
Prospective analysis of the prognostic value of prenatal MRI measurement of cystic volume ratio in fetal congenital cystic adenomatoid malformation
Introduction
Fetal congenital cystic adenomatoid malformation (CCAM) is the most common lung dysplasia in fetuses (1,2), but its prognosis is difficult to determine (3). Some lesions may shrink and even disappear, while some may continue to enlarge, resulting in pulmonary dysplasia, edema, and even death (4). The cystic volume ratio (CVR) value can be used to judge the proportion of normal lung tissue in CCAM fetuses and the degree of lung development. CVR can objectively compare fetuses of different gestational ages individually and individually, CCAM is mainly screened by prenatal ultrasound examination, which may, however, cause missed diagnosis and misdiagnosis; meanwhile prenatal magnetic resonance imaging (MRI) can more clearly visualize the fetal lung structure (5). Only a few reports exist that have evaluated the prognosis of fetal CCAM through prenatal MRI examination in China, with this lack being mainly attributable to the dearth in hospitals capable of conducting prenatal MRI examinations. Our team has thus been engaging in research on prenatal MRI examination for the diagnosis of fetal lung masses for many years (6-8). In this study, the value of prenatal MRI examination in the measurement of CVR for the prognosis of CCAM was evaluated. We present the following article in accordance with the STARD reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-973/rc).
Methods
General materials
The follow-up data of fetuses with CCAM confirmed by pathological results through computed tomography (CT) examination and/or operations after birth in Huzhou Maternity & Child Health Care Hospital and Anhui Provincial Children’s Hospital from June 2016 to December 2020 were collected. All fetuses underwent prenatal MRI examination within 24 to 48 h after prenatal ultrasonography. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committees of Huzhou Maternity & Child Health Care Hospital (No. 2016-009) and Anhui Provincial Children’s Hospital (No. EYLL-2018-013), and all pregnant women voluntarily signed the informed consent prior to examination.
MRI examination
Using a Siemens Avanto 1.5T MR imaging system (Siemens, Munich, Germany). Gradient field strength of 45 mT∙m−1∙s−1, a 32-channel phased array heart coil, and 1–2 excitations. We employed 3 MRI sequences: (I) true fast imaging with steady-state precession (true FISP) sequences, repeat time (TR) of 3.6–4.2 ms, an echo time (TE) of 1.0–2.0 ms, a reversal angle of 90°, and a scan time of 0.5–2.0 s per layer; (II) half-Fourier acquisition single-shot turbo spin-echo (HASTE) sequences with a TR of 1,150–1,500 ms, a TE of 42–145 ms, a reversal angle of 160°; and (III) 2-dimensional FLASH T1WI (TFL) sequences with a TR of 1,680–2,000 ms, a TE of 2.9–4.5 ms, an inversion angle of 15°.
An Achieva Nova Dual MR Imager (Philips, Amsterdam, the Netherland) with a 4-channel abdominal surface coil and 1–2 excitations was used for the following: (I) single-shot fast spin-echo (SSFSE) sequences, with a TR of 12,000.0 ms, a TE of 120.0 ms, and a flip angle of 80°; (II) and a balanced fast field echo (B-FFE) sequence, with the TR and TE values equal to the minimum values set by the system, a flip angle of 90°.
First, a localization scan of the coronal plane in the lower abdomen was carried out, which was followed by routine brain, chest, and abdomen cross-sectional, sagittal, and coronal plane.
Prenatal ultrasonography
Ultrasonography was completed with a Voluson 730 Experd 4D Ultrasound Machine (GE Healthcare, Chicago, IL, USA) and an X300 Color Doppler Ultrasound Diagnostic System (Siemens), with a convex array probe at a frequency of 4.0–8.0 MHz.
CCAM classification methods
Traditionally, CCAM has been classified into 3 types according to the classification method described by Sanders (9): type I or macrocystic CCAM, consisting of a single or several cysts with a diameter of ≥20 mm; type II or minicystic CCAM, consisting of a single or several cysts with a diameter of <10 mm; and type III or microcystic CCAM, consisting of a single or several cysts with a diameter of <5 mm or noncystic solid lesions. One study research has shown that type I and type II are different in terms of cyst size but have similar appearance on imaging, with the large and small cysts often being mixed (10). In line with the classification methods of other scholars (10-12), CCAM was divided into the macrocystic type (diameter ≥5 mm) and the microcystic type (diameter <5 mm) in this study.
