
Clinical impact of antineutrophil cytoplasmic antibody positivity on the occurrence of interstitial lung disease in patients with polymyositis/dermatomyositis
Introduction
Idiopathic inflammatory myopathy (IIM) is a cluster of chronic autoimmune diseases that are characterized by typical inflammation of the muscle fibres.
IIM includes five types of myopathies; namely, polymyositis (PM), dermatomyositis (DM), overlap myositis, sporadic inclusion body myositis, and necrotising autoimmune myopathy (1). Among these IIMs, both PM and DM mainly present symmetrical proximal muscle weakness (2). Compared to PM, DM has two distinct features: typical cutaneous changes and two peak ages, as DM occurs in both young patients in their teens and adults in their 40s and 50s, whereas PM mainly occurs in older patients (3).
In 1975, Bohan and Peter proposed the classification criteria for PM/DM (the Bohan and Peter criteria). The typical cutaneous changes such as heliotrope rash with periorbital oedema and violaceous erythema and Gottron’s sign enabled the distinction of DM from PM. Conversely, in the absence of typical cutaneous changes, histological confirmation of muscle inflammation by muscle biopsy was considered essential for the classification as definite PM or DM (4,5). However, the Bohan and Peter criteria include no specific descriptions of histological findings, and they do not mention autoantibodies. Accordingly, there has been a demand for new classification criteria to overcome these limitations.
In 2017, the European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR) proposed the new classification criteria for adult and juvenile idiopathic inflammatory myopathies, which are based on the scoring system (the 2017 EULAR/ACR criteria) (6). Although the use of the 2017 EULAR/ACR criteria in classifying PM/DM are currently encouraged, the Bohan and Peter criteria remain widely used because of their convenience and significant concordance rate with the 2017 EULAR/ACR criteria (7).
Interstitial lung disease (ILD) is a relatively common systemic complication of PM/DM. ILD occurs in 19–40% of PM/DM patients, and non-specific interstitial pneumonia (NSIP) on high-resolution computed tomography (HRCT) is more common than usual interstitial pneumonia (UIP) (8,9). In addition, a meta-analysis reported a global prevalence rate of ILD in patients with PM/DM of 41.0% with higher rates in Asian patients compared to those in American and European patients (42.0%, 35.0%, and 26.0%, respectively) (10). Anti-Jo 1 is associated with ILD in PM/DM, and a previous study reported that 72.5% of anti-Jo 1-positive patients with antisynthetase syndrome exhibited ILD (11).
Anti-Jo 1 is a representative aminoacyl transfer RNA (tRNA) synthetase and is strongly associated with antisynthetase syndrome characterised by ILD, myositis, Raynaud’s’ phenomenon, and arthritis (12). A study reported that anti-Jo 1 antibodies were found in 15–25% of PM/DM patients (13). Anti-Jo 1 status reportedly predicted the clinical course of ILD in PM/DM patients; moreover, anti-Jo 1-positive ILD patients showed a different prognosis from that of anti-Jo 1-negative ILD patients (11,14,15). Therefore, we expected anti-Jo 1 status to be significantly associated with an increase in the occurrence of ILD.
Antineutrophil cytoplasmic antibody (ANCA) is a group of autoantibodies targeting neutrophil-specific proteins including myeloperoxidase (MPO) and proteinase 3 (PR3), that plays an important role in the classification ANCA-associated vasculitis (AAV) (16,17). Despite the lack of knowledge of the shared pathophysiological mechanism between PM/DM and AAV, there have been several reports regarding overlap syndrome between these conditions (18,19). In contrast, ILD is an established manifestation of AAV in addition to PM/DM (20,21). The reported prevalence rate of ILD in AAV ranges from 7% to 47%, and UIP confirmed on HRCT was more common than other ILD patterns (21-23). Until now, the ANCA positivity rate and the association between ANCA positivity and ILD occurrence in PM/DM patients without overlapping syndromes of AAV and PM/DM have not been reported. Hence, this study investigated the clinical impact of ANCA positivity on the occurrence of ILD in patients with probable and definite PM/DM who met both the Bohan and Peter and 2017 EULAR/ACR criteria. We present the following article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-604/rc).
Methods
Patients
The electronic medical records of 79 patients with PM/DM were retrospectively reviewed. The inclusion criteria were (I) patients who were initially classified as having PM/DM at the Division of Rheumatology, Department of Internal Medicine, Yonsei University College of Medicine, Severance Hospital, between January 2005 and June 2021; (II) patients who fulfilled the Bohan and Peter criteria for probable and definite PM/DM (4,5); (III) patients who met the 2017 EULAR/ACR criteria for probable and definite PM/DM or who were reclassified according to those criteria (6); (IV) patients whose medical records were sufficiently completed to allow the collection of clinical and laboratory data at the time of PM/DM diagnosis; (V) patients with available results of both ANCA tests such as an indirect immunofluorescence assay (IIFA) for perinuclear (P)-ANCA and cytoplasmic (C)-ANCA and an antigen-specific immunoassay for MPO-ANCA and PR3-ANCA (24).
