
The effect of early vs. late CT-guided stereotactic hematoma aspiration on neurological function recovery in patients with hypertensive cerebral hemorrhage in the basal ganglia: a retrospective comparative cohort study
Introduction
Hypertensive cerebral hemorrhage is called hemorrhagic stroke. The incidence rate and the mortality rate of this disease are high (1). Some patients with hypertensive cerebral hemorrhage may suffer from severe neurological dysfunction after the traditional treatment (2).
The operation mode, operation time, bleeding volume, bleeding site and bleeding speed may affect the recovery of nerve function after operation. Stereotactic hematoma aspiration is a minimally invasive treatment for cerebral hemorrhage (3-5). It only requires a 4–5 cm incision in the scalp, the drilling of a hole (about 1 cm in diameter) in the skull, and the selection of a drainage tube with an outer diameter of 2–3 mm to puncture the hematoma cavity. During the operation, part of the liquefied hematoma is extracted to achieve decompression. Urokinase is then injected into the cavity every day after the operation to dissolve the residual hematoma and release it. Usually, the blood accumulated in the brain can be drained in 3 to 5 days. However, while this method is simple and effective, it requires a high level of puncture technology and accuracy. Thus, it needs to be performed under the guidance of computed tomography (CT).
Previous studies have confirmed that CT-guided stereotactic hematoma aspiration has a positive effect on the neurological rehabilitation of patients with hypertensive cerebral hemorrhage (6,7). However, the optimal timing for CT-guided stereotactic hematoma aspiration is not yet known. It is believed that a hematoma caused by an intracranial hemorrhage compressing the brain nerve damages the nerve. The longer the period of compression, the lower the possibility of nerve function recovery. Thus, the operation should be performed as soon as possible to remove the hematoma and reduce the secondary damage to the surrounding tissues (8). However, an early operation may increase the risk of hematoma expansion and are easy to cause rebleeding.
This study aimed to explore the effect of the timing of CT-guided stereotactic hematoma aspiration on the recovery of neurological function in patients with hypertensive cerebral hemorrhage in the basal ganglia region. We present the following article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-995/rc).
Methods
Data collection
The data of 110 patients with hypertensive cerebral hemorrhage in the basal ganglia admitted to the Union Hospital Tongji Medical College Huazhong University of Science and Technology from January 2021 to December 2021 were retrospectively collected in this retrospective comparative cohort study. Based on the timing of the operation, the patients were allocated to the early treatment group (within 24 hours, n=50) and the late treatment group (after 24 hours, n=60).
To be eligible for inclusion in this study, patients had to meet the following inclusion criteria: (I) have an acute hypertensive cerebral hemorrhage in the basal ganglia (bleeding volume: 30–50 mL); (II) have had the disease <24 h; (III) agree to receive hematoma aspiration; (IV) be aged 20–80 years; and (V) have complete clinical data. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had a traumatic cerebral hemorrhage; (II) had a malignant tumor; (III) had another major intracranial disease, such as a cerebrovascular malformation; (IV) had an infectious disease; (V) had an immune system disease; (VI) had coagulation dysfunction; (VII) had limb dysfunction; and/or (VIII) had any surgical contraindications.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Union Hospital Tongji Medical College Huazhong University of Science and Technology (No. 20220027). Individual consent for this retrospective analysis was waived.
Treatment strategy
(I) The early treatment group: CT-guided stereotactic hematoma aspiration was performed within 24 h of the onset of the disease. The patient’s head was marked before the operation, and a thin-layer axial CT scan of the brain was then performed (layer spacing: 1 mm). The data were imported into computer software to complete the fusion of the CT images of the head and the 3-dimensional reconstruction. The center point of the largest layer of the hematoma was selected as the puncture center. After avoiding the important functional area, the maximum length of the hematoma was selected as the puncture path. An incision of about 4 cm was made in the head skin at the cranial entry point, a hole (about 8 mm in diameter) was drilled, the drainage tube was slowly placed into the preset target point of the hematoma cavity through the positioning guide, and the drainage tube was then clamped and connected to the drainage bag after part of the hematoma had been removed. (II) The late treatment group: CT-guided stereotactic hematoma aspiration was performed 24 h after the onset of the disease. The surgical procedure was the same as that of the early treatment group.
