
Efficacy and safety of adjunctive nebulized colistin sulfate for multidrug-resistant Gram-negative bacteria pneumonia: a retrospective comparative cohort study
Introduction
In recent years, with the widespread clinical application of broad-spectrum antibiotics and the poor control of nosocomial infection, the rate of bacterial resistance has increased year by year, which has become a serious global problem. particularly, the detection rate of multidrug-resistant (MDR) bacteria represented by Gram-negative bacteria (GNB) has increased rapidly, posing a huge challenge to clinical anti-infective treatment. Among them, carbapenem-resistant Klebsiella pneumoniae (CR-KP), carbapenem-resistant Acinetobacter baumannii (CR-AB), and carbapenem-resistant Pseudomonas aeruginosa (CR-PA) infections are particularly dangerous, and in the absence of effective antibacterial drugs, the mortality rate can be as high as 45–50% (1-4). Patients who are infected with MDR-GNB need aggressive and immediate treatment when pathogens are suspected. Empirical therapy often includes carbapenems alone or in combination with cefoperazone/sulbactam and tigecycline. However, treatment is not always successful due to increased resistance (5). Therefore, in the face of such a severe drug resistance status and limited treatment options, polymyxins have returned to the clinic and are used for first-line treatment (6,7).
Polymyxins, which mainly include polymyxin B, colistimethate sodium (CMS), and colistin sulfate, began to be used clinically in the late 1950s. There are many studies verifying their effectiveness for MDR-GNB (4,5). However, because of the poor renal reserve function in elderly patients, the potential nephrotoxicity and neurotoxic adverse reactions of polymyxins are still the main factors limiting their widespread clinical application (8,9). In addition, a study reported that the concentration distribution of intravenous polymyxins in the lung tissue is insufficient, especially in the case of large airway secretions or pulmonary edema, and the antibacterial effect cannot be effectively achieved (10). Due to the limitations of the above treatments, inhaled antibiotics are used to minimize nephrotoxicity and enhance the efficacy of intravenous antibiotics (11). Currently, most studies use nebulized CMS as an adjunctive or alternative therapy, but few studies have reported the effectiveness and safety of colistin sulfate. Therefore, the purpose of this study was to evaluate the efficacy and safety of nebulized colistin sulfate in patients who were infected with MDR-GNB pneumonia. We hope this study can provide an effective and safe option for clinicians facing difficulties in treating MDR-GNB. We present the following article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-984/rc).
Methods
Study design and patient inclusion criteria
This retrospective study was conducted between December 2019 and October 2021 in Chongqing University Fuling Hospital, which is a 1200-bed tertiary hospital in Chongqing prefecture, China. A total of 203 patients who were infected with MDR GNB pneumonia were selected. A flow chart for the inclusion and exclusion of study patients is shown in Figure 1. Based on whether patients received nebulized colistin sulfate, patients were divided into 2 groups: the NCIA group (nebulized colistin sulfate in combination with other intravenous antibiotics) and the IA group (intravenous antibiotics without nebulized colistin sulfate). We confirm that all methods were carried out in accordance with relevant guidelines and regulations. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the medical ethics committee of Chongqing University Fuling Hospital (No. 2022CQSFLZXYYEC-003) and individual consent for this retrospective analysis was waived.
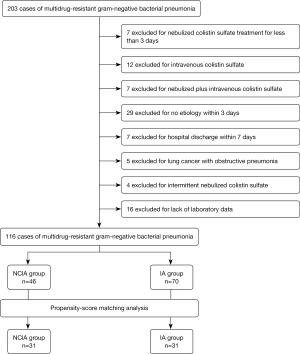
The inclusion criteria were as follows: (I) age >18 years old; (II) new onset and/or progressive pulmonary infiltrates on chest radiography; (III) MDR GNB in sputum or bronchial alveolar lavage fluid (BALF) were cultured within 3 days of admission.
The exclusion criteria were as follows: (I) pregnancy, perinatal period, and feeding period; (II) patients who underwent nebulized colistin sulfate treatment for less than 3 days or died within 48 hours; (III) acute or chronic renal insufficiency; (IV) lung cancer with obstructive pneumonia; (V) intravenous colistin sulfate; (VI) lack of laboratory data.
Definition of MDR-GNB pneumonia
A sputum sample and BALF were collected from each patient for bacterial culture and DNA quantification by real-time polymerase chain reaction (PCR) or metagenomic next generation sequencing (mNGS). The antimicrobial susceptibility of isolated bacterial pathogens was assessed on the basis of the minimum inhibitory concentration (MIC) according to the Clinical and Laboratory Standards Institute guidelines (12). MDR-GNB infection was diagnosed according to previously published international guidelines, namely, GNB is resistant to 3 or more than 3 kinds of antibiotics on the drug susceptibility test (13). The computed tomography (CT) imaging diagnosis of pneumonia was made by a pulmonologist and a radiologist.
