
Performance of mid-upper arm circumference and other prognostic indices based on inflammation and nutrition in oncology outpatients: a tertiary cancer center study
Introduction
Cancer cachexia is defined as ‘a multifactorial syndrome characterized by an ongoing loss of skeletal muscle mass (with or without loss of fat mass) that cannot be reversed by conventional nutritional support and leads to progressive functional decline’ in patients with advanced cancer (1). Although cachexia is often defined by involuntary weight loss, not all cachexic patients experience weight loss, and skeletal muscle depletion is a strong weight-independent prognostic factor (2,3). There is growing evidence that a systemic inflammatory response is a major factor of muscle wasting and functional decline in patients with cachexia (4-8). One of the sensitive measures of the systemic inflammatory response is C-reactive protein (CRP), and various studies have demonstrated the prognostic value of CRP in patients with cancer (9-11). The Glasgow Prognostic Score (GPS) and modified GPS (mGPS) are the most widely studied inflammation-based prognostic indices incorporating CRP (12-15). These indices simply combine CRP level and albumin level to predict prognosis. In GPS, which was originally derived from inoperable non-small-cell lung cancer (NSCLC) patients with a median survival of approximately 12 months, patients with both an elevated CRP (>1.0 mg/dL) and hypoalbuminemia (<3.5 g/dL) are allocated a score of 2, whereas patients with a low CRP (≤1.0 mg/dL) and normal albumin (≥3.5 g/dL) are assigned a score of 0 (12,13). Patients with only one of these abnormalities are scored 1. GPS was modified to mGPS after a further study, which showed that hypoalbuminemia without an elevated CRP was rare and that hypoalbuminemia itself was not associated with poor survival (14). However, this study investigated operable colorectal cancer patients with years of survival. Although both GPS and mGPS are considered strong prognostic factors along with performance status in advanced cancer patients with months of survival (13,15-17), only one study directly compared the performance of GPS to that of mGPS (18). In that study, hypoalbuminemia was associated with poorer survival in patients with operable or inoperable NSCLC, and GPS was superior to mGPS in predicting survival, especially in patients with inoperable NSCLC.
Other inflammation and nutrition-based prognostic indices that incorporate hematological components have also been widely investigated. These indices include the Prognostic Nutritional Index (PNI), neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), and Prognostic Index (PI) (19-24). In the Glasgow Inflammation Outcome Study, the investigators compared the performance of mGPS, NLR, PLR, PI, and PNI in more than twenty-seven thousand patients with cancer. The mGPS demonstrated the greatest area under the receiver operating characteristic curve (AUROC) (25). However, this study included all types of cancer regardless of stage, thus, it is unclear which indices are superior in patients with advanced cancer.
Recently, the CRP/albumin ratio (CAR) has been reported to be related to poor outcomes in various types of cancer (26-29). The prognostic value of the CAR was also explored in advanced cancer patients receiving palliative care (30,31). Recently, our group reported that a simple anthropometric measure, ‘mid-upper arm circumference (MUAC)’, is an independent prognostic factor that can estimate 3-month survival in medical oncology outpatients (32).
The objective of this study was to compare the performance of known inflammation and nutrition-based prognostic indices with MUAC, which is also a well-known, but a relatively novel prognostic factor for advanced cancer (32,33), in oncology outpatients having an expected survival of less than a year. In particular, we focused on comparing the GPS, mGPS and MUAC. We have hypothesized that MUAC will show comparable performance with GPS/mGPS. GPS/mGPS were chosen because they have distinct cutoff points and are the most well-established inflammation and nutrition-based prognostic indices. Both GPS and mGPS were included because it is still not well-established which index shows better performance in patients with advanced cancer. We present the following article in accordance with the STARD reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-481/rc).
Methods
Patients
This study was part of a prospective cohort study that enrolled 200 patients with advanced cancer at a tertiary cancer center from March 2016 to January 2019 (32). In brief, patients were deemed eligible if their oncologists estimated their survival to be less than a year. All patients were 18 years of age or older and were diagnosed with advanced cancer. We defined advanced cancer as metastatic or recurrent disease or progressive locally advanced disease for which curative treatment was not possible. Major exclusion criteria were patients with hematologic malignancies, patients with an expected survival of less than 1 month, and patients unable to communicate. The study was conducted in accordance with the Declaration of Helsinki as revised in 2013. Written informed consent was obtained from each patient prior to enrollment. The protocol was approved by the Institutional Review Board (IRB) of Seoul National University Bundang Hospital (No. B-1601/332-302).
