests, P≤0.05 (*) was deemed significant, P≤0.01 (**) very significant, and P≤0.001 (***) extremely significant. ACG, atrophic chronic gastritis; BMI, body mass index; CNY, Chinese Yuan; OR, odd ratio; CI, confidence interval.
Table 4
| Variables | β | SE | Walds | P value | OR (95% CI) |
|---|---|---|---|---|---|
| Poor sleep quality | 1.462 | 0.507 | 8.321 | 0.004** | 4.32 (1.60–11.65) |
| Smoking | −1.929 | 0.783 | 6.065 | 0.014* | 0.15 (0.03–0.68) |
| Weekly exercise time, hours | −0.119 | 0.057 | 4.372 | 0.037* | 0.89 (0.79–0.99) |
For these tests, P≤0.05 (*) was deemed significant, P≤0.01 (**) was very significant. ACG, atrophic chronic gastritis; CI, confidence interval; OR, odds ratio; SE, standard error.
Table 5
| Variables | β | SE | Walds | P value | OR (95% CI) |
|---|---|---|---|---|---|
| Poor sleep quality | 3.173 | 0.906 | 12.270 | <0.001*** | 23.89 (4.05–141.05) |
| High salt diet | 1.937 | 0.673 | 8.285 | 0.004** | 6.94 (1.86–25.96) |
| Family history of tumors | 1.809 | 0.776 | 5.431 | 0.020* | 6.10 (1.33–27.93) |
| Abdominal pain | 1.490 | 0.629 | 5.600 | 0.018* | 4.44 (1.29–15.23) |
For these tests, P≤0.05 (*) was deemed significant, P≤0.01 (**) was very significant, and P≤0.001 (***) extremely significant. ACG, atrophic chronic gastritis; CI, confidence interval; OR, odds ratio; SE, standard error.
Table 6
| Variables | β | SE | Walds | P value | OR (95% CI) |
|---|---|---|---|---|---|
| Poor sleep quality | 3.066 | 0.945 | 10.529 | 0.001*** | 21.46 (3.37–136.80) |
| High salt diet | 1.918 | 0.666 | 8.301 | 0.004** | 6.81 (1.87–25.09) |
| Family history of tumors | 2.024 | 0.814 | 6.180 | 0.013* | 7.57 (1.54–37.35) |
| Abdominal pain | 1.689 | 0.660 | 6.552 | 0.010** | 5.42 (1.49–19.74) |
For these tests, P≤0.05 (*) was deemed significant, P≤0.01 (**) was very significant, and P≤0.001 (***) extremely significant. ACG, atrophic chronic gastritis; CI, confidence interval; OR, odds ratio; SE, standard error.
Table 7
| Variables | β | SE | Walds | P value | OR (95% CI) |
|---|---|---|---|---|---|
| Poor sleep quality | 2.252 | 0.829 | 7.379 | 0.007** | 9.51 (1.87–48.30) |
| Weekly exercise time, hours | −0.242 | 0.102 | 5.632 | 0.018* | 0.785 (0.64–0.96) |
For these tests, P≤0.05 (*) was deemed significant, P≤0.01 (**) was very significant. ACG, atrophic chronic gastritis; CI, confidence interval; OR, odds ratio; SE, standard error.
Table 8
| Variables | β | SE | Walds | P value | OR (95% CI) |
|---|---|---|---|---|---|
| Poor sleep quality | 3.016 | 1.453 | 4.312 | 0.038* | 20.42 (1.18–351.92) |
For these tests, P≤0.05 (*) was deemed significant. ACG, atrophic chronic gastritis; CI, confidence interval; OR, odds ratio; SE, standard error.
Development and validation of risk prediction models
Logistic regression models were constructed for multivariate analyses and created the following formula (details of the assignment are shown in Table 9):
Table 9
| Variables | Symbol | Assignment |
|---|---|---|
| Poor sleep quality | x1 | No =0, Yes =1 |
| Cigarette smoking | x2 | No =0, Yes =1 |
| Weekly exercise time, hours | x3 | 1 hour =1, 2 hours =2… |
| High salt diet | x4 | No =0, Yes =1 |
| Family history of tumors | x5 | No =0, Yes =1 |
| Abdominal pain | x6 | No =0, Yes =1 |
P values closer to 1 indicate a greater possibility of anxiety/depression in ACG patients, whereas P values closer to 0 are associated with a lower possibility anxiety/depression. The scores of ACG patients were calculated using the formulation of predictive models. The receiver operating characteristic (ROC) curve was displayed to validate the predictive accuracy of the model.