Data collection of baseline characteristics
Data on demographic characteristics and baseline variables were retrieved from lianzhong electronic medical record database. Patient characteristics [age, sex, Charlson comorbidity index (CCI) points, sequential organ failure assessment (SOFA) points, clinical pulmonary infection score (CPIS) points, smoking history, pulmonary disease, comorbidity], temperature, laboratory data, clinical outcome, mechanical ventilation (MV) or not, intensive care unit (ICU) stay or not, and we collected pathogenic bacteria isolated by sputum culture on admission. The severity of pneumonia was assessed by the simplified CPIS (14-16) and the SOFA score (17-19) on the day of admission. Patients’ comorbidities were evaluated by the CCI (20). The collected laboratory data were as follows: leukocytes, neutrophil%, procalcitonin (PCT), C-reactive protein (CRP), albumin, creatinine, bilirubin, and PaO2/FiO2 (P/F) ratio.
Bacteria detection rate and microbiological eradication definition
The definition of bacteria clearance was as follows: the original pathogenic bacteria were not found in the sputum and BALF after administration of bacterial culture or DNA quantification by real-time PCR. The definition of uncleared was as follows: pathogenic bacteria were still cultured at the time of discharge. The bacteria detection rate was defined as the ratio of the number of cases with bacteria detected to the sum of cases (the sum of cases did not include those not submitted for inspection). The microbiological eradication rate was defined as the ratio of the number of cases of eradication to the sum of the number of cases of eradication, persistence, and recurrence.
Therapeutic regimens
All patients in this study were treated with intravenous antibiotics, such as carbapenems, cefoperazone/sulbactam, and tigecycline, which were determined by specialized clinicians according to the clinical condition of the patient. The patients in the NCIA group were also treated with nebulized colistin sulfate (trade name: FengWeiLing, Shanghai First Biochemical Pharmaceutical Co., Ltd., China). A total of 250,000 international units of colistin sulfate in 5 mL 0.45% saline was administered through the Aerogen Pro mesh nebulizer (Aerogen Ltd., Galway Business Park, Dangan, Galway, Ireland) for 20 min every 12 h, with a duration of ≥3 days.
Outcomes and safety assessment
The primary outcomes were favorable clinical outcomes, bacteria detection rate, and safety. The clinical response to treatment was classified as cure (resolution of symptoms and free from antibiotics), improvement (partial resolution of symptoms but not free from antibiotics), or failure (persistent symptoms or death). Both cure and improvement were defined as clinically favorable outcomes (21). The secondary outcomes were days of hospital stay, days of ICU stay, days of MV, days of antibiotic therapy, and 28-day all-cause mortality.
Adverse reactions related to colistin sulfate were closely observed, including airway hyperresponsiveness, tetter, skin pigmentation, acute renal injury, acute liver injury, and hematotoxicity.
We collected the outcomes and adverse reactions at day 7, day 14, and the last day. The last day was defined as the day antibiotic treatment ceased.
Propensity score matching (PSM) analysis
PSM was performed using the logistic regression model to generate a propensity score for balancing the baseline characteristics and potential confounders between the 2 groups. One-on-one paired PSM analysis with caliper <0.2 was performed by SPSS version 26 for Windows (SPSS Inc., Chicago, IL, USA). In this study, patients were matched one-to-one by propensity score using the covariates of age, sex, CCI (<3, ≥3), SOFA score (<3, ≥3), CPIS (<6, ≥6), and existence of effusion as the confounding variables for PSM. Then, the matched and adjusted data were analyzed.
Statistical analyses
Categorical variables were expressed as percentages, and continuous variables were expressed as mean ± standard deviation (SD) or quartiles (25th to 75th). The variable distribution was assessed by the Kolmogorov-Smirnov test. The Chi-square or Fisher’s exact test (two-tailed) was used to compare categorical variables, while the unpaired Student’s t-test or Mann-Whitney U test was used to compare continuous variables. Statistical analyses involved the use of SPSS version 26 for Windows (SPSS Inc., Chicago, IL, USA). A P value of <0.05 was considered statistically significant.
Results
Demographic characteristics and disease severity before and after PSM
Table 1 shows the demographic characteristics of the NCIA (n=46) and IA (n=70) groups before PSM. All patients were infected with MDR-GNB. Before PSM, there was no significant difference in sex, age, and CCI between the 2 groups. Furthermore, we evaluated the severity of disease by parameters including the SOFA score, MV, ICU stay, and CPIS, but there was no significant difference between the 2 groups except in CPIS. After PSM, 31 patients were selected from each group. Table 2 shows patient characteristics and laboratory data in both the NCIA and IA groups. There was no significant difference between groups in terms of comorbidity and laboratory data, including leukocytes, neutrophil%, PCT, CRP, albumin, creatinine, bilirubin, and P/F ratio. The baseline characteristics were well-balanced between the 2 matched groups.