The exclusion criteria were (I) patients with concomitant serious medical conditions such as malignancies, infectious diseases requiring hospitalisation, and other autoimmune diseases related to ANCA positivity, including AAV or primary sclerosing cholangitis (16,17,25); (II) patients who had not been followed up for 3 months or more; (III) patients who had ever received immunosuppressive drugs for the treatment of PM/DM or ILD before PM/DM diagnosis; (IV) patients to whom drugs that cause ANCA positivity such as propylthiouracil or hydralazine were administered (26,27).
Of the 79 patients, three patients were excluded because they had only IIFA results for ANCA. Of the remaining 76 patients, one patient was excluded because the PM was accompanied by concomitant primary sclerosing cholangitis. Finally, this study included and analysed 75 PM/DM patients.
Clinical and laboratory data
Regarding variables at the time of PM/DM diagnosis, age and sex were collected as demographic data, and ANCA types and their positivity were also confirmed. The results of ANCA tests were accepted when tests were performed within 7 days of PM/DM diagnosis. The numbers of patients satisfying components constituting the Bohan and Peter criteria and the 2017 EULAR/ACR criteria, and the laboratory results including creatine phosphokinase (CPK) (IU/L), lactate dehydrogenase (LDH) (IU/L), and aldolase (sigma U/mL) are shown in Table 1.
Table 1
| Variables | Values |
|---|---|
| At the time of diagnosis | |
| Demographic data | |
| Age (years) | 50.0 (20.0) |
| Male gender | 16 (21.3) |
| ANCA positive | |
| ANCA positivity | 12 (16.0) |
| MPO-ANCA (or P-ANCA) positive | 11 (14.7) |
| PR3-ANCA (or C-ANCA) positive | 1 (1.3) |
| Subtype | |
| PM | 29 (38.7) |
| DM | 46 (61.3) |
| Bohan and Peter criteria components | |
| Symmetrical proximal muscle weakness | 71 (94.7) |
| Muscle biopsy consistent with PM/DM [62]* | 51 (82.3) |
| Elevated muscle enzyme | 74 (98.7) |
| Abnormal EMG [73]** | 65 (89.0) |
| Dermatologic feature | 44 (58.7) |
| Definite PM according to the Bohan and Peter criteria (of 29 PM patients) | 22 (75.9) |
| Definite DM according to the Bohan and Peter criteria (of 46 DM patients) | 37 (80.4) |
| 2017 EULAR/ACR criteria components | |
| Age of onset of first symptom18–40 years | 20 (26.7) |
| Age of onset of first symptom ≥40 years | 55 (73.3) |
| Objective symmetric weakness, usually progressive, of the proximal upper extremities | 52 (69.3) |
| Objective symmetric weakness, usually progressive, of the proximal lower extremities | 67 (89.3) |
| Neck flexors are relatively weaker than neck extensors | 8 (10.7) |
| In the legs, proximal muscle are relatively weaker than distal muscles | 41 (54.7) |
| Heliotrope rash | 14 (18.7) |
| Gottron’s papules | 16 (21.3) |
| Gottron’s sign | 10 (13.3) |
| Dysphagia or esophageal dysmotility | 7 (9.3) |
| Anti-Jo 1 antibody positive | 26 (34.7) |
| Elevated serum levels of CPK or LDH or AST or ALT | 74 (98.7) |
| Endomysial infiltration of mononuclear cells surrounding, but not invading, myofibers [62]* | 16/62 (25.8) |
| Perimysial and/or perivascular infiltration of mononuclear cells [62]* | 16/62 (25.8) |
| Perifascicular atrophy [62]* | 11/62 (17.7) |
| Rimmed vacuoles [62]* | 2/62 (3.2) |
| Total score | |
| Definite PM according to the 2017 EULAR/ACR criteria (of 29 PM patients) | 9 (31.0) |
| Definite DM according to the 2017 EULAR/ACR criteria (of 46 DM patients) | 29 (63.0) |
| PM/DM-related laboratory results | |
| CPK (IU/L) | 543.0 (3,193.0) |
| LDH (IU/L) | 472.0 (455.0) |
| Aldolase (sigma U/mL) | 21.8 (38.1) |
| During follow-up | |
| ILD | 32 (42.7) |
| Follow-up duration based on ILD (months) | 19.0 (71.0) |
Values are expressed as a median (interquartile range) or n (%). *, muscle biopsy was performed in 62 patients; **, EMG was performed in 73 patients. PM/DM, polymyositis/dermatomyositis; ANCA, antineutrophil cytoplasmic antibody; MPO, myeloperoxidase; P, perinuclear; PR3, proteinase 3; C, cytoplasmic; EMG, electromyography; CPK, creatine phosphokinase; LDH, lactate dehydrogenase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; EULAR, the European League Against Rheumatism; ACR, American College of Rheumatology; ILD, interstitial lung disease.