Prognostic indices
Demographic data and clinical information were obtained from the electronic medical records. All patients went through routine blood tests after enrollment. Patients were assessed by the Eastern Cooperative Oncology Group (ECOG) performance status and a trained registered nurse measured the MUAC of each patient at the time of enrollment. The circumference of the upper arm was measured at the midpoint between the acromial process of the scapula and the olecranon process of the ulna.
Inflammation and nutrition-based prognostic indices including GPS, mGPS, PNI, NLR, and CAR were calculated for each patient. Based on previous studies, GPS was calculated as follows: CRP ≤1.0 mg/dL and albumin ≥3.5 g/dL =0; CRP >1.0 mg/dL or albumin <3.5 g/dL =1; CRP >1.0 mg/dL and albumin <3.5 g/dL =2 (11). mGPS was calculated as follows: CRP ≤1.0 mg/dL =0; CRP >1.0 mg/dL and albumin ≥3.5 g/dL =1; CRP >1.0 mg/dL and albumin <3.5 g/dL =2 (12). PNI was calculated as 10 × albumin (g/dL) + 0.005 × lymphocyte (/mm3) (17). NLR was defined as the division of neutrophil count by lymphocyte count and CAR was defined as the division of CRP by albumin level (20-22,26-31).
Statistical analysis
Patient characteristics, laboratory results, and prognostic indices were summarized using descriptive statistics. The sample size of 200 was determined based on Harrell’s study (34). Survival time was calculated using the Kaplan-Meier method. Overall survival was defined as the time from enrollment to the time of death. Alive patients were dealt as with censored cases at the last follow-up. Univariate analyses were performed to identify significant prognostic factors using the log-rank test. The discriminatory ability of the prognostic indices for estimating 12- and 24-week survival was measured using the AUROC. We calculated the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), AUROC, and hazard ratio (HR) to compare the performance of GPS, mGPS, and MUAC. The cutoff points of MUAC were determined according to the Youden’s index (35). All tests were two-sided and a P value <0.05 was considered significant. Confidence intervals (CIs) were calculated at the 95% confidence level. JMP version 14 for Windows (SAS Institute Inc., Cary, NC, USA) was used for all statistical analysis.
Results
In total, 200 ambulatory patients with advanced cancer were included in this study, all outpatients of medical oncology clinics. Baseline characteristics and laboratory results are summarized in Table 1. With a median follow-up period of 33 weeks, 159 (79.5%) patients died, and the median overall survival time was 32.4 weeks (95% CI: 5.6–142.7).
Table 1
| Characteristics | Values |
|---|---|
| Age, years (mean ± SD) | 64.4±11.6 |
| Sex, n (%) | |
| Male | 128 (64.0) |
| Female | 72 (36.0) |
| Primary cancer site, n (%) | |
| Lung | 67 (33.5) |
| Kidney/bladder/prostate/testis | 29 (14.5) |
| Colon/rectum | 28 (14.0) |
| Stomach | 20 (10.0) |
| Breast | 18 (9.0) |
| Soft tissue | 6 (3.0) |