Table 1
| Variables | Before matching | After matching | |||||
|---|---|---|---|---|---|---|---|
| NCIA group (n=46) | IA group (n=70) | P value (χ2) | NCIA group (n=31) | IA group (n=31) | P value (χ2) | ||
| Age (years), n (%) | 0.447 (0.506) | 1.000 (0.000) | |||||
| ≥70 | 37 (80.4) | 51 (72.9) | 24 (77.4) | 24 (77.4) | |||
| <70 | 9 (19.6) | 19 (27.1) | 7 (22.6) | 7 (22.6) | |||
| Sex, n (%) | 0.452 (0.565) | 0.335 (0.930) | |||||
| Male | 37 (80.4) | 59 (84.3) | 27 (87.1) | 23 (74.2) | |||
| Female | 9 (19.6) | 11 (15.7) | 4 (12.9) | 8 (25.8) | |||
| CCI, n (%) | 0.721 (0.128) | 0.335 (0.930) | |||||
| ≥3 | 28 (60.9) | 39 (55.7) | 18 (58.1) | 23 (74.2) | |||
| <3 | 18 (39.1) | 31 (44.3) | 13 (41.9) | 8 (25.8) | |||
| ICU, n (%) | 0.255 (1.297) | 0.780 (0.078) | |||||
| Yes | 35 (76.1) | 45 (64.3) | 21 (67.7) | 23 (74.2) | |||
| No | 11 (23.9) | 25 (35.7) | 10 (32.3) | 8 (25.8) | |||
| MV, n (%) | 0.797 (0.066) | 0.184 (1.761) | |||||
| Yes | 31 (67.4) | 50 (71.4) | 17 (54.8) | 23 (74.2) | |||
| No | 15 (32.6) | 20 (28.6) | 14 (45.2) | 8 (25.8) | |||
| SOFA (points), n (%) | 1.000 (0.000) | 0.705 (0.144) | |||||
| ≥2 | 40 (87.0) | 61 (87.1) | 26 (83.9) | 28 (90.3) | |||
| 0–1 | 6 (13.0) | 9 (12.9) | 5 (16.1) | 3 (9.7) | |||
| CPIS (points), n (%) | 0.000 (12.963) | 0.362 (0.830) | |||||
| ≥6 | 41 (89.1) | 39 (55.7) | 26 (83.9) | 22 (71.0) | |||
| <6 | 5 (10.9) | 31 (44.3) | 5 (16.1) | 9 (29.0) | |||
PSM, propensity score matching; NCIA, nebulized colistin sulfate in combination with intravenous antibiotics; IA, intravenous antibiotics without nebulized colistin sulfate; CCI, Charlson comorbidity index; ICU, intensive care unit; MV, mechanical ventilation; SOFA, sequential organ failure assessment; CPIS, clinical pulmonary infection score.
Table 2
| Variables | NCIA group (n=31) | IA group (n=31) | P value |
|---|---|---|---|
| Age (years), (mean ± SD) | 78.26±9.342 | 77.23±11.218 | 0.695 (t=0.394) |
| Gender (male), n (%) | 27 (87.1) | 23 (74.2) | 0.335 (χ2=0.930) |
| Smoking history, n (%) | 18 (58.1) | 17 (54.8) | 0.798 (χ2=0.066) |
| Pulmonary disease, n (%) | |||
| Pleural effusion | 14 (45.2) | 9 (29.0) | 0.293 (χ2=1.106) |
| Bronchiectasis | 3 (9.7) | 5 (16.1) | 0.705 (χ2=0.144) |
| Pneumoconiosis | 1 (3.2) | 0 (0.0) | 1.000 (χ2=0.000) |
| Interstitial lung disease | 0 (0.0) | 0 (0.0) | |
| Tuberculosis | 0 (0.0) | 0 (0.0) | |
| Pulmonary embolism | 2 (6.5) | 5 (16.1) | 0.422 (χ2=0.644) |
| COPD | 3 (9.7) | 3 (9.7) | 1.000 (χ2=0.000) |
| Comorbidity, n (%) | |||
| Coronary heart disease | 1 (3.2) | 1 (3.2) | 1.000 (χ2=0.000) |
| Diabetes mellitus | 7 (22.6) | 3 (9.7) | 0.300 (χ2=1.073) |
| Moderate or severe kidney disease | 8 (25.8) | 3 (9.7) | 0.184 (χ2=1.768) |
| Collagen vascular disease | 0 (0.0) | 0 (0.0) | |
| Cerebrovascular disease | 10 (32.3) | 16 (51.6) | 0.198 (χ2=1.656) |
| Hemiplegia | 7 (22.6) | 9 (29.0) | 0.843 (χ2=0.341) |
| HIV | 0 (0.0) | 0 (0.0) | |
| Malignancy | 4 (12.9) | 4 (12.9) | 1.000 (χ2=0.000) |
| Moderate or severe hepatic disease | 1 (3.2) | 1 (3.2) | 1.000 (χ2=0.000) |
| Peptic ulcer | 0 (0.0) | 3 (9.7) | 0.237 (χ2=1.401) |
| Disease severity (points) | |||
| SOFA score at day 1 | 4 [2–6] | 5 [3–6] | 0.313 (Z=1.009) |
| CPIS at day 1 | 7 [6–8] | 7 [5–8] | 0.937 (Z=0.079) |
| MV, n (%) | 0.471 (χ2=1.506) | ||
| IPPV | 6 (31.6) | 4 (16.7) | |
| NPPV | 10 (52.6) | 14 (58.3) | |
| IPPV + NPPV | 3 (15.8) | 6 (25.0) | |
| Laboratory data analysis | |||
| Leukocytes | 8.77 (6.20–11.52) | 10.50 (7.87–17.48) | 0.104 (Z=1.626) |
| Neutrophil % | 84.90 (73.70–92.10) | 86.00 (78.70–91.20) | 0.811 (Z=0.239) |
| PCT | 0.195 (0.076–1.470) | 0.244 (0.118–1.940) | 0.297 (Z=1.043) |
| C-reactive protein | 54.50 (15.60–99.60) | 67.00 (23.55–160.00) | 0.357 (Z=0.921) |
| Albumin | 31.60 (27.20–35.50) | 33.40 (27.95–35.65) | 0.493 (Z=0.685) |
| Creatinine | 73.10 (48.80–106.20) | 77.10 (45.20–112.00) | 0.827 (Z=0.218) |
| Bilirubin | 12.10 (9.33–20.88) | 14.45 (10.34–26.26) | 0.278 (Z=1.084) |
| P/F ratio | 231.03 (153.33–296.00) | 217.24 (179.31–254.00) | 0.598 (Z=0.528) |
PSM, propensity score matching; COPD, chronic obstructive pulmonary disease; NCIA, nebulized colistin sulfate in combination with intravenous antibiotics; IA, intravenous antibiotics without nebulized colistin sulfate; HIV, human immunodeficiency virus; SOFA, sequential organ failure assessment; CPIS, clinical pulmonary infection score; MV, mechanical ventilation; IPPV, invasive positive pressure ventilation; NPPV, non-invasive positive pressure ventilation; PCT, procalcitonin; P/F, PaO2/FiO2.