Regarding variables during follow-up, all-cause mortality and ILD were investigated as poor outcomes. The follow-up duration based on all-cause mortality was defined as the period from the time of PM/DM diagnosis to death for the deceased patients and from diagnosis to the last visit for the surviving patients. The follow-up duration based on ILD was defined as the period from the time of PM/DM diagnosis to the occurrence of ILD in PM/DM patients with ILD and to the last visit for those without ILD.
ANCA measurement
Myeloperoxidase (MPO)-ANCA and proteinase 3 (PR3)-ANCA were measured using the novel anchor-coated highly sensitive (hs) Phadia ELiA (Thermo Fisher Scientific/Phadia, Freiburg, Germany) and human native antigens, on the Phadia250 analyser. We used immunoassays as the primary screening method for ANCA; however, when patients were found to be negative for ANCA by an antigen-specific assay but positive for perinuclear (P)-ANCA or cytoplasmic (C)-ANCA by an indirect immunofluorescence assay, they were considered to have MPO-ANCA or PR3-ANCA when AAV was strongly suspected based on the clinical and laboratory features (28).
Definitions of probable and definite PM and DM
In terms of the Bohan and Peter criteria, patients satisfying three out of the five components were classified as having probable PM/DM, and while those satisfying 4 or more components were classified as having definite PM/DM. In terms of the 2017 EULAR/ACR criteria, patients with a total aggregated score of ≥5.5 without biopsy or ≥6.7 with biopsy were defined as probable PM/DM. Patients with a total aggregated score of ≥7.5 without biopsy or ≥8.7 with biopsy were categorised as definite PM/DM (4-6).
Evaluation of ILD
Chest HRCT was performed to determine ILD occurrence when ILD was suspected on chest radiography. Since lung biopsy was not performed in all patients, the HRCT findings were roughly divided into UIP and non-UIP (8,29). HRCT features frequently seen in UIP include honeycombing, traction bronchiectasis, and traction bronchiolectasis, which may be seen with the concurrent presence of ground-glass opacification and fine reticulation (29). Among the results of pulmonary function tests (PFT), both the forced volume capacity (FVC) and diffusing capacity of the lung for carbon monoxide (DLCO), which reflect the severity of ILD, were collected (9).
Definition of AAV
We used the 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides and the 2007 algorithm for classifying AAV proposed by the European Medicine Agency to diagnose AAV (16,17).
Statistical analyses
All statistical analyses were performed using IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, NY, USA). Continuous variables are expressed as medians with interquartile ranges, whereas categorical variables are expressed as numbers (percentages). Significant differences between the two categorical variables were analysed using the Chi-square and Fisher’s exact tests. Significant differences between two continuous variables were compared using the Mann-Whitney U test. Comparison of the cumulative survivals rates between the two groups was analysed by the Kaplan-Meier survival analysis with the log-rank test. P values less than 0.05 were considered statistically significant.
Ethical statement
The present study was approved by the Institutional Review Board (IRB) of Severance Hospital (Seoul, Korea; No. 4-2021-1057) and conducted in accordance with the Declaration of Helsinki (as revised in 2013). Given the retrospective study design and the use of anonymized patient data, the requirement for written informed consent was waived.
Results
Characteristics
Among the variables collected at the time of PM/DM diagnosis, the median age was 50.0 years and 21.3% of patients were male. ANCA was detected in 12 patients (16.0%), 11 of whom had MPO-ANCA (or P-ANCA). Meanwhile, anti-Jo 1 was positive in 26 patients (34.7%). The numbers of patients satisfying each component of the two classification criteria are summarised in Table 1. In particular, in terms of the 2017 EULAR/ACR criteria, the median total score was 8.6. Of the 75 patients, 29 (38.7%) and 46 (61.3%) were classified as having PM and DM, respectively. Of the 29 PM patients, 22 (75.9%) were classified as having definite PM and seven (24.1%) as probable PM based on the Bohan and Peter criteria, while 9 patients (31.0%) were classified as having definite PM and 20 (69.0%) as probable PM based on the 2017 EULAR/ACR criteria. In contrast, of the 46 DM patients, 37 (80.4%) were classified as having definite DM and nine (19.6%) as probable DM based on the Bohan and Peter criteria, and 29 (63.0%) were categorised as having definite DM and 17 (37.0%) as probable DM based on the 2017 EULAR/ACR criteria. The median CPK, LDH and aldolase values were 543.0 IU/L, 472.0 IU/L and 21.8 sigma U/mL, respectively.
Among the variables collected during the median follow-up duration of 19.0 months, ILD occurred in 32 patients, 24 of whom had ILD at the time of PM/DM diagnosis (Table 1).