CVR calculation and classification methods
Through regarding the lesion as an ellipse, prenatal MRI was used to measure the longest diameter, the widest diameter, and the highest diameter, and then the following equation was used to calculate its volume: volume = the longest diameter × the widest diameter × the highest diameter × 0.52. In order to eliminate the deviation of lesion volume of different gestational ages, CVR was used to measure the changes in lesions of different gestational ages according to the following formula: CVR = lesion volume/head circumference (13,14). With reference to the relevant literature, we divided the (15) CCAM cases into a CVR ≥1.26 group and a CVR <1.26 group.
Image analysis
MRI images were analyzed by 2 experienced associate chief radiologists using the double-blind method according to lesion signal, location, size, blood supply vessels, and heart position, amniotic fluid volume. In cases of disagreement, the radiologists arrived at a consensus through consultation.
Statistical analysis
Data were statistically analyzed using SPSS 22.0 software (IBM Corp., Armonk, NY, USA). Normally distributed measurement data are expressed as and were compared using the t test. Measurement data failing to conform to a normal distribution are expressed by the median (the 25th to 75th percentile) [M (P25-P75)] and were compared with the nonparametric test. Enumeration data are expressed as cases or percentages and were compared with the chi-squared test. Received operating characteristic curve (ROC) was drawn between CVR groups with associated symptoms and clinical prognosis, and the area under the curve (AUC) was calculated. Logistical regression analysis was used to identify the independent prognosis factors associated with CCAM. A P value <0.05 was considered to indicate a statistically significant difference.
Results
Comparison of general clinical data
In all, 51 CCAM cases were collected, with 11 of these being placed in the CVR ≥1.26 group (including 10 cases of macrocystic type and 1 case of microcystic type; Figure 1) and 40 in the CVR <1.26 group (including 24 cases of macrocystic type and 16 case of microcystic type; Figure 2). There was no statistical difference between the 2 groups of pregnant women in age, time of pregnancy, parity, or gestational weeks according to the prenatal MRI examination (P>0.05; Table 1).
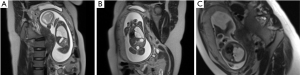
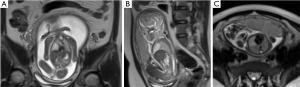
Table 1
| Group | Cases | Age (years), mean ± SD | Times of pregnancy, mean ± SD | Parity, M [P25–P75] | Gestational weeks during prenatal MRI examination, mean ± SD |
|---|---|---|---|---|---|
| CVR ≥1.26 | 11 | 29.36±5.00 | 2.27±0.90 | 1 [1–1] | 26.00±2.72 |
| CVR <1.26 | 40 | 27.03±4.31 | 2.28±1.04 | 1 [1–1] | 26.10±3.34 |
| Statistics value | 1.540* | −0.007* | −0.412△ | −0.091* | |
| P value | 0.130 | 0.995 | 0.680 | 0.928 |
* is the t-test; △ is the chi-square test. MRI, magnetic resonance imaging; CVR, cystic volume ratio.
Comparison of lesion location and prenatal symptoms between CVR and CCAM
The incidence rate of large vessel or heart displacement and increase in amniotic fluid in the CVR ≥1.26 group was greater than that in the CVR <1.26 group (P<0.05; Table 2). Between CVR groups, the AUC of large vessel or heart displacement was 0.734, and that of termination of pregnancy was 0.702 (Figure 3).
Table 2
| Group | Cases | Left [cases (%)] | Large vessel or heart displacement [cases (%)] | Increase in amniotic fluid [cases (%)] |
|---|---|---|---|---|
| CVR ≥1.26 | 11 | 6 (54.55) | 9 (81.82) | 4 (36.36) |
| CVR <1.26 | 40 | 24 (60.00) | 14 (35.00) | 1 (2.50) |
| χ2 | 0.106 | 7.638 | 7.686 | |
| P value | 0.745 | 0.006 | 0.001 |
CVR, cystic volume ratio; CCAM, congenital cystic adenomatoid malformation.