Therapeutic efficacy
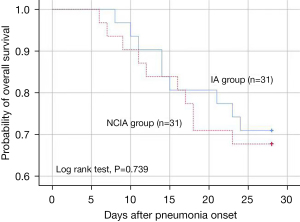
Table 3 shows the comparison of therapeutic efficacy between the NCIA and IA groups after PSM. There were significant differences in favorable clinical outcomes on days 7 (67.7% vs. 32.3%, P=0.005) and 14 (71% vs. 41.9%, P=0.045), and the bacteria detection rate on days 7, 14, and the last day. Only 5 patients (16.7%) were identified as pathogenic on the last day in the NCIA group. There were also significant differences in the following aspects: days of hospital stay (17 vs. 23 days, P=0.01), antipyretic time (0.5 vs. 7.5 days, P=0.037), days of antibiotic therapy (14 vs. 23 days, P=0.002), and SOFA score on day 7 (2.5 vs. 5, P=0017). In addition, there was also a significant difference in CPIS on days 7 and 14. However, there were no significant differences in both groups in terms of days of ICU stay, days of MV, and 28-day all-cause mortality. In Table 4, we observed that nebulized colistin sulfate was an independent factor for 28-day all-cause mortality [adjusted hazard ratio (aHR) =0.499, 95% CI: 0.264–0.946, P=0.033], favorable clinical outcomes on days 7 and 14, and microbiological eradication on days 7, 14, and the last day by multivariate analysis. In Figure 2, Kaplan-Meier analysis of 28-day all-cause survival showed no significant differences between the NCIA and IA groups.
Table 3
| Variables | NCIA group (n=31) | IA group (n=31) | P value |
|---|---|---|---|
| SOFA score after day 7 (points) | 2.50 (1.00–4.00) | 5.00 (2.00–7.00) | 0.017 (Z=2.386) |
| SOFA score after day 14 (points) | 3.00 (1.00–4.50) | 3.50 (2.00–5.25) | 0.256 (Z=1.136) |
| SOFA score on the last day (points) | 2.00 (1.00–6.75) | 3.00 (2.00–7.00) | 0.513 (Z=0.654) |
| CPIS after day 7 (points) | 5.00 (2.75–6.00) | 7.00 (5.00–9.00) | 0.004 (Z=2.845) |
| CPIS after day 14 (points) | 2.00 (1.00–3.00) | 4.00 (2.00–6.50) | 0.005 (Z=2.809) |
| CPIS on the last day (points) | 1.00 (0.00–4.25) | 2.00 (1.00–7.00) | 0.081 (Z=1.747) |
| Favorable clinical outcomes, n (%) | |||
| After day 7 | 21 (67.7) | 10 (32.3) | 0.005 (χ2=7.806) |
| After day 14 | 22 (71.0) | 13 (41.9) | 0.045 (χ2=4.481) |
| On the last day | 21 (67.7) | 17 (54.8) | 0.297 (χ2=1.088) |
| Days of hospital stay (d) | 17.00 (11.00–20.00) | 23.00 (14.00–34.00) | 0.010 (Z=2.579) |
| Days of ICU stay (d) | 12.00 (8.00–18.00) | 14.00 (10.00–29.50) | 0.265 (Z=1.115) |
| Days of mechanical ventilation (d) | 9.00 (6.00–16.00) | 13.00 (10.00–21.50) | 0.058 (Z=1.899) |
| Mortality (day 1 to 28), n (%) | 10 (32.3) | 14 (45.2) | 0.434 (χ2=0.612) |
| Antipyretic time (d) | 0.50 (0.00–9.50) | 7.50 (1.25–13.75) | 0.037 (Z=2.085) |
| Days of antibiotic therapy (d) | 14.00 (9.00–19.00) | 23.00 (14.00–33.00) | 0.002 (Z=3.158) |
| Imaging absorption, n (%) | 0.392 (χ2=3.000) | ||
| Complete | 6 (19.4) | 3 (9.7) | |
| Partial | 18 (58.1) | 18 (58.1) | |
| Not absorbed | 6 (19.4) | 10 (32.3) | |
| Bacteria detection rate by culture or RT-PCR/mNGS, n (%) | |||
| After day 7 | 18 (60.0) | 26 (83.9) | 0.049 (χ2=4.322) |
| After day 14 | 8 (25.8) | 22 (71.0) | 0.006 (χ2=8.563) |
| On the last day | 5 (16.7) | 15 (48.4) | 0.013 (χ2=6.961) |
| Adverse reactions, n (%) | |||
| Acute kidney injury | 5 (16.1) | 3 (9.7) | 0.707 (χ2=0.144) |
| Acute liver injury | 1 (3.2) | 3 (9.7) | 0.612 (χ2=0.267) |
| Platelet decline | 6 (19.4) | 5 (16.1) | 0.740 (χ2=0.111) |
| Anemia | 4 (12.9) | 13 (41.9) | 0.021 (χ2=5.187) |
| Tetter | 0 (0.0) | 0 (0.0) | |
| Skin pigmentation | 0 (0.0) | 0 (0.0) | |
| Airway hyperresponsiveness | 1 (3.2) | 0 (0.0) | 1.000 (χ2=0.000) |
PSM, propensity score matching; NCIA, nebulized colistin sulfate in combination with intravenous antibiotics; IA, intravenous antibiotics without nebulized colistin sulfate; SOFA, sequential organ failure assessment; CPIS, clinical pulmonary infection score; ICU, intensive care unit; RT-PCR, real-time polymerase chain reaction; mNGS, metagenomic next generation sequencing.