Model 1 Anxiety
The following formula was created:
The difference was statistically significant on the likelihood ratio test (P<0.001). The maximum Youden’s index was used to determine the optimal cut-off value. The area under the ROC curve was 0.803, 95% CI: 0.722–0.885. Maximum Youden’s index was 0.508 and the cut-off was 0.508. At this cut-off, the sensitivity was 72.1%, and the specificity was 78.7%. The ROC curve showed good predictive power of our features (Figure 2A).
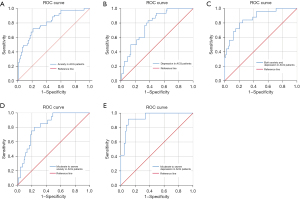
Model 2 Depression
The following formula was created:
The difference was statistically significant on the likelihood ratio test (P<0.001). The area under the ROC curve was 0.773, 95% CI: 0.687–0.860. Maximum Youden’s index was 0.458 and the cut-off was 0.458. At this cut-off, the sensitivity was 83.3%, and the specificity was 62.5%. The ROC curve showed good predictive power of our features (Figure 2B).
Model 3 Both anxiety and depression
The following formula was created:
The difference was statistically significant on the likelihood ratio test (P<0.001). The area under the ROC curve was 0.865, 95% CI: 0.786–0.944. Maximum Youden’s index was 0.519 and the cut-off was 0.519. At this cut-off, the sensitivity was 96.0%, and the specificity was 55.9%. The ROC curve showed good predictive power of our features (Figure 2C).
Model 4 Moderate to severe anxiety
The following formula was created:
The difference was statistically significant on the likelihood ratio test (P<0.001). The optimal cut-off value was determined by Youden’s index. The area under the ROC curve was 0.826, 95% CI: 0.744–0.907, and Youden’s index was 0.565. At this cut-off, the sensitivity was 80.0%, and the specificity was 76.5%. The ROC curve showed good predictive power of our features (Figure 2D).
Model 5 Moderate to severe depression
The following formula was created:
The difference was statistically significant on the likelihood ratio test (P<0.001). The optimal cut-off value was determined by Youden’s index. The area under the ROC curve was 0.940, 95% CI: 0.892–0.989, and Youden’s index was 0.893. At this cut-off, the sensitivity was 100%, and the specificity was 89.3%. The ROC curve showed good predictive power of our features (Figure 2E).
Discussion
Our research assessed the state of anxiety and depression of ACG patients by questionnaires and further explored potential risk factors for anxiety and depression.
We found that ACG patients had serious anxiety and depression. Nearly half of ACG patients with anxiety or depression suffered from moderate to severe anxiety or depression. Given the impact of anxiety and depression on gastrointestinal function, this is a cautionary result. In further analysis, we found that poor sleep quality is a key risk factor for the generation and progression of anxiety and depression in ACG patients, particularly depression. For anxiety, exercise and smoking can affect the appearance and progression of anxiety in ACG patients. For depression, a high-salt diet, a family history of cancer, and long-term abdominal pain were likely to affect the appearance and progression of depression in ACG patients. We developed a risk prediction model for ACG patients, which allows clinicians to score anxiety and depression in ACG patients.
ACG patients suffer from various forms of psychological distress, and protective factors should be enhanced cumulatively to protect against psychological distress (9). Previous work showed that anxiety and depression are independent risk factors for ACG in the young and middle-aged population (21). This study found that ACG patients with poor sleep quality were more likely to present with both anxiety and depression and to have aggravated anxiety and depression. Poor sleep causes abnormal corticotropin releasing factor (CRF) regulation (22) and impaired medial prefrontal cortex activity (23), which leads to anxiety and depression in ACG patients. With a longer duration of disease, anxiety and depression induce central nervous system (CNS), homeostatic disturbances, and gastrointestinal dysfunction and eventually cause ACG progression (24). Therefore, sleep quality may indeed be a crucial element for ACG patients’ sleep quality. Early and appropriate intervention can be exerted for ACG patients who suffer from poor sleep quality.