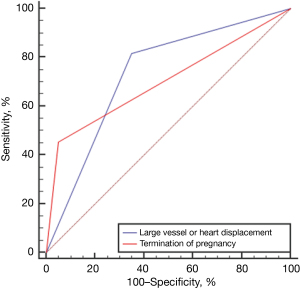
Comparison between CVR and postpartum prognosis
In the CVR ≥1.26 group, there were 6 live births and 5 pregnancy terminations; meanwhile, in the CVR <1.26 group there were 38 live births, and 2 pregnancy terminations. For liveborn CCAM infants, the incidence rate of dyspnea in the CVR ≥1.26 group was greater than that in the CVR <1.26 group (P<0.05), and the rate of termination of pregnancy in the CVR ≥1.26 group was also greater than that in the CVR <1.26 Group (P<0.05; Table 3). The AUC between CVR and dyspnea after birth was 0.811 (Figure 4).
Table 3
| Group | Liveborn | Termination of pregnancy [cases (%)] | ||
|---|---|---|---|---|
| Boy [cases (%)] | Dyspnea [cases (%)] | Lesion disappeared [cases (%)] | ||
| CVR ≥1.26 | 2 (33.33) | 5 (83.33) | 0 (0.00) | 5 (45.45) |
| CVR <1.26 | 25 (65.79) | 8 (21.05) | 8 (21.05) | 2 (5.26) |
| χ2 | 2.302 | 6.896 | 0.453 | 8.752 |
| P value | 0.129 | 0.009 | 0.501 | 0.003 |
CVR, cystic volume ratio.
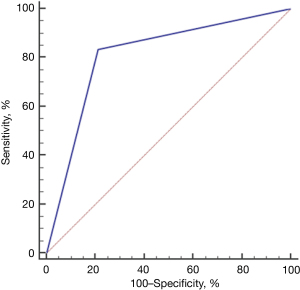
Comparison between macrocystic and microcystic CCAM and accompanying symptoms and postpartum prognosis
In the 51 CCAM cases, there were 34 macrocystic cases and 17 microcystic cases; The CVR in macrocystic cases was greater than that of microcystic cases (P<0.05; Table 4).
Table 4
| Group | Cases | Large vessel or heart displacement [cases (%)] | Increase in amniotic fluid [cases (%)] | CVR | Lesion disappeared [cases (%)] |
|---|---|---|---|---|---|
| Macrocystic | 34 | 18 (52.94) | 5 (14.71) | 0.55 (0.34–1.31) | 5 (14.71) |
| Microcystic | 17 | 5 (29.41) | 0 (0.00) | 0.34 (0.17–0.57) | 3 (17.65) |
| Statistics value | 2.534◇ | 2.772◇ | −2.329△ | 0.074◇ | |
| P value | 0.111 | 0.244 | 0.020 | 0.785 |
◇ is the chi-square test; △ is the non-parametric test. CCAM, congenital cystic adenomatoid malformation; CVR, cystic volume ratio.
Risk factors associated with the postpartum prognosis of CCAM
Univariate analysis indicated that CVR, gestational weeks, and large vessel or heart displacement were factors associated with the postpartum prognosis of CCAM (Table 5). Furthermore, logistical regression analysis identified that CVR is an independent factor associated with the postpartum prognosis of CCAM (Table 6).
Table 5
| Parameter | Variable | Postpartum prognosis | P | |
|---|---|---|---|---|
| Live birth | Terminations | |||
| Age (years) | <27 | 19 | 2 | 0.466 |
| ≥27 | 25 | 5 | ||
| Gestational weeks (week) | <26 | 17 | 6 | 0.020 |
| ≥26 | 27 | 1 | ||
| Pregnancy time (n) | <2 | 10 | 2 | 0.735 |
| ≥2 | 34 | 5 | ||
| CCAM type | Microcystic | 16 | 1 | 0.250 |
| Macrocystic | 28 | 6 | ||
| CVR | <1.26 | 6 | 5 | 0.001 |
| ≥1.26 | 38 | 2 | ||
| Large vessel or heart displacement | No | 27 | 1 | 0.020 |
| Yes | 17 | 6 | ||
CCAM, congenital cystic adenomatoid malformation; CVR, cystic volume ratio.