| Esophagus | 5 (2.5) |
| Ovary/cervix | 4 (2.0) |
| Liver/biliary-tract | 4 (2.0) |
| Pancreas | 4 (2.0) |
| Head/neck | 4 (2.0) |
| Others | 11 (5.5) |
| Undergoing chemotherapy (yes), n (%) | 131 (65.5) |
| ECOG performance status, n (%) | |
| 0 | 7 (3.5) |
| 1 | 125 (62.5) |
| 2 | 55 (27.5) |
| 3 | 13 (6.5) |
| 4 | 0 (0.0) |
| Laboratory result, median [Q1–Q3] | |
| WBC (/μL) | 6,045 [4,300–8,250] |
| Hemoglobin (g/dL) | 11.0 [9.8–12.2] |
| Platelet (×103/μL) | 240.5 [176.3–315.3] |
| Neutrophil (%) | 65.8 [56.9–74.0] |
| Lymphocyte (%) | 20.65 [14.8–29.0] |
| Total bilirubin (mg/dL) | 0.5 [0.4–0.7] |
| Albumin (mg/dL) | 3.8 [3.4–4.1] |
| AST (IU/L) | 25 [20.0–36.8] |
| ALT (IU/L) | 19 [13–27] |
| LDH (IU/L) | 222 [179–286] |
| Uric acid (mg/dL) | 4.9 [4.2–6.0] |
| CRP (mg/dL) | 0.89 [0.3–2.7] |
| BUN (mg/dL) | 15 [11–19] |
| Creatinine (mg/dL) | 0.8 [0.6–1.1] |
| MUAC, cm (mean ± SD) | 26.5±3.8 |
| Mortality at last follow-up, n (%) | 159 (79.5) |
| Median survival time, weeks (95% CI) | 32.4 (5.6–142.7) |
SD, standard deviation; ECOG, Eastern Cooperative Oncology Group; WBC, white blood cell; AST, aspartate transaminase; ALT, alanine transaminase; LDH, lactate dehydrogenase; CRP, C-reactive protein; BUN, blood urea nitrogen; MUAC, mid-upper arm circumference; CI, confidence interval.
Table 2 shows the calculated or measured scores of prognostic indices. The number of patients with GPS scores of 0, 1, and 2 were 103 (51.8%), 54 (27.1%), and 42 (21.1%), respectively. For the mGPS, the numbers of patients with scores of 0, 1, and 2 were 115 (57.8%), 42 (21.1%), and 42 (21.1%), respectively. The median values of PNI, NLR, and CAR were 43.81, 3.23, and 0.23, respectively. The median value of MUAC was 26.5 (range, 14–39) cm. Figure 1 shows the distribution of MUAC.
Table 2
| Prognostic indices | Values |
|---|---|
| GPS† | 0 [0–1] |
| CRP ≤1.0 mg/dL and Alb ≥3.5 g/dL | 103 (51.8%) |
| CRP >1.0 mg/dL or Alb <3.5 g/dL | 54 (27.1%) |
| CRP >1.0 mg/dL and Alb <3.5 g/dL | 42 (21.1%) |
| mGPS† | 1 [0–1] |
| CRP ≤1.0 mg/dL and Alb ≥3.5 g/dL | 103 (51.8%) |
| CRP ≤1.0 mg/dL and Alb <3.5 g/dL | 12 (6.0%) |
| CRP >1.0 mg/dL and Alb ≥3.5 g/dL | 42 (21.1%) |
| CRP >1.0 mg/dL and Alb <3.5 g/dL | 42 (21.1%) |
| PNI‡ | 43.81 [39.04–47.85] |
| NLR | 3.23 [1.96–5.08] |
| CAR | 0.23 [0.08–0.74] |
| MUAC, cm | 26.50 [24.00–28.75] |
Data are presented as median [Q1–Q3] or n (%). †, missing value (n=1); ‡, PNI = 10 × albumin (g/dL) + 0.005 × lymphocyte (/mm3). GPS, Glasgow Prognostic Score; mGPS, modified Glasgow Prognostic Score; PNI, Prognostic Nutritional Index; NLR, neutrophil/lymphocyte ratio; CAR, C-reactive protein/albumin ratio; MUAC, mid-upper arm circumference; CRP, C-reactive protein; Alb, serum albumin.
The AUROC for estimating 12- and 24-week survival was similarly high for all indices analyzed (Table 3). The AUROCs for estimating 12-week survival and 24-week survival with GPS, mGPS, PNI, NLR, CAR, and MUAC, were between 0.68 and 0.77, and 0.67 and 0.74, respectively.