Smoking was previously considered to be an independent risk factor for many diseases. However, we found that smoking contributes to alleviate anxiety in ACG patients. Nicotine improves patients’ dysphoria by increasing the excitability of nerve cells and stimulating the brain’s reward mechanism (25,26). Smoking relieves patients’ anxiety in the short term, but in the long run, smoking is probably not a wise option. With increasing amounts and duration of smoking, the accumulation of nicotine and other injurious agents in tobacco leads to gastric mucosal injury and other severe diseases (27). Physicians could illustrate the potential harm caused by smoking and convince patients to stop smoking. Gradually reducing the number of cigarettes smoked could engage more ACG patients in quitting. For ACG patients who already suffer from anxiety, engaging in moderate aerobic exercise (such as fast walking, cycling, or swimming) each week may potentially exert some benefits in anxiety (28). One study has found exercise allows ACG patients to experience physical improvements and changes in interpersonal relationships and enhances patients’ self-confidence (29). It also promotes an increase in the secretion of neurotransmitter chemicals and endorphins in the body (30). Furthermore, it contributes to the relief of anxiety and reduces their risk of developing moderate-to-severe anxiety disorder in ACG patients.
Depression is highly prevalent among ACG patients (31), and a high-salt diet may cause in this population. A study showed that depression is associated with disturbances of mitochondrial function. A high-salt diet may lead to abnormal mitochondrial biosynthesis, which could lead to increased free radical production, inflammation, and insulin resistance, eventually leading to depression (32). Based on our results, dietary habits may influence depression in ACG patients. Doctors could give patients targeted advice on dietary habits. In addition, family history of cancer is one of the key messages in the ACG patient’s medical history. ACG patients with a family history of cancer may be unusually sensitive to ACG, thus inducing depression. Making patients acknowledge ACG through illness education is perhaps an effective way to relief depression. For ACG patients who suffer from long-term abdominal pain, appropriate intervention could be used for ACG patients who suffer from abdominal pain.
In this study, we established a risk model for the prediction of anxiety and depression in ACG patients that showed relatively high predictive value. The area under the ROC curve over 0.7 was considered indicative of “fair” discriminative ability, 0.8 as “good”, and over 0.9 as “excellent” (33). It is generally accepted that the discriminative ability of a risk index should be greater than 0.8 for a prediction model to be considered clinically relevant (34).
Poor sleep quality, cigarette smoking and weekly exercise time were included in Model 1. The area under ROC curve was 0.803, indicating a good predictive ability. The sensitivity and specificity of the model were 72.1% and 78.7%, respectively, suggesting a good screening effect, which may also help to diagnose anxiety in patients with ACG. Poor sleep quality, high salt diet, family history of tumours and abdominal pain were included in Model 2. In ACG patients, the area under the ROC curve was 0.773, showing the good predictive capacity of the model. Sensitivity and specificity for this model were 83.3% and 62.5%, respectively, indicating the model has a good screening effect for depression, but may lead to a high rate of misdiagnosis. In Model 3, factors consistent with Model 2 were included. The area under the ROC curve was 0.865, which suggested a good predictive ability of the model. Sensitivity and specificity for this model were 96.0% and 55.9%, respectively, showing that the model has a good screening effect for both anxiety and depression but diagnostic value is less than satisfactory. In Model 4, poor sleep quality and cigarette smoking were included. The area under the ROC curve was 0.826, indicating the good predictive ability of the model. Sensitivity and specificity for this model were 80.0% and 76.5%, suggesting that the model has a good screening effect and contributes to diagnostic for moderate to severe anxiety in ACG patients. In Model 5, poor sleep quality was included. The area under the ROC curve was 0.940, showing excellent predictive ability of the model. Sensitivity and specificity for this model were 100.0% and 89.3%, indicating good results in both screening and diagnosis.