Table 6
| Parameter | B | SE | P | Exp (B) | 95% CI |
|---|---|---|---|---|---|
| CVR | 2.558 | 1.125 | 0.023 | 12.908 | 1.424−117.028 |
| Gestational weeks (week) | −1.506 | 1.089 | 0.167 | 0.222 | 0.026−1.874 |
| Large vessel or heart displacement | 1.151 | 1.271 | 0.365 | 3.162 | 0.262−38.196 |
CCAM, congenital cystic adenomatoid malformation; CVR, cystic volume ratio.
Discussion
Compared with ultrasonography, MRI has unique advantages of a large visual field, multiple parameters, and high resolution of soft tissue; it is also not restricted by fetal position or maternal figure and can better visualize the details of the normal anatomy and abnormal lesions of the fetal chest; therefore, it has been adopted as an important supplement to obstetrical ultrasonography (10,16). Fetal MRI examination is usually performed based on True FISP/B-FFE and HASTE/SSFSE sequences, which, with the fast scanning speed, can shorten the imaging time and greatly reduce the number of artifacts in fetuses and pregnant women. In addition, by virtue of the high image resolution, it can clearly obtain the images of fetal organs. For obtaining high-quality images, the pregnant women may only need to hold their breath, without the need of any sedative. Based on True FISP/B-FFE and HASTE/SSFSE sequences, the fetal lungs can show uniform high signals. The difference between the two is that the fetal heart and large vessels show high signal on the True FISP/B-FFE sequence, which can clearly show the structure of the 4 chambers of the heart and the large vessels, and can clearly show the changes in the compression of the heart and large vessels by the lesions; meanwhile, in HASTE/SSFSE, the heart may show the “black blood” signal, which can only display the position and size of the fetal heart but not the internal structure. However, due to the role of the bright “water”, HASTE/SSFSE can display the shape, boundary, and internal structure of the fetal lung more clearly, while better distinguishing the fetal lung lesions from the surrounding normal lung tissues. In the case of HASTE/SSFSE, CCAM produces high signals while supplying vessels produce low signals, so it can be used to better find the source blood vessels of the lesions. In recent years, the application of prenatal MRI to screening prenatal diseases has become more widespread (17).
CVR has become an important indicator for evaluating CCAM lung development and changes in patients’ conditions (12). Studies have found that a CVR >1.6 is associated with pulmonary edema in 90% of fetuses with CCAM, and when the threshold of CVR is set to 2.0 (18,19), the specificity and positive predictive value are higher than 1.6 (20). However, in real-world clinical practice, when CVR is >1.6, the incidence of CCAM is low while the induction rate is high, making it difficult to perform follow-up on the clinical prognosis. Therefore, some scholars reduced the threshold of CVR before conducting their grouping studies. Ehrenberg-Buchner et al. (21) performed a grouping study based on 64 fetuses and found that when the CVR was >1.0, the risk of postpartum respiratory insufficiency increased. Ruchonnet-Metrailler et al. (22) performed a grouping study based on 89 fetuses and found that when the CVR was >0.84 and the amniotic fluid was increased or accompanying peritoneal dropsy was present, the possibility of severe postpartum dyspnea was greater. Currently, the measurement of CVR by prenatal MRI is rarely reported either in China or internationally. In this study, CCAM cases were divided into the CVR ≥1.26 group and the CVR <1.26 group according to the method of An et al. (15). It was found that the incidence of displacement of large vessels/heart and increase of amniotic fluid in the CVR ≥1.26 group was greater than that in the CVR <1.26 group (P<0.05). This was mainly because the larger mass could more easily compress and push the large vessels and heart, making the mediastinum move to the opposite side; when the mass oppressed the esophagus and veins, this would more likely cause an increase of amniotic fluid and fetal edema (23). One study found that a higher CVR could increase the incidence rate of postpartum respiratory symptoms (3). In our study, the incidence of dyspnea among CCAM liveborn infants in the CVR ≥1.26 group was greater than that in the CVR <1.26 group (P<0.05) mainly because the larger lesions compressed the normal lung tissues and aggravated the disease.