Table 4
| NCIA (vs. IA) | aHR (95% CI)/aOR (95% CI) | P value |
|---|---|---|
| 28-day all-cause mortalitya | 0.499 (0.264–0.946) | 0.033 |
| Favorable clinical outcomes after 7 daysb | 4.410 (1.520–12.792) | 0.006 |
| Favorable clinical outcomes after 14 daysb | 3.636 (1.075–12.303) | 0.038 |
| Microbiological eradication after 7 daysb | 3.467 (1.040–11.556) | 0.043 |
| Microbiological eradication after 14 daysb | 6.600 (1.763–24.714) | 0.005 |
| Microbiological eradication on the last dayb | 4.687 (1.425–15.421) | 0.011 |
a, adjusted hazard ratios (aHRs) and 95% CIs were derived from Cox regression analysis. b, adjusted odds ratios (aORs) and 95% CIs were derived from logistic regression analysis. PSM, propensity score matching; NCIA, nebulized colistin sulfate in combination with intravenous antibiotics; IA, intravenous antibiotics without nebulized colistin sulfate.
Nephrotoxicity
In Table 3, we assessed nephrotoxicity by acute kidney injury (AKI) development after the initiation of combination therapy. A total of 16.1% and 9.7% of patients in the NCIA group and control group, respectively, developed AKI, but we did not observe significant differences between these 2 groups. There was no significant difference in acute liver injury and platelet decline. In our study, 1 patient in the NCIA group developed airway hyperresponsiveness (P=1.000). Tetter and skin pigmentation did not occur in both groups.
Discussion
Polymyxin B and colistin have become effective antibiotics commonly used to control MDR-GNB infection at home and abroad (4). However, there are 2 preparations of colistin, colistin sulfate, and CMS, and the proportion of major components of colistin in clinical use varies among different brands, resulting in unstable clinical efficacy (5,22-24). At present, the use of nebulized colistin as an adjuvant or alternative therapy is a hot spot in the study of MDR-GNB pulmonary infection, and a large number of studies have proven the effectiveness and safety of nebulized CMS. The United States and France even wrote aerosolized CMS into the guidelines for antibiotic use after MDR-GNB infection by hospital-acquired pneumonia (HAP) or ventilator-associated pneumonia (VAP) (25-29). It is noteworthy that since the clinical application of colistin sulfate in China, there have been few studies on the efficacy and safety of nebulized colistin sulfate in the treatment of MDR-GNB pneumonia.
Palmer et al. showed that aerosolized CMS significantly reduced the CPIS in patients with severe pulmonary infection of MDR-GNB compared with the control group (30). The results of our study showed that, compared with the IA group, the CPIS of the NCIA group was significantly reduced on the 7th and 14th day of aerosolized colistin sulfate, and the SOFA score was added to assess the condition. The results showed that the SOFA score of the NCIA group was also significantly decreased on the 7th day. The clinical response rate was up to 67.7% and 71% on day 7 and 14, respectively, providing further support that aerosolized colistin sulfate improves the condition of patients.
Polymyxins, by adhering to cell membranes, change the structure of cell membranes to make them more permeable, leading to bacterial death and neutralizing endotoxins released by GNB (31). Nebulized colistin was first used to enhance the clearance of pseudomonas aeruginosa in patients with cystic pulmonary fibrosis, and its effect on airway clearance of MDR-GNB in non-cystic fibrosis bronchiectasis, chronic obstructive pulmonary disease (COPD), VAP, and the lower respiratory tract was subsequently confirmed (11,32-34). The results of our study showed that compared with the IA group, the bacterial detection rate of the NCIA group decreased significantly on day 7 (60% vs. 83.9%), day 14 (25.8% vs. 71%), and the day when antibiotics were discontinued (16.7% vs. 48.4%). In contrast, the bacterial clearance rate of sputum increased, which was consistent with the above study.