Comparison analyses according to ANCA positivity
All patients with PM/DM were divided into two groups according to ANCA positivity: patients with ANCA and those without ANCA. Among the variables collected at diagnosis, PM/DM patients with ANCA less frequently exhibited abnormal electromyography (EMG) findings compared to patients without ANCA (66.7% vs. 93.4%, P=0.021). In contrast, PM/DM patients with ANCA more frequently presented with Gottron’s sign compared to patients without ANCA (41.7% vs. 7.9%, P=0.007), despite the lack of significant difference in the proportions of DM patients. Anti-Jo 1 was detected more often in PM/DM patients without ANCA than those with (39.7% vs. 8.3%, P=0.048). The levels of CPK, LDH, and aldolase did not differ significantly between the groups. Among the variables collected during follow-up, ILD occurred more frequently in PM/DM patients with ANCA than those without ANCA (75.0% vs. 36.5%, P=0.023). Conversely, the follow-up duration based on ILD was also significantly shorter in PM/DM patients with ANCA (P=0.033) (Table 2).
Table 2
| Variables | PM/DM patients without ANCA (N=63) | PM/DM patients with ANCA (N=12) | P value |
|---|---|---|---|
| At the time of diagnosis | |||
| Demographic data | |||
| Age (years) | 48.5 (22.0) | 54 (9.0) | 0.140 |
| Male gender | 15 (23.8) | 1 (8.3) | 0.442 |
| Subtype | |||
| PM | 24 (38.1) | 5 (41.7) | 1.000 |
| DM | 39 (61.9) | 7 (58.3) | 1.000 |
| PM/DM-related manifestations based on Bohan and Peter criteria | |||
| Symmetrical proximal muscle weakness | 61 (96.8) | 10 (83.3) | 0.118 |
| Muscle biopsy consistent with PM/DM* | 42/51 (82.4) | 9/11 (81.8) | 1.000 |
| Elevated muscle enzyme | 63 (100.0) | 11 (91.7) | 0.160 |
| Abnormal EMG** | 57/61 (93.4) | 8/12 (66.7) | 0.021 |
| Dermatologic feature | 37 (58.7) | 7 (58.3) | 1.000 |
| Parameters based on 2017 EULAR classification criteria for idiopathic inflammatory myositis | |||
| Age of onset of first symptom 18–40 years | 19 (30.2) | 1 (8.3) | 0.164 |
| Age of onset of first symptom ≥40 years | 44 (69.8) | 11 (91.7) | 0.164 |
| Objective symmetric weakness, usually progressive, of the proximal upper extremities | 46 (73.0) | 6 (50.0) | 0.170 |
| Objective symmetric weakness, usually progressive, of the proximal lower extremities | 56 (88.9) | 11 (91.7) | 1.000 |
| Neck flexors are relatively weaker than neck extensors | 6 (9.5) | 2 (16.7) | 0.606 |
| In the legs, proximal muscle are relatively weaker than distal muscles | 32 (50.8) | 9 (75.0) | 0.205 |
| Heliotrope rash | 13 (20.6) | 1 (8.3) | 0.444 |
| Gottron’s papules | 13 (20.6) | 3 (25.0) | 0.711 |
| Gottron’s sign | 5 (7.9) | 5 (41.7) | 0.007 |
| Dysphagia or esophageal dysmotility | 7 (11.1) | 0 (0) | 0.589 |
| Anti-Jo 1 antibody positive | 25 (39.7) | 1 (8.3) | 0.048 |
| Elevated serum levels of CPK or LDH or AST or ALT | 63 (100.0) | 11 (91.7) | 0.160 |
| Endomysial infiltration of mononuclear cells surrounding, but not invading, myofibers* | 13/51 (25.5) | 3/11 (27.3) | 1.000 |
| Perimysial and/or perivascular infiltration of mononuclear cells* | 11/51 (21.6) | 5/11 (45.5) | 0.132 |
| Perifascicular atrophy* | 10/51 (19.6) | 1/11 (9.1) | 0.671 |
| Rimmed vacuoles* | 2/51 (3.9) | 0/11 (0) | 1.000 |
| Total score | 8.9 (3.1) | 7.9 (4.7) | 0.767 |
| PM/DM-related laboratory results | |||
| CPK (IU/L) | 638.5 (3,106.3) | 380.0 (3,226.0) | 0.826 |
| LDH (IU/L) | 495.5 (429.0) | 434.0 (587.0) | 0.301 |
| Aldolase (sigma U/mL) | 22.3 (35.9) | 12.3 (55.9) | 0.179 |
| During follow-up | |||
| ILD | 23 (36.5) | 9 (75.0) | 0.023 |
| Follow-up duration based on ILD (months) | 34.0 (85.0) | 2.5 (26.0) | 0.033 |
Values are expressed as a median (interquartile range) or N (%). *, muscle biopsy was performed in 62 patients; **, EMG was performed in 73 patients. PM/DM, polymyositis/dermatomyositis; ANCA, antineutrophil cytoplasmic antibody; EMG, electromyography; CPK, creatine phosphokinase; LDH, lactate dehydrogenase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; EULAR, the European League Against Rheumatism; ILD, interstitial lung disease.
Comparisons of ILD occurrence according to the presence of ANCA and anti-Jo 1
Contrary to the finding of the significantly higher occurrence of ILD in ANCA-positive patients, we observed no significant difference in the occurrence of ILD between 26 PM/DM patients with anti-Jo 1 and 49 patients without (50.0% vs. 38.8%, P=0.350) (Table 3).