In our research, all ACG patients underwent gastroscopy and biopsy for histopathology observation, which ensures consistency across this study. This is the first analysis of anxiety and depression in ACG patients in Guangzhou, China. To the best of our knowledge, this is the first study to analyse risk factors for anxiety and depression in ACG patients. Patients were also stratified based on scale score (moderate-to-severe), and risk and protective factors for the progression of anxiety and depression were analysed. We also integrated these risk factors to construct an effective clinical prediction model for the first time. The relationship between risk factors and anxiety and depression can be visualized in the formula. It may be useful for assessing the potential risk of developing anxiety and depression in ACG patients for health care workers. Doctors could purposefully focus on relieving anxiety and depression in ACG patients and intervene for ACG patients with potential risks of anxiety and depression in a timely manner. This approach could also avoid the adverse effects of anxiety and depression on ACG as much as possible, allowing better results with drug treatment, decreasing the cost burden of treatment for ACG patients, and reducing the chances of other diseases caused by anxiety and depression.
Our study also has some limitations. Firstly, in view of the small sample size, its clinical relevance could be questioned, and additional studies with larger sample sizes are needed to validate these findings. Secondly, our risk prediction models have some limitations. Some models have low specificity and may not be as accurate in diagnosis, which may lead to misdiagnosis. Another limitation is that the prediction model was not externally validated. Thirdly, this study is a single-centre investigation, and patients in different areas may reach different conclusions because of different environmental exposures and dietary habits. We will continue to include more ACG patients and follow these patients to collect more data. The severity of ACG will be stratified according to pathological biopsy results, and the association of anxiety and depression with ACG will be further explored.
Conclusions
For ACG patients, the situation in the field of anxiety and depression is gloomy. This study searched for risk factors for anxiety and depression and developed a risk prediction model for anxiety and depression in ACG patients. The prediction model is meaningful in the early identification and management of anxiety and depression in ACG patients and provide meaningful suggestions for ACG patients.
Acknowledgments
The author would like to appreciate Yijun Chen, Yinghua Liu and Hanqing Chen from the First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China, for their valuable discussions and feedback.
Funding: This work was supported by the National Natural Science Foundation of China (Nos. 32171370 and 11505193), the Natural Science Foundation of Guangdong Province (No. 2022A1515010415), and the Research Foundation of Guangzhou First People’s Hospital (No. KY09040029).
Footnote
Reporting Checklist: The authors have completed the STROBE and TRIPOD reporting checklists. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-730/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-730/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-730/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fang JY, Du YQ, Liu WZ, et al. Chinese Consensus Opinion on Chronic Gastritis (2017, Shanghai). Shanghai Medical Journal 2017;40:705-8.
- Adamu MA, Weck MN, Gao L, et al. Incidence of chronic atrophic gastritis: systematic review and meta-analysis of follow-up studies. Eur J Epidemiol 2010;25:439-48. [Crossref] [PubMed]
- Shah SC, Piazuelo MB, Kuipers EJ, et al. AGA Clinical Practice Update on the Diagnosis and Management of Atrophic Gastritis: Expert Review. Gastroenterology 2021;161:1325-1332.e7. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015;64:1353-67. [Crossref] [PubMed]
- Geeraerts B, Vandenberghe J, Van Oudenhove L, et al. Influence of experimentally induced anxiety on gastric sensorimotor function in humans. Gastroenterology 2005;129:1437-44. [Crossref] [PubMed]
- Takeoka A, Tayama J, Kobayashi M, et al. Psychological effects of Helicobacter pylori-associated atrophic gastritis in patients under 50 years: A cross-sectional study. Helicobacter 2017; [Crossref] [PubMed]
- Chung J, Ju G, Yang J, et al. Prevalence of and factors associated with anxiety and depression in Korean patients with newly diagnosed advanced gastrointestinal cancer. Korean J Intern Med 2018;33:585-94. [Crossref] [PubMed]
- Sun Y, Wang S, Qi M, et al. Psychological distress in patients with chronic atrophic gastritis: the risk factors, protection factors, and cumulative effect. Psychol Health Med 2018;23:797-803. [Crossref] [PubMed]
- Guideline for primary care of chronic gastritis (2019). Chinese Journal of General Practitioners 2020;19:768-75.
- Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest 2015;125:926-38. [Crossref] [PubMed]
- Taché Y, Martinez V, Million M, et al. Stress and the gastrointestinal tract III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. Am J Physiol Gastrointest Liver Physiol 2001;280:G173-7. [Crossref] [PubMed]
- Liu D, Jiang XY, Zhou LS. Enriched environment on the intestinal mucosal barrier and brain-gut axis in rats with colorectal cancer. Exp Biol Med (Maywood) 2018;243:1185-98. [Crossref] [PubMed]
- Wang Y, Chen R, Zhang L. Evaluation of the reliability and validity of the generalized anxiety disorder 7-item scale among inpatients in general hospital. Journal of Clinical Psychiatry 2018;28:168-71.