Outcome measures
The following data were collected and studied, including: (I) general data: age, gender, cerebral hemorrhage volume, hemorrhage ruptured into ventricle, Glasgow Coma Scale (GCS) score (9), hypertension grade, and comorbidities (e.g., hyperlipidemia, and diabetes); (II) Operation duration; (III) National Institute of Health Stroke Scale (NIHSS) scores before operation, 3 months after operation, and 6 months after operation (10). (IV) Glasgow Outcome Scale (GOS) score (11): a score of 5 represented a good recovery (i.e., a return to normal life, despite slight defects); a score of 4 represented a moderate disability (i.e., disabled but able to live independently and work under protection); a score of 3 represented a severe disability (i.e., disabled, requiring care in daily life); a score of 2 represented a persistent vegetative state and only a minimal survival response (e.g., eyes open with the sleep/wake cycle); and a score of 1 represented death. (V) Complications: pulmonary infection, intracranial infection, rebleeding, and deep venous thrombosis of lower limbs.
Statistical analysis
The statistical analysis was performed using SPSS 26.0. A 2-tailed P value <0.05 was considered statistically significant. The measurement data with normal distribution are expressed as the mean ± standard deviation. An independent samples t-test was applied to compare the measurement data between the 2 groups. The enumeration data are expressed as the number (percentage) [n (%)]. A Pearson χ2 test was used to compare the measurement data between the 2 groups.
Results
Preoperative clinical features of the 2 groups
There were no significant differences in terms of age, gender, amount of cerebral hemorrhage, hemorrhage ruptured into ventricle rate, GCS score, hypertension grade, hyperlipidemia, and diabetes between the 2 groups (P>0.05; see Table 1).
Table 1
| Category | Early treatment group (n=50) | Late treatment group (n=60) | T/χ2 value | P value |
|---|---|---|---|---|
| Age (year) (mean ± standard deviation) | 56.44±15.13 | 55.50 ± 14.14 | 0.336 | 0.737 |
| Gender n (%) | 0.710 | 0.400 | ||
| Male | 26 (52.00) | 36 (60.00) | ||
| Female | 24 (48.00) | 24 (40.00) | ||
| Amount of cerebral hemorrhage (mL) (mean ± standard deviation) | 40.16±6.45 | 40.25±6.32 | 0.074 | 0.941 |
| Hemorrhage ruptured into ventricle n (%) | 0.148 | 0.700 | ||
| Yes | 7 (14.00) | 10 (16.67) | ||
| No | 43 (86.00) | 50 (83.33) | ||
| GCS score (mean ± standard deviation) | 10.30±1.67 | 10.68±1.61 | 1.223 | 0.224 |
| Hypertension grade n (%) | 0.044 | 0.835 | ||
| Grade I | 2 (4.00) | 3 (5.00) | ||
| Grade II | 48 (96.00) | 57 (95.00) | ||
| Hyperlipidemia n (%) | 0.002 | 0.964 | ||
| Yes | 9 (18.00) | 11 (18.33) | ||
| No | 41 (82.00) | 49 (81.67) | ||
| Diabetes n (%) | 0.208 | 0.648 | ||
| Yes | 6 (12.00) | 9 (15.00) | ||
| No | 44 (88.00) | 51 (85.00) |
GCS, Glasgow Coma Scale.
Comparison of operation duration between the 2 groups
No difference was found in terms of operation duration between the 2 groups (see Table 2).