Table 3
| Variables | ILD | P value |
|---|---|---|
| ANCA | 0.023 | |
| Without (N=63) | 23 (36.5) | |
| With (N=12) | 9 (75.0) | |
| Anti-Jo 1 | 0.350 | |
| Without (N=49) | 19 (38.8) | |
| With (N=26) | 13 (50.0) |
Values are expressed as N (%). ILD, interstitial lung disease; PM/DM, polymyositis/dermatomyositis; ANCA, antineutrophil cytoplasmic antibody.
Among 75 PM/DM patients, one patient (1.3%) had both ANCA and anti-Jo 1, 11 patients (14.7%) had only ANCA without anti-Jo 1, only anti-Jo 1 without ANCA was detected in 25 patients (33.3%), and neither ANCA nor anti-Jo 1 was detected in 38 patients (50.7%). We observed significant difference in the occurrence of ILD between 38 ANCA-negative, anti-Jo 1-negative PM/DM patients and 11 ANCA-positive, anti-Jo 1-negative patients (28.9% versus 72.7%, P=0.014) (Table 4). Other than that, no statistical significance was observed for ILD occurrence among the 4 groups.
Table 4
| Anti-Jo 1 (−) | Anti-Jo 1 (+) | P value | |
|---|---|---|---|
| ANCA (−) | 11/38 (28.9%) | 12/25 (48.0%) | 0.124 |
| ANCA (+) | 8/11 (72.7%) | 1/1 (100.0%) | >0.999 |
| P value | 0.014 | >0.999 |
ILD, interstitial lung disease; PM/DM, polymyositis/dermatomyositis; ANCA, antineutrophil cytoplasmic antibody.
Comparisons of FVC, DLCO, and UIP according to the presence of ANCA and anti-Jo 1
To assess the results of ILD-related variables according to the presence of ANCA and anti-Jo 1, 32 PM/DM patients were further analysed. PM/DM-ILD patients with ANCA showed a higher FVC than those without ANCA (55.5% vs. 76.0%, P=0.002), but we observed no significant differences in DLCO and UIP between the two groups. None of the three variables differed significantly between PM/DM-ILD patients with anti-Jo 1 and those without (Table 5).
Table 5
| Variables | ANCA | Anti-Jo 1 | |||||
|---|---|---|---|---|---|---|---|
| Without (N=23) | With ANCA (N=9) | P value | Without (N=19) | With ANCA (N=13) | P value | ||
| FVC, % | 55.5 (17.0) | 76.0 (26.0) | 0.002 | 64.5 (32.0) | 61.0 (14.0) | 0.226 | |
| DLCO, % | 56.5 (31.0) | 67.0 (21.0) | 0.173 | 60.0 (22.0) | 64.0 (32.0) | 0.824 | |
| UIP | 6 (26.1) | 5 (55.6) | 0.213 | 7 (36.8) | 4 (30.8) | 1.000 | |
Values are expressed as a median (interquartile range) or n (%). HRCT, high-resolution computed tomography; PM/DM, polymyositis/dermatomyositis; ILD, interstitial lung disease; ANCA, antineutrophil cytoplasmic antibody; FVC, forced volume capacity; DLCO, diffusing capacity of the lung for carbon monoxide; UIP, usual interstitial pneumonia.
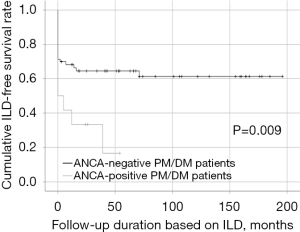
Comparisons of cumulative ILD-free survival rates
ANCA-positive PM/DM patients exhibited a significantly lower cumulative ILD-free survival rate than ANCA-negative PM/DM patients (P=0.009) (Figure 1). PM/DM patients with anti-Jo 1 tended to exhibit a lower cumulative ILD-free survival rate than those without, but the difference was not statistically significant (P=0.392) (Figure S1).
When classified into four groups according to the presence and absence of ANCA and anti-Jo 1, ANCA-positive and anti-Jo 1-positive patients had the highest cumulative occurrence rate of ILD, followed by ANCA-positive anti-Jo 1-negative patients (Figure S2). We divided the 75 patients into two groups, PM and DM, and investigated the cumulative ILD-free survival rates between them. In both groups, patients with ANCA tended to exhibit a lower cumulative ILD-free survival rate compared to that in patients without ANCA, and PM patients showed a result closer to statistical significance than DM patients (P=0.059 vs. P=0.082); however, the differences were not statistically significant (Figure S3).
Comparisons of cumulative survival rates
When we investigated the clinical effect of the presence or absence of ILD on all-cause mortality, PM/DM patients with ILD showed a tendency to exhibit a lower patients’ survival rate than those without ILD; however, it was not statistically significant (P=0.062) (Figure S4A). In addition, when only PM/DM-ILD patients were included in the analysis of the effects of UIP and non-UIP on all-cause mortality, the cumulative patients’ survival rates between PM/DM-ILD patients with UIP and those with non-UIP did not differ significantly (Figure S4B). Furthermore, examination of the differences in the prognosis of death between patients with ILD at the time of and after PM/DM diagnosis in 32 PM-DM-ILD patients showed no significant difference in the cumulative patients’ survival rates (Figure S4C).