Aerosolized colistin can deliver an effective amount of drugs directly to the respiratory system, presenting a high concentration in lung tissue faster than intravenous injection. This provides a rapid and effective bactericidal effect, shortening the duration of intravenous antibiotic use and hospital stay, thus effectively improving bacterial resistance rates (29,30,35,36). Our study showed that the time from fever to normal body temperature (0.5 vs. 7.5 days) and antibiotic use time (14 vs. 23 days) were significantly shorter in the NCIA group than in the IA group, and the length of hospital stay was reduced (17 vs. 23 days).
Currently, there are different opinions as to whether aerosolized colistin can shorten the MV time and ICU stay time of MDR-GNB severe pulmonary infection. Solé-Lleonart et al. compared aerosolized and intravenous administration of CMS and found that aerosolized CMS did not reduce MV and ICU stay time (37). However, the results of a prospective randomized trial by Abdellatif et al. showed that aerosolized CMS reduced MV time and ICU stay compared with intravenous colistin administration (35). In our study, the NCIA group did not have a shortened duration of MV or ICU stay. We first consider the differences in experimental design that lead to different research results; secondly, the differences in the atomization device used and the atomization dose cannot be ignored. understanding the mechanisms that determine pulmonary parenchymal deposition and the use of appropriate doses are critical issues for treatment efficiency. In order to optimize lung deposition in ventilated patients, mesh nebulizers and specific ventilator settings should be used to provide continuous atomization rather than breathing-driven atomization to reduce the flow rate and inertial impact of atomized particles (38). At the same time, experiments showed that alveolar deposition of nebulized antibiotics decreased as distal bronchioles were blocked by suppurative embolism, and the histological severity of pneumonia and pulmonary ventilation disappeared (39). In our study, the NCIA group was given mesh nebulizer atomization, excluding the influence of the atomization device on the study results. However, some non-invasive ventilation patients in the NCIA group had signs of invasive MV. Their family members refused endotracheal intubation, meaning that they could not carry out effective lung ventilation, which reduced the alveolar deposition of colistin sulfate. Secondly, colistin is a concentration-dependent antibiotic, and low-dose use is associated with poor prognosis of MDR-GNB infection (40). However, at present, the dose of aerosolized colistin has not been standardized. Pharmaco Kinetics/Pharmaco Dynamics (PK/PD) analysis concluded that alveolar concentration was significantly higher after aerosolized administration of CMS than intravenous administration (29). However, since colistin sulfate has been clinically applied in China, no PK/PD study has been conducted in patients on severe MV for MDR-GNB pulmonary infection. Therefore, whether the dose is appropriate for such patients is a problem that requires further in vitro tests and clinical exploration in the future.
It is worth mentioning that many studies have shown that aerosolized colistin does not improve 28-day all-cause mortality, which is considered to be associated with inappropriate dosing, co-occurrence of end-stage structural lung disease, and use as salvage rather than initial treatment (28,37,41,42). Interestingly, in our study, there was no improvement in 28-day all-cause mortality in the NCIA group, but a significant reduction in the risk of death (HR =0.499; 95% CI: 0.264–0.964; P=0.033). Therefore, once MDR-GNB infection is confirmed and drug sensitivity to colistin sulfate is identified, it is recommended to start adjuvant therapy with nebulized colistin sulfate as soon as possible. We are also eager to see future multicenter studies that standardize aerosolized doses to reduce the risk of death in MDR-GNB infected patients.
It is known that the potential nephrotoxicity of intravenous polymyxins remains a major factor limiting in their widespread clinical use (43,44), while aerosolized colistin is not associated with an increased risk of renal impairment, and its safety has been confirmed in numerous studies (45-48). In our study, AKI, acute liver injury, and thrombocytopenia occurred in a small number of cases in both the NCIA group and IA group, but the differences were not statistically significant. No neurotoxicity and skin pigmentation were found, indicating that aerosolized colistin sulfate does not increase the risk of adverse reactions. Solé-Lleonart et al. suggested that aerosolized CMS was safe in terms of nephrotoxicity and neurotoxicity, but increased respiratory complications by 9% (95% CI: 0.01–0.18; I2=52%), especially in patients with hypoxemia (37). In this study, 1 patient had airway hyperresponsiveness in the process of atomization, but the difference was not statistically significant between groups, which was considered to be related to the small sample size.
In conclusion, our study showed that nebulized colistin sulfate, as an adjunct to systemic antibiotic therapy in Chinese MDR-GNB pneumonia patients, can improve clinical efficacy, increase the bacterial clearance rate, shorten intravenous antibiotic use time and hospital stay, and reduce the risk of death with high safety. However, due to the limitation of detection conditions, we failed to monitor the concentration of colistin sulfate in the blood and airway secretions of the NCIA group in a timely manner, so the relationship between clinical efficacy and drug concentration could not be well observed. Secondly, due to the problems of this being a single center and small sample size study, the accuracy of the conclusion needs to be verified by multicenter and large-sample studies in the future.