Table 2
| Category | Early treatment group (n=50) | Late treatment group (n=60) | T value | P value |
|---|---|---|---|---|
| Operation duration (min) (mean ± standard deviation) | 94.56±14.75 | 94.03±14.50 | 0.188 | 0.851 |
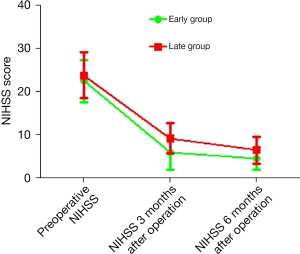
Comparison of the NIHSS scores at different time points between the 2 groups
There was no difference in the preoperative NIHSS scores of the patients in the 2 groups (22.50±4.90 vs. 23.83±5.35, P=0.179). Compared to patients in the late treatment group, the NIHSS scores of patients in the early treatment group were significantly lower 3 and 6 months after the operation (5.90±4.02 vs. 9.23±3.47, P<0.001; 4.54±2.56 vs. 6.50±3.07, P<0.001, respectively; see Table 3 and Figure 1).
Table 3
| Category | Early treatment group (n=50) | Late treatment group (n=60) | T value | P value |
|---|---|---|---|---|
| Preoperative NIHSS (mean ± standard deviation) | 22.50±4.90 | 23.83±5.35 | 1.353 | 0.179 |
| 3 months after operation NIHSS (mean ± standard deviation) | 5.90±4.02 | 9.23±3.47 | 4.669 | <0.001 |
| 6 months after operation NIHSS (mean ± standard deviation) | 4.54±2.56 | 6.50±3.07 | 3.591 | <0.001 |
NIHSS, National Institute of Health Stroke Scale.
Comparison of the GOS scores between the 2 groups
The GOS scores of patients in the early treatment group were significantly better than those of patients in the late treatment group (P=0.035; see Table 4).
Table 4
| Category | Early treatment group (n=50) | Late treatment group (n=60) | χ2 value | P value |
|---|---|---|---|---|
| GOS score n (%) | 8.617 | 0.035 | ||
| 5 | 26 (52.00) | 17 (28.33) | ||
| 4 | 15 (30.00) | 21 (35.00) | ||
| 3 | 9 (18.00) | 19 (31.67) | ||
| 2 | 0 (0.00) | 3 (5.00) | ||
| 1 | 0 (0.00) | 0 (0.00) |
GOS, Glasgow Outcome Scale.
Comparison of postoperative complications between the 2 groups
No significant difference was found in the incidence of postoperative pulmonary infection, intracranial infection, rebleeding, and lower extremity deep venous thrombosis between the 2 groups (P>0.05; see Table 5).
Table 5
| Category | Early treatment group (n=50) | Late treatment group (n=60) | χ2 value | P value |
|---|---|---|---|---|
| Pulmonary infection, n (%) | 3 (6.00) | 4 (6.67) | 0.020 | 0.887 |
| Intracranial infection, n (%) | 1 (2.00) | 2 (3.33) | 0.026 | 0.873 |
| Intracranial infection rebleeding, n (%) | 1 (2.00) | 2 (3.33) | 0.026 | 0.873 |
| Lower extremity deep venous thrombosis, n (%) | 4 (8.00) | 4 (6.67) | 0.010 | 0.920 |
Discussion
In 1978, Backlund et al. first reported using CT-guided stereotactic aspiration and drainage to treat cerebral hematoma. Comparisons have shown that CT-guided stereotactic aspiration is significantly superior to craniotomy and conservative treatment in reducing complications, decreasing mortality, and improving patients’ quality of life (9). However, the intracranial anatomical structure is very important, and a puncture side injury can have serious consequences.
As stereotactic intracranial hematoma aspiration requires a very high level of puncture accuracy, CT was used to guide the stereotactic intracranial hematoma aspiration. This study explored the effect of the timing of CT-guided stereotactic hematoma aspiration on the neurological recovery of patients with hypertensive cerebral hemorrhage in the basal ganglia. The results showed that early CT-guided stereotactic hematoma aspiration significantly improved the neurological function recovery of patients with hypertensive cerebral hemorrhage in the basal ganglia.
In recent years, the incidence rate of hypertension has increased year by year, and the incidence rate of hypertensive cerebral hemorrhage has also been on the rise (10-12). A cerebral hemorrhage in the basal ganglia is the most common type of hypertensive cerebral hemorrhage (13). Imaging and other indicators have important diagnostic and therapeutic significance in a variety of diseases (14-16).