Application of the AAV criteria to ANCA-positive patients
We applied two criteria, the 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides and the 2007 algorithm for classifying AAV proposed by the European Medicine Agency, to ANCA-positive PM/DM patients (16,17). However, none of them was classified as having AAV. As the inclusion criteria included only patients without overlap syndrome of AAV and PM/DM.
Discussion
We thought that anti-Jo 1 might be involved in the induction of ILD in PM/DM patients. However, the results in our analysis of 75 PM/DM patients showed that ANCA positivity was associated with an increased occurrence rate of ILD (Figure 1), while anti-Jo 1 positivity was not.
We propose several hypotheses to explain this discrepancy. First, the presence of ANCA may have diminished the strength of the association between anti-Jo 1 positivity and the occurrence of ILD in terms of pathological mechanism. In Table 4, when ANCA negative, anti Jo-1 positive patients tend to have more ILD than anti Jo-1 negative patients, although not statistically significant. Among the possible pathological mechanisms by which anti-Jo 1 could affect the occurrence of ILD, immune complexes including anti-Jo 1 and RNA have been reported as possible endogenous inducers that could promote IFN-alpha production in plasmacytoid dendritic cells; thus, increased IFN-alpha might play an important role in the occurrence and progression of ILD (30,31). In terms of the pathophysiology of ILD in AAV, three hypotheses have been proposed. First, ILD might occur because of repeated episodes of diffuse alveolar haemorrhage resulting from pulmonary capillaritis. Second, ILD might be a consequence of direct damage to vascular inflammation by MPO-ANCA. Finally, intrinsic factors such as existing lung diseases or environmental factors such as cigarette smoking might activate the endothelial cells of the lung capillaries and accelerate MPO expression, leading to an increase in circulating MPO-ANCA (32-35). Therefore, it may be impossible to explain the results of this study through the pathological mechanism because of the lack of known common features between anti-Jo 1 and ANCA in participating in the occurrence of ILD occurrence.
Second, ANCA production may play an antagonistic role with anti-Jo 1 production. In Table 4, when ANCA positive, Anti-Jo-1 positive rate is less than that when ANCA negative. These results suggest that it may be difficult for ANCA and anti-Jo 1 to exist simultaneously in PM/DM patients.
Third, the anti-Jo 1 positive rate may have been lower than that of the general cohort of PM/DM patients. The reported occurrence rate of ILD in anti-Jo 1-positive PM/DM patients was approximately 55% (36). In this study, the occurrence rate of ILD in PM/DM patients with anti-Jo 1 was 50.0%. Therefore, anti-Jo 1 played a role in the development of ILD properly as usual. A previous study reported the anti-Jo 1 positivity rates ranging from 15% to 30% of PM/DM patients (37). In this study, the anti-Jo 1 positivity rate was 34.6%. Therefore, the anti-Jo 1 positivity rate and the occurrence rate of ILD in anti-Jo 1-positive PM/DM patients were consistent with those of previous studies. As the evidence refutes the three hypotheses presented above, further studies are needed on the role of ANCA in the pathogenesis and the association between ANCA and anti-Jo 1 positivity in PM/DM patients.
We also evaluated which subtype of IIM was more susceptible to the effect of ANCA positivity on the occurrence of ILD. No IIM subtypes was more strongly associated with ANCA positivity and the occurrence of ILD (Figure S3).
ILD is a major risk factor for mortality in PM/DM patients. The three most common causes of mortality in PM/DM patients were pulmonary infection (35%), ILD (21%), and both infection and ILD (25%) (38). When we investigated the clinical effect of the presence or absence of ILD on all-cause mortality, PM/DM patients with ILD have a lower survival rate than those without ILD, but there is no statistical significance (Figure S4A).
In this study, the degree of restrictive pulmonary insufficiency based on FVC in PM/DM patients with ANCA was better than those without ANCA. However, we observed no significant differences in the degree of DLCO and the frequency of UIP between the two groups. We assumed two reasons for this discrepancy: first, only ILD occurrence and UIP patterns were investigated on HRCT, and the analysis of the three-dimensional invasion area of the actual ILD was not performed using the automated reconstruction of HRCT findings; and second, ANCA was tested within 7 days of PM/DM diagnosis, while PFT were performed when the occurrence of ILD was confirmed on HRCT. Therefore, the discrepancy might be due to a time gap between the time of ANCA tests and that of PFT and HRCT performance.