In about 15% of CCAM cases, the disease disappears before delivery (24), but postnatal CT sometimes indicates that the supposedly “vanished” lesion on prenatal ultrasonography did not actually disappear after birth. Rather, due the normal lung tissue squeezing the lesion and reducing its echo intensity, the boundary with the normal lung tissue becomes unclear, especially microcystic CCAM; therefore, CCAM found by ultrasonography should be examined by prenatal MRI to further confirm or correct the diagnosis of prenatal ultrasonography. Previous studies on the disappearance of CCAM lesions have only included a small number of cases (25,26), and the reports on the disappearance in macrocystic or microcystic cases demonstrated low precision. In this study, CCAM lesions disappeared in 8 cases, including in 5 macrocystic cases and 3 microcystic cases, all in the CVR <1.26 group. Due to the small number of cases, no further analysis was performed in this study. It was found that the CVR of the macrocystic case was significantly larger than that in microcystic the cases because macrocystic CCAM had some solid lesions, and the volume was larger due to the varying sizes of the cysts.
In conclusion, Prenatal symptoms and postpartum prognosis were worse than CVR <1.26 when fetal CVR ≥1.26 measured by prenatal MRI; the CVR of macrocystic CCAM was significantly greater than that of microcystic CCAM. The measurement of the CVR of CCAM through prenatal MRI examination can help clinicians make more accurate prognoses and configure more appropriate treatment plans. Prenatal MRI thus has high practical value in prenatal consultation, evaluation, and postpartum treatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-973/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-973/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-973/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committees of Huzhou Maternity & Child Health Care Hospital (No. 2016-009) and Anhui Provincial Children’s Hospital (No. EYLL-2018-013), and all pregnant women voluntarily signed the informed consent prior to examination.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen Y, Zhao B, Xi F, et al. The prenatal ultrasonic character and postnatal follow-up of 227 microcystic and macrocystic congenital cystic adenomatoid malformations. J Obstet Gynaecol 2021;41:562-8. [Crossref] [PubMed]
- Han PH, Jiang KM, Zhong TH, et al. Characteristics of MR imaging manifestations and changes in fetus with congenital cystic adenomatoid malformation. Radiologic Practice 2018;33:754-7.
- Zhang HC, Tian JZ, Chen ZP, et al. Prenatal Ultrasound measurement of cystic volume ratio for prognosis evaluation in congenital cystic adenomatoid malformation. Chinese Journal of Medical Imaging 2016;24:367-70.
- Adin ME. Ultrasound as a screening tool in the follow-up of asymptomatic congenital cystic adenomatoid malformation. Ultrasound 2016;24:175-9. [Crossref] [PubMed]
- Chen WJ, Huang H, Liu YX, et al. Prenatal MRI and postnatal CT manifestations of pulmonary sequestration with congenital cystic adenomatoid malformation. Journal of Practical Radiology 2019;35:89-93.
- Li Z, Zhu M, Dong SZ, et al. Clinical value of prenatal MRI in the diagnosis and differential denomato of fetal bronchopulmonary sequestration. Zhonghua Fu Chan Ke Za Zhi 2016;51:23-6. [PubMed]
- Li Z, Luo ZQ. Prenatal MRI in the differential diagnosis of bronchopulmonary sequestration and congenital cystic adenomatoid malformation application value. Chinese Journal of Birth Health & Heredity 2017;25:101-3.