Acknowledgments
Funding: This research was funded by the Chongqing Regional Key Discipline Construction Project (No. zdxk201702).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-984/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-984/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-984/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the medical ethics committee of Chongqing University Fuling Hospital (No. 2022CQSFLZXYYEC-003) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chinese Research Hospital Association of Critical Care Medicine, Chinese Research Hospital Association of Evidence Base And Translational Infectious Diseases. Chinese expert consensus on polymyxins in the clinical practice. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2019;31:1194-8. [PubMed]
- Tsuji BT, Pogue JM, Zavascki AP, et al. International Consensus Guidelines for the Optimal Use of the Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 2019;39:10-39. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Li J, Nation RL, Turnidge JD, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 2006;6:589-601. [Crossref] [PubMed]
- Roberts KD, Azad MA, Wang J, et al. Antimicrobial Activity and Toxicity of the Major Lipopeptide Components of Polymyxin B and Colistin: Last-line Antibiotics against Multidrug-Resistant Gram-negative Bacteria. ACS Infect Dis 2015;1:568-75. [Crossref] [PubMed]
- Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis 2005;40:1333-41. [Crossref] [PubMed]
- Rabanal F, Cajal Y. Recent advances and perspectives in the design and development of polymyxins. Nat Prod Rep 2017;34:886-908. [Crossref] [PubMed]
- Fiaccadori E, Antonucci E, Morabito S, et al. Colistin Use in Patients With Reduced Kidney Function. Am J Kidney Dis 2016;68:296-306. [Crossref] [PubMed]
- Kelesidis T, Falagas ME. The safety of polymyxin antibiotics. Expert Opin Drug Saf 2015;14:1687-701. [Crossref] [PubMed]
- Smith BS, Yogaratnam D, Levasseur-Franklin KE, et al. Introduction to drug pharmacokinetics in the critically ill patient. Chest 2012;141:1327-36. [Crossref] [PubMed]
- Vardakas KZ, Voulgaris GL, Samonis G, et al. Inhaled colistin monotherapy for respiratory tract infections in adults without cystic fibrosis: a systematic review and meta-analysis. Int J Antimicrob Agents 2018;51:1-9. [Crossref] [PubMed]
- Humphries R, Bobenchik AM, Hindler JA, et al. Overview of Changes to the Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing, M100, 31st Edition. J Clin Microbiol 2021;59:e0021321.
- Falagas ME, Karageorgopoulos DE. Pandrug resistance (PDR), extensive drug resistance (XDR), and multidrug resistance (MDR) among Gram-negative bacilli: need for international harmonization in terminology. Clin Infect Dis 2008;46:1121-2; author reply 1122. [Crossref] [PubMed]
- Shen F, Wu Y, Wang Y, et al. Performance of clinical pulmonary infection score induces the duration and defined daily doses of antibiotics in patients with bacterial severe pneumonia in intensive care unit. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2019;31:556-61. [PubMed]
- Gunalan A, Sistla S, Sastry AS, et al. Concordance between the National Healthcare Safety Network (NHSN) Surveillance Criteria and Clinical Pulmonary Infection Score (CPIS) Criteria for Diagnosis of Ventilator-associated Pneumonia (VAP). Indian J Crit Care Med 2021;25:296-8. [Crossref] [PubMed]
- Rosbolt MB, Sterling ES, Fahy BG. The utility of the clinical pulmonary infection score. J Intensive Care Med 2009;24:26-34. [Crossref] [PubMed]
- Fernando SM, Tran A, Taljaard M, et al. Prognostic Accuracy of the Quick Sequential Organ Failure Assessment for Mortality in Patients With Suspected Infection: A Systematic Review and Meta-analysis. Ann Intern Med 2018;168:266-75. [Crossref] [PubMed]
- Kashyap R, Sherani KM, Dutt T, et al. Current Utility of Sequential Organ Failure Assessment Score: A Literature Review and Future Directions. Open Respir Med J 2021;15:1-6. [Crossref] [PubMed]
- Pawar RD, Shih JA, Balaji L, et al. Variation in SOFA (Sequential Organ Failure Assessment) Score Performance in Different Infectious States. J Intensive Care Med 2021;36:1217-22. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Wang SH, Yang KY, Sheu CC, et al. Efficacies of Colistin-Carbapenem versus Colistin-Tigecycline in Critically Ill Patients with CR-GNB-Associated Pneumonia: A Multicenter Observational Study. Antibiotics (Basel) 2021;10:1081. [Crossref] [PubMed]
- He J, Ledesma KR, Lam WY, et al. Variability of polymyxin B major components in commercial formulations. Int J Antimicrob Agents 2010;35:308-10. [Crossref] [PubMed]
- He H, Li JC, Nation RL, et al. Pharmacokinetics of four different brands of colistimethate and formed colistin in rats. J Antimicrob Chemother 2013;68:2311-7. [Crossref] [PubMed]
- Brink AJ, Richards GA, Colombo G, et al. Multicomponent antibiotic substances produced by fermentation: implications for regulatory authorities, critically ill patients and generics. Int J Antimicrob Agents 2014;43:1-6. [Crossref] [PubMed]