- Sun XY, Li YX, Yu CQ, et al. Reliability and validity of depression scales of Chinese version: a systematic review. Chinese Journal of Epidemiology 2017;38:110-6. [PubMed]
- Hinz A, Klein AM, Brähler E, et al. Psychometric evaluation of the Generalized Anxiety Disorder Screener GAD-7, based on a large German general population sample. J Affect Disord 2017;210:338-44. [Crossref] [PubMed]
- Negeri ZF, Levis B, Sun Y, et al. Accuracy of the Patient Health Questionnaire-9 for screening to detect major depression: updated systematic review and individual participant data meta-analysis. BMJ 2021;375: [Crossref] [PubMed]
- Peterson DE, Boers-Doets CB, Bensadoun RJ, et al. Management of oral and gastrointestinal mucosal injury: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann Oncol 2015;26:v139-51. [Crossref] [PubMed]
- Mallick S, Benson R, Rath GK. Radiation induced oral mucositis: a review of current literature on prevention and management. Eur Arch Otorhinolaryngol 2016;273:2285-93. [Crossref] [PubMed]
- Scientific Research Report on dietary guidelines for Chinese Residents (2021). ACTA Nutrimenta SINICA;43:1-2.
- Wang H, Zhou QH, Jiang DT, et al. Analysis of Risk Factors of Chronic Atrophic Gastritis in Young and Middle-aged. Guide of China Medicine 2020;18:33-4.
- Szelenberger W, Soldatos C. Sleep disorders in psychiatric practice. World Psychiatry 2005;4:186-90. [PubMed]
- Ben Simon E, Rossi A, Harvey AG, et al. Overanxious and underslept. Nat Hum Behav 2020;4:100-10. [Crossref] [PubMed]
- Zeng W, Yang F, Shen WL, et al. Interactions between central nervous system and peripheral metabolic organs. Sci China Life Sci 2022;65:1929-58. [Crossref] [PubMed]
- Chang GQ, Karatayev O, Leibowitz SF. Prenatal exposure to nicotine stimulates neurogenesis of orexigenic peptide-expressing neurons in hypothalamus and amygdala. J Neurosci 2013;33:13600-11. [Crossref] [PubMed]
- Walters CL, Cleck JN, Kuo YC, et al. Mu-opioid receptor and CREB activation are required for nicotine reward. Neuron 2005;46:933-43. [Crossref] [PubMed]
- Bennett JR. Smoking and the gastrointestinal tract. Gut 1972;13:658-65. [Crossref] [PubMed]
- Alpert JS. Cardiology patient page. Nutritional advice for the patient with heart disease: what diet should we recommend for our patients? Circulation 2011;124:e258-60. [Crossref] [PubMed]
- Hayden RM, Allen GJ. Relationship between aerobic exercise, anxiety, and depression: convergent validation by knowledgeable informants. J Sports Med Phys Fitness 1984;24:69-74. [PubMed]
- Dishman RK, Berthoud HR, Booth FW, et al. Neurobiology of exercise. Obesity (Silver Spring) 2006;14:345-56. [Crossref] [PubMed]
- Zhao X, Wu M, Zhang D, et al. The relationship of interpersonal sensitivity and depression among patients with chronic atrophic gastritis: The mediating role of coping styles. J Clin Nurs 2018;27:e984-91. [Crossref] [PubMed]
- Marx W, Lane M, Hockey M, et al. Diet and depression: exploring the biological mechanisms of action. Mol Psychiatry 2021;26:134-50. [Crossref] [PubMed]
- Kisa NG, Kisa E, Cevik BE. Prediction of Mortality in Patients After Oncologic Gastrointestinal Surgery: Comparison of the ASA, APACHE II, and POSSUM Scoring Systems. Cureus 2021;13:e13684. [Crossref] [PubMed]
- Hong W, Earnest A, Sultana P, et al. How accurate are vital signs in predicting clinical outcomes in critically ill emergency department patients. Eur J Emerg Med 2013;20:27-32. [Crossref] [PubMed]