- Li Z, Lv YD, Fang R, et al. Usefulness of prenatal MRI examination in the differential diagnosis of fetal congenital cystic adenomatoid malformation and bronchopulmonary sequestration. World J Clin Cases 2021;9:822-9. [Crossref] [PubMed]
- Sanders RC. Prenatal ultrasonic detection of anomalies with a lethal or disastrous outcome. Radiol Clin North Am 1990;28:163-77. [Crossref] [PubMed]
- Xu XF, Yu H, Wang NF, et al. Diagnostic value of MRI in the fetal congenital cystic adenomatoid malformation of the lung. Journal of Practical Radiology 2016;32:251-4.
- Lin KW, Zhou ZF, Shi YQ, et al. The value of MRI in prenatal diagnosis of congenital cystic adenomatoid malformation and bronchopulmonary sequestration. Journal of Medical Imaging 2020;30:1056-62.
- Sun ZY, Xia LM, Chen XL, et al. Congenital cystic adenomatoid malformation of fetus: manifestations and diagnosis of MRI. Chinese Journal of Radiology 2007;41:490-2.
- Yang WL, Li P. Research advances of fetal conditions and prognosis for congenital cystic denomatoid malformation and pulmonary sequestration. Chin J Pediatr Surg 2019;40:269-72.
- Crombleholme TM, Coleman B, Hedrick H, et al. Cystic adenomatoid malformation volume ratio predicts outcome in prenatally diagnosed cystic adenomatoid malformation of the lung. J Pediatr Surg 2002;37:331-8. [Crossref] [PubMed]
- An P, Wang Y, Feng W, et al. Congenital Cystic Adenomatoid Malformation Volume Ratio in Prenatal Assessment of Prognosis of Fetal Pulmonary Sequestrations. Curr Med Sci 2019;39:658-62. [Crossref] [PubMed]
- Li Z, Lv Y, He P, et al. Clinical value of prenatal MRI for diagnosis of isolated ventriculomegaly and prediction of early postnatal developmental outcomes. Prenat Diagn 2019;39:124-9. [Crossref] [PubMed]
- Guo J, Huang CY, Liu P, et al. Application of MRI in the diagnosis of fetal lung malformation. Anhui Medical and Pharmaceutical Journal 2016;20:2087-9.
- Shamas AG, Bohara K. Congenital cystic adenomatoid malformation of the lung (CCAM), a retrospective clinical audit and literature review in a tertiary centre in Scotland over a period of 14 years. J Obstet Gynaecol 2017;37:19-24. [Crossref] [PubMed]
- Liu YH, Liao RZ, Liu SH, et al. Prenatal diagnosis of fetal congenital cystic adenomatoid malformation of the lung by color doppler ultrasound. Journal of Clinical Ultrasound in Medicine 2016;18:785-6.
- An P, Xiong Y. Research progress of congenital cystic adenomatoid malformation. Prog Obstet Gynecol 2018;27:477-9.
- Ehrenberg-Buchner S, Stapf AM, Berman DR, et al. Fetal lung lesions: can we start to breathe easier? Am J Obstet Gynecol 2013;208:151.e1-7. [Crossref] [PubMed]
- Ruchonnet-Metrailler I, Leroy-Terquem E, Stirnemann J, et al. Neonatal outcomes of prenatally diagnosed congenital pulmonary malformations. Pediatrics 2014;133:e1285-91. [Crossref] [PubMed]
- Lian XH, Ye FY, Lv GR. The effect of prenatal ultrasonography in diagnosis and prognosis of congenital cyst adenomatous malformation. Journal of Clinical Ultrasound in Medicine 2020;22:349-52.
- Khalek N, Johnson MP. Management of prenatally diagnosed lung lesions. Semin Pediatr Surg 2013;22:24-9. [Crossref] [PubMed]
- Zhong SL, Deng YQ, Zhang DR. The analysis of ultrasonic features and follow-up outcome in 99 cases with fetal congenital cystic adenomatoid malformations. Chinese Journal of Clinical Obstetrics and Gynecology 2018;19:525-8.
- Cook J, Chitty LS, De Coppi P, et al. The natural history of prenatally diagnosed congenital cystic lung lesions: long-term follow-up of 119 cases. Arch Dis Child 2017;102:798-803. [Crossref] [PubMed]
(English Language Editor: J. Gray)