- Rello J, Solé-Lleonart C, Rouby JJ, et al. Use of nebulized antimicrobials for the treatment of respiratory infections in invasively mechanically ventilated adults: a position paper from the European Society of Clinical Microbiology and Infectious Diseases. Clin Microbiol Infect 2017;23:629-39. [Crossref] [PubMed]
- Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61-e111. [Crossref] [PubMed]
- Leone M, Bouadma L, Bouhemad B, et al. Hospital-acquired pneumonia in ICU. Anaesth Crit Care Pain Med 2018;37:83-98. [Crossref] [PubMed]
- Zheng JY, Huang SS, Huang SH, et al. Colistin for pneumonia involving multidrug-resistant Acinetobacter calcoaceticus-Acinetobacter baumannii complex. J Microbiol Immunol Infect 2020;53:854-65. [Crossref] [PubMed]
- Boisson M, Jacobs M, Grégoire N, et al. Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and colistin after aerosol delivery and intravenous administration of CMS in critically ill patients. Antimicrob Agents Chemother 2014;58:7331-9. [Crossref] [PubMed]
- Palmer LB, Smaldone GC. Reduction of bacterial resistance with inhaled antibiotics in the intensive care unit. Am J Respir Crit Care Med 2014;189:1225-33. [Crossref] [PubMed]
- Molina J, Cordero E, Pachón J. New information about the polymyxin/colistin class of antibiotics. Expert Opin Pharmacother 2009;10:2811-28. [Crossref] [PubMed]
- Bruguera-Avila N, Marin A, Garcia-Olive I, et al. Effectiveness of treatment with nebulized colistin in patients with COPD. Int J Chron Obstruct Pulmon Dis 2017;12:2909-15. [Crossref] [PubMed]
- Tabernero Huguet E, Gil Alaña P, Alkiza Basañez R, et al. Inhaled colistin in elderly patients with non-cystic fibrosis bronchiectasis and chronic Pseudomonas aeruginosa bronchial infection. Rev Esp Geriatr Gerontol 2015;50:111-5. [Crossref] [PubMed]
- Falagas ME, Siempos II, Rafailidis PI, et al. Inhaled colistin as monotherapy for multidrug-resistant gram (-) nosocomial pneumonia: a case series. Respir Med 2009;103:707-13. [Crossref] [PubMed]
- Abdellatif S, Trifi A, Daly F, et al. Efficacy and toxicity of aerosolised colistin in ventilator-associated pneumonia: a prospective, randomised trial. Ann Intensive Care 2016;6:26. [Crossref] [PubMed]
- Bassetti S, Tschudin-Sutter S, Egli A, et al. Optimizing antibiotic therapies to reduce the risk of bacterial resistance. Eur J Intern Med 2022;99:7-12. [Crossref] [PubMed]
- Solé-Lleonart C, Rouby JJ, Blot S, et al. Nebulization of Antiinfective Agents in Invasively Mechanically Ventilated Adults: A Systematic Review and Meta-analysis. Anesthesiology 2017;126:890-908. [Crossref] [PubMed]
- Rouby JJ, Sole-Lleonart C, Rello J, et al. Ventilator-associated pneumonia caused by multidrug-resistant Gram-negative bacteria: understanding nebulization of aminoglycosides and colistin. Intensive Care Med 2020;46:766-70. [Crossref] [PubMed]
- Rouby JJ, Bouhemad B, Monsel A, et al. Aerosolized antibiotics for ventilator-associated pneumonia: lessons from experimental studies. Anesthesiology 2012;117:1364-80. [Crossref] [PubMed]
- Ismail B, Shafei MN, Harun A, et al. Predictors of polymyxin B treatment failure in Gram-negative healthcare-associated infections among critically ill patients. J Microbiol Immunol Infect 2018;51:763-9. [Crossref] [PubMed]
- Tang R, Luo R, Wu B, et al. Effectiveness and safety of adjunctive inhaled antibiotics for ventilator-associated pneumonia: A systematic review and meta-analysis of randomized controlled trials. J Crit Care 2021;65:133-9. [Crossref] [PubMed]
- Lu Q, Luo R, Bodin L, et al. Efficacy of high-dose nebulized colistin in ventilator-associated pneumonia caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Anesthesiology 2012;117:1335-47. [Crossref] [PubMed]
- Jafari F, Elyasi S. Prevention of colistin induced nephrotoxicity: a review of preclinical and clinical data. Expert Rev Clin Pharmacol 2021;14:1113-31. [Crossref] [PubMed]
- Arrayasillapatorn N, Promsen P, Kritmetapak K, et al. Colistin-Induced Acute Kidney Injury and the Effect on Survival in Patients with Multidrug-Resistant Gram-Negative Infections: Significance of Drug Doses Adjusted to Ideal Body Weight. Int J Nephrol 2021;2021:7795096. [Crossref] [PubMed]
- Lu Q, Girardi C, Zhang M, et al. Nebulized and intravenous colistin in experimental pneumonia caused by Pseudomonas aeruginosa. Intensive Care Med 2010;36:1147-55. [Crossref] [PubMed]
- Athanassa ZE, Markantonis SL, Fousteri MZ, et al. Pharmacokinetics of inhaled colistimethate sodium (CMS) in mechanically ventilated critically ill patients. Intensive Care Med 2012;38:1779-86. [Crossref] [PubMed]
- Vardakas KZ, Mavroudis AD, Georgiou M, et al. Intravenous plus inhaled versus intravenous colistin monotherapy for lower respiratory tract infections: A systematic review and meta-analysis. J Infect 2018;76:321-7. [Crossref] [PubMed]
- Haworth CS, Foweraker JE, Wilkinson P, et al. Inhaled colistin in patients with bronchiectasis and chronic Pseudomonas aeruginosa infection. Am J Respir Crit Care Med 2014;189:975-82. [Crossref] [PubMed]