Another interesting finding was that PM/DM patients with ANCA more frequently showed Gottron’s sign at diagnosis compared to patients without ANCA. Since Gottron’s sign is a typical cutaneous change in DM patients, only DM patients were selected and the frequency of Gottron’s sign according to the presence or absence of ANCA was investigated again. Of the 46 DM patients, 5 of 7 DM patients with ANCA and 5 of 39 without ANCA exhibited Gottron’s sign (71.4% vs. 12.8%, P=0.003). Although Gottron’s sign has been previously reported in patients with overlap syndrome of AAV and IIM, patients with overlap SD were not included in this study, so the possibility that Gottron’s sign was confused with the cutaneous manifestation of AAV could be excluded (18).
This study is the first demonstrate the clinical impact of ANCA positivity on the occurrence of ILD in patients with PM/DM based on both the Bohan and Peter and the 2017 EULAR/ACR criteria. However, this study has several limitations. The number patients included in this study was not large enough to generalise the results owing to the limitations of the single-centre study and the exclusion of patients lacking ANCA test results at diagnosis. Moreover, as a limitation inherent to its retrospective study design, this study could not perform serial and regular ANCA testing, PFT, and HRCT; thus, it was impossible to determine the direct association between ANCA positivity and the occurrence of ILD. Anti-MDA5 antibody was known to be associated with the development of ILD in PM/DM patient, but this test was not performed in this study.
In conclusion, the results of our study demonstrated that ANCA positivity at the time of PM/DM diagnosis might be an important risk factor for ILD in PM/DM patients. Therefore, we suggest that physicians perform ANCA tests at the time of PM/DM diagnosis, and pay close attention to regular monitoring of the occurrence of ILD in patients with ANCA during follow-up.
Acknowledgments
Funding: This work was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare (No. HI14C1324); the Handok Inc., Seoul, Republic of Korea (No. HANDOK 2021-006); and CELLTRION PHARM, Inc. Chungcheongbuk-do, Republic of Korea (No. NCR 2019-6).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-604/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-604/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-604/coif). The authors report that this work was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare (No. HI14C1324); the Handok Inc., Seoul, Republic of Korea (No. HANDOK 2021-006); and Celltrion Pharm, Inc. Chungcheongbuk-do, Republic of Korea (No. NCR 2019-6). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The present study was approved by the Institutional Review Board (IRB) of Severance Hospital (Seoul, Korea; No. 4-2021-1057) and conducted in accordance with the Declaration of Helsinki (as revised in 2013). Given the retrospective study design and the use of anonymized patient data, the requirement for written informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ashton C, Paramalingam S, Stevenson B, et al. Idiopathic inflammatory myopathies: a review. Intern Med J 2021;51:845-52. [Crossref] [PubMed]
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet 2003;362:971-82. [Crossref] [PubMed]
- Findlay AR, Goyal NA, Mozaffar T. An overview of polymyositis and dermatomyositis. Muscle Nerve 2015;51:638-56. [Crossref] [PubMed]
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975;292:344-7. [Crossref] [PubMed]
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med 1975;292:403-7. [Crossref] [PubMed]
- Lundberg IE, Tjärnlund A, Bottai M, et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis 2017;76:1955-64. [Crossref] [PubMed]
- Yoo J, Ahn SS, Jung SM, et al. Reclassification of Korean patients with polymyositis and dermatomyositis based on the Bohan and Peter criteria by the 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies. Korean J Intern Med 2021;36:441-6. [Crossref] [PubMed]
- Marie I, Hachulla E, Chérin P, et al. Interstitial lung disease in polymyositis and dermatomyositis. Arthritis Rheum 2002;47:614-22. [Crossref] [PubMed]
- Panagopoulos P, Goules A, Hoffmann-Vold AM, et al. Natural history and screening of interstitial lung disease in systemic autoimmune rheumatic disorders. Ther Adv Musculoskelet Dis 2021;13:1759720X211037519.