Stereotactic hematoma puncture and drainage is a minimally invasive method in the treatment of cerebral hemorrhage. Using the stereotactic hematoma aspiration method, the puncture needle or suction tube is placed in the center of the hematoma under the guidance of CT, magnetic resonance imaging, etc. Beyond simple aspiration, the blood clot can also be broken by an ultrasonic surgical aspirator and then aspirated, or thrombolytic drugs can be injected into the hematoma cavity to facilitate postoperative drainage. The biggest difference between this operation and other surgical methods is that it is less traumatic, the bone window is less than 10 mm, and there is no need to perform skull repair after extubation.
At present, hematoma aspiration is used for the diagnosis and treatment of intracerebral hemorrhage (3), but the timing of the operation has a great effect on the recovery of patients after surgery. Our findings suggest that the NIHSS score and GOS score of patients in the early treatment group were significantly improved.
The basal ganglia, also known as the basal nucleus region, is a gray matter nucleus group buried in the deep part of the bilateral cerebral hemisphere and is also the main structure of the extrapyramidal system. It is mainly divided into the caudate nucleus, lenticular nucleus, platen nucleus, and amygdala. The lenticular nucleus is be divided into putamen and globus pallidus. These nuclei play an important role in the brain and are the areas in which nerve cell bodies are concentrated. When cerebral hemorrhage in the basal ganglia occurs, hemiplegia can occur (17-19). The continuous compression of a hematoma on a nerve can easily lead to irreversible nerve injury, and an edema will form after cerebral hemorrhage, which can further aggravate the damage to nerve function (20-22).
The timely aspiration of a hematoma helps to relieve the compression of the nerve and improve the prognosis of patients. This study showed that the rate of intracranial rebleeding in the early treatment group did not increase significantly, which indicated that early stereotactic hematoma aspiration under CT guidance has a positive effect on hypertensive cerebral hemorrhage in the basal ganglia and is worthy of promotion.
This study was a retrospective clinical study with a relatively small number of cases. A large number of multicenter clinical studies need to be conducted to further confirm the effect of the timing of stereotactic hematoma aspiration on the recovery of neurological function in patients with hypertensive cerebral hemorrhage in the basal ganglia.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-995/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-995/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-995/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Union Hospital Tongji Medical College Huazhong University of Science and Technology (No. 20220027). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zia E, Hedblad B, Pessah-Rasmussen H, et al. Blood pressure in relation to the incidence of cerebral infarction and intracerebral hemorrhage. Hypertensive hemorrhage: debated nomenclature is still relevant. Stroke 2007;38:2681-5. [Crossref] [PubMed]
- Wei LJ, Lin C, Xue XS, et al. The effect of hematoma puncture drainage before decompressive craniectomy on the prognosis of hypertensive intracerebral hemorrhage with cerebral hernia at a high altitude. Chin J Traumatol 2021;24:328-32. [Crossref] [PubMed]
- Kellner CP, Chartrain AG, Nistal DA, et al. The Stereotactic Intracerebral Hemorrhage Underwater Blood Aspiration (SCUBA) technique for minimally invasive endoscopic intracerebral hemorrhage evacuation. J Neurointerv Surg 2018;10:771-6. [Crossref] [PubMed]
- Kim MH, Song JH, Kim SH, et al. A new trend in operative technique for intracerebral hemorrhage: a comparative study of stereotactic endoscopic removal and stereotactic catheter drainage. Neurosurg Focus 1996;1:e2-discussion e2. [Crossref] [PubMed]
- Niizuma H, Suzuki J. Stereotactic aspiration of putaminal hemorrhage using a double track aspiration technique. Neurosurgery 1988;22:432-6. [Crossref] [PubMed]
- Miller CM, Vespa PM, McArthur DL, et al. Frameless stereotactic aspiration and thrombolysis of deep intracerebral hemorrhage is associated with reduced levels of extracellular cerebral glutamate and unchanged lactate pyruvate ratios. Neurocrit Care 2007;6:22-9. [Crossref] [PubMed]
- Deng C, Ji Y, Song W, et al. Clinical effect of minimally invasive aspiration and drainage of intracranial hematoma in the treatment of cerebral hemorrhage. Pak J Med Sci 2022;38:95-9. [PubMed]
- Zhou H, Zhang Y, Liu L, et al. A prospective controlled study: minimally invasive stereotactic puncture therapy versus conventional craniotomy in the treatment of acute intracerebral hemorrhage. BMC Neurol 2011;11:76. [Crossref] [PubMed]
- Nakajima M, Okada Y, Sonoo T, et al. Development and validation of a novel method for converting the Japan Coma Scale to Glasgow Coma Scale. J Epidemiol 2022; [Crossref] [PubMed]
- Alexandre AM, Valente I, Pedicelli A, et al. Mechanical thrombectomy in acute ischemic stroke due to large vessel occlusion in the anterior circulation and low baseline National Institute of Health Stroke Scale score: a multicenter retrospective matched analysis. Neurol Sci 2022;43:3105-12. [Crossref] [PubMed]
- Khari S, Zandi M, Yousefifard M. Glasgow Coma Scale Versus Physiologic Scoring Systems in Predicting the Outcome of ICU admitted Trauma Patients; a Diagnostic Accuracy Study. Arch Acad Emerg Med 2022;10:e25. [PubMed]
- Hong S, Daniels B, van Leeuwen MT, et al. Incidence and risk factors of hypertension therapy in Australian cancer patients treated with vascular signalling pathway inhibitors. Discov Oncol 2022;13:6. [Crossref] [PubMed]
- Wityk RJ, Caplan LR. Hypertensive intracerebral hemorrhage. Epidemiology and clinical pathology. Neurosurg Clin N Am 1992;3:521-32. [Crossref] [PubMed]
- Chen Y, Wang J, Zhang X, et al. Correlation between apparent diffusion coefficient and pathological characteristics of patients with invasive breast cancer. Ann Transl Med 2021;9:143. [Crossref] [PubMed]
- Qi A, Li Y, Yan S, et al. Effect of postoperative chemotherapy on blood glucose and lipid metabolism in patients with invasive breast cancer. Gland Surg 2021;10:1470-7. [Crossref] [PubMed]
- Teng D, Xia S, Hu S, et al. miR-887-3p Inhibits the Progression of Colorectal Cancer via Downregulating DNMT1 Expression and Regulating P53 Expression. Comput Intell Neurosci 2022;2022:7179733. [Crossref] [PubMed]
- Wu Y, Zhang S, Dong Y, et al. Therapeutic Effect of Electronic Endoscopic Hematoma Removal on Hypertensive Basal Ganglia Cerebral Hemorrhage Based on Smart Medical Technology. J Healthc Eng 2021;2021:7486249. [Crossref] [PubMed]
- Sun W, Hu Q, Wang J, et al. Prognostic value of early glycosylated hemoglobin and blood glucose levels in patients with basal ganglia cerebral hemorrhage. J Int Med Res 2019; Epub ahead of print. [Crossref] [PubMed]
- Wang XM, Zhang YG, Li AL, et al. Expressions of serum inflammatory cytokines and their relationship with cerebral edema in patients with acute basal ganglia hemorrhage. Eur Rev Med Pharmacol Sci 2016;20:2868-71. [PubMed]
- Macdonald RL. Commentary: Low-Dose Intravenous Heparin Infusion After Aneurysmal Subarachnoid Hemorrhage Is Associated With Decreased Risk of Delayed Neurological Deficit and Cerebral Infarction. Neurosurgery 2021;88:E234-5. [Crossref] [PubMed]
- Kole MJ, Wessell AP, Ugiliweneza B, et al. Low-Dose Intravenous Heparin Infusion After Aneurysmal Subarachnoid Hemorrhage is Associated With Decreased Risk of Delayed Neurological Deficit and Cerebral Infarction. Neurosurgery 2021;88:523-30. [Crossref] [PubMed]
- Seule M, Muroi C, Sikorski C, et al. Monitoring of cerebral hemodynamics and oxygenation to detect delayed ischemic neurological deficit after aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl 2013;115:57-61. [Crossref] [PubMed]