- Sun KY, Fan Y, Wang YX, et al. Prevalence of interstitial lung disease in polymyositis and dermatomyositis: A meta-analysis from 2000 to 2020. Semin Arthritis Rheum 2021;51:175-91. [Crossref] [PubMed]
- Marie I, Josse S, Hatron PY, et al. Interstitial lung disease in anti-Jo-1 patients with antisynthetase syndrome. Arthritis Care Res (Hoboken) 2013;65:800-8. [Crossref] [PubMed]
- Witt LJ, Curran JJ, Strek ME. The Diagnosis and Treatment of Antisynthetase Syndrome. Clin Pulm Med 2016;23:218-26. [Crossref] [PubMed]
- Satoh M, Tanaka S, Ceribelli A, et al. A Comprehensive Overview on Myositis-Specific Antibodies: New and Old Biomarkers in Idiopathic Inflammatory Myopathy. Clin Rev Allergy Immunol 2017;52:1-19. [Crossref] [PubMed]
- Yoshifuji H, Fujii T, Kobayashi S, et al. Anti-aminoacyl-tRNA synthetase antibodies in clinical course prediction of interstitial lung disease complicated with idiopathic inflammatory myopathies. Autoimmunity 2006;39:233-41. [Crossref] [PubMed]
- Tillie-Leblond I, Wislez M, Valeyre D, et al. Interstitial lung disease and anti-Jo-1 antibodies: difference between acute and gradual onset. Thorax 2008;63:53-9. [Crossref] [PubMed]
- Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1-11. [Crossref] [PubMed]
- Watts R, Lane S, Hanslik T, et al. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann Rheum Dis 2007;66:222-7. [Crossref] [PubMed]
- Yuste C, Rapalai M, Pritchard BA, et al. Overlap between dermatomyositis and ANCA vasculitides. Clin Kidney J 2014;7:59-61. [Crossref] [PubMed]
- Panda PK, Suri TM, Sood R, et al. Overlap syndrome: juvenile dermatomyositis and perinuclear antineutrophil cytoplasmic autoantibody vasculitis, a case report and review of literature. Int J Rheum Dis 2017;20:2219-24. [Crossref] [PubMed]
- Mukhtyar C, Lee R, Brown D, et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis 2009;68:1827-32. [Crossref] [PubMed]
- Bhamra K, Luqmani R. Damage assessment in ANCA-associated vasculitis. Curr Rheumatol Rep 2012;14:494-500. [Crossref] [PubMed]
- Maillet T, Goletto T, Beltramo G, et al. Usual interstitial pneumonia in ANCA-associated vasculitis: A poor prognostic factor. J Autoimmun 2020;106:102338. [Crossref] [PubMed]
- Alba MA, Flores-Suárez LF, Henderson AG, et al. Interstital lung disease in ANCA vasculitis. Autoimmun Rev 2017;16:722-9. [Crossref] [PubMed]
- Bossuyt X, Cohen Tervaert JW, Arimura Y, et al. Position paper: Revised 2017 international consensus on testing of ANCAs in granulomatosis with polyangiitis and microscopic polyangiitis. Nat Rev Rheumatol 2017;13:683-92. [Crossref] [PubMed]
- Stinton LM, Bentow C, Mahler M, et al. PR3-ANCA: a promising biomarker in primary sclerosing cholangitis (PSC). PLoS One 2014;9:e112877. [Crossref] [PubMed]
- Yu F, Zhao MH, Zhang YK, et al. Anti-endothelial cell antibodies (AECA) in patients with propylthiouracil (PTU)-induced ANCA positive vasculitis are associated with disease activity. Clin Exp Immunol 2005;139:569-74. [Crossref] [PubMed]
- Yokogawa N, Vivino FB. Hydralazine-induced autoimmune disease: comparison to idiopathic lupus and ANCA-positive vasculitis. Mod Rheumatol 2009;19:338-47. [Crossref] [PubMed]
- McAdoo SP, Medjeral-Thomas N, Gopaluni S, et al. Long-term follow-up of a combined rituximab and cyclophosphamide regimen in renal anti-neutrophil cytoplasm antibody-associated vasculitis. Nephrol Dial Transplant 2019;34:63-73. [Crossref] [PubMed]
- Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:e44-68. [Crossref] [PubMed]
- Eloranta ML, Barbasso Helmers S, Ulfgren AK, et al. A possible mechanism for endogenous activation of the type I interferon system in myositis patients with anti-Jo-1 or anti-Ro 52/anti-Ro 60 autoantibodies. Arthritis Rheum 2007;56:3112-24. [Crossref] [PubMed]
- Lundberg IE, Helmers SB. The type I interferon system in idiopathic inflammatory myopathies. Autoimmunity 2010;43:239-43. [Crossref] [PubMed]
- Hervier B, Pagnoux C, Agard C, et al. Pulmonary fibrosis associated with ANCA-positive vasculitides. Retrospective study of 12 cases and review of the literature. Ann Rheum Dis 2009;68:404-7. [Crossref] [PubMed]
- Guilpain P, Chéreau C, Goulvestre C, et al. The oxidation induced by antimyeloperoxidase antibodies triggers fibrosis in microscopic polyangiitis. Eur Respir J 2011;37:1503-13. [Crossref] [PubMed]
- Tzelepis GE, Kokosi M, Tzioufas A, et al. Prevalence and outcome of pulmonary fibrosis in microscopic polyangiitis. Eur Respir J 2010;36:116-21. [Crossref] [PubMed]
- Churg A, Zay K, Shay S, et al. Acute cigarette smoke-induced connective tissue breakdown requires both neutrophils and macrophage metalloelastase in mice. Am J Respir Cell Mol Biol 2002;27:368-74. [Crossref] [PubMed]
- Shappley C, Paik JJ, Saketkoo LA. Myositis-Related Interstitial Lung Diseases: Diagnostic Features, Treatment, and Complications. Curr Treatm Opt Rheumatol 2019;5:56-83. [Crossref] [PubMed]
- Mileti LM, Strek ME, Niewold TB, et al. Clinical characteristics of patients with anti-Jo-1 antibodies: a single center experience. J Clin Rheumatol 2009;15:254-5. [Crossref] [PubMed]
- Wu C, Wang Q, He L, et al. Hospitalization mortality and associated risk factors in patients with polymyositis and dermatomyositis: A retrospective case-control study. PLoS One 2018;13:e0192491. [Crossref] [PubMed]