Table 3
| Prognostic indices | AUROC for 12-week survival (95% CI) | AUROC for 24-week survival (95% CI) |
|---|---|---|
| GPS | 0.75 (0.66–0.82) | 0.70 (0.62–0.76) |
| mGPS | 0.74 (0.65–0.82) | 0.67 (0.60–0.74) |
| PNI | 0.74 (0.62–0.82) | 0.71 (0.63–0.78) |
| NLR | 0.68 (0.57–0.77) | 0.67 (0.58–0.74) |
| CAR | 0.77 (0.68–0.84) | 0.74 (0.67–0.80) |
| MUAC | 0.71 (0.60–0.80) | 0.72 (0.64–0.79) |
AUROCs, area under the receiver operating characteristic curves; CI, confidence interval; GPS, Glasgow Prognostic Score; mGPS, modified Glasgow Prognostic Score; PNI, Prognostic Nutritional Index; NLR, neutrophil/lymphocyte ratio; CAR, C-reactive protein/albumin ratio; MUAC, mid-upper arm circumference.
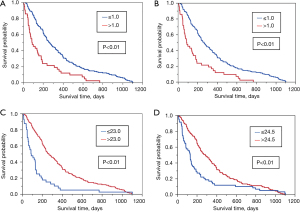
The sensitivity, specificity, PPV, NPV, AUROC, and HR were calculated to compare the performance of GPS, mGPS, and MUAC, for estimating 12- and 24-week survival (Table 4). Both the median and mean values of MUAC were 26.5 [standard deviation (SD) 3.8; range, 14–39] cm, and the cutoff points according to the Youden’s index were 23.0 cm for 12-week survival and 24.5 cm for 24-week survival. The AUROCs of GPS, mGPS, and MUAC for estimating 12-week survival were 0.75 (95% CI: 0.66–0.82), 0.74 (95% CI: 0.65–0.82), and 0.72 (95% CI: 0.64–0.79), respectively. For 24-week survival, the AUROCs were 0.70 (95% CI: 0.62–0.76), 0.67 (95% CI: 0.60–0.74), and 0.72 (95% CI: 0.64–0.79), respectively. The specificity was highest for estimating 12-week survival with MUAC (86.0%) and the sensitivity was highest for estimating 12-week survival with GPS (81.1%). There were no significant differences between the AUROCs of GPS, mGPS and MUAC. Kaplan-Meier survival curves for GPS, mGPS, and MUAC all showed statistically significant survival differences (Figure 2).
Table 4
| Time frame | Prognostic indices | Cutoff points | Prevalence† (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUROC (95% CI) | HR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| 12-week | GPS | 1 | 157/199 (78.9) | 81.1 | 59.3 | 31.3 | 93.2 | 0.75 (0.66–0.82) | 2.25 (1.59–3.20) |
| mGPS | 1 | 157/199 (78.9) | 75.7 | 65.4 | 33.3 | 90.6 | 0.74 (0.65–0.82) | 2.25 (1.59–3.20) | |
| MUAC | 23.0 | 40/193 (20.7) | 50.0 | 86.0 | 45.0 | 88.2 | 0.71 (0.60–0.80) | 2.33 (1.62–3.34) | |
| 24-week | GPS | 1 | 157/199 (78.9) | 69.7 | 65.0 | 55.2 | 77.7 | 0.70 (0.62–0.76) | 2.25 (1.59–3.20) |
| mGPS | 1 | 157/199 (78.9) | 60.5 | 69.1 | 54.8 | 73.9 | 0.67 (0.60–0.74) | 2.25 (1.59–3.20) | |
| MUAC | 24.5 | 59/193 (30.6) | 52.7 | 83.2 | 66.1 | 73.9 | 0.72 (0.64–0.79) | 1.63 (1.19–2.23) |
†, prevalence is defined death events in each time frame per study population. AUROCs, area under the receiver operating characteristic curves; GPS, Glasgow Prognostic Score; mGPS, modified Glasgow Prognostic Score; MUAC, mid-upper arm circumference; PPV, positive predictive value; NPV, negative predictive value; CI, confidence interval; HR, hazard ratio.
Discussion
We found that inflammation and nutrition-based prognostic indices showed similar accuracies in estimating 12- and 24-week survival of medical oncology outpatients in this secondary analysis of a prospective cohort study. Interestingly, a relatively novel prognostic factor for advanced cancer patients, MUAC, showed comparable performance with GPS and mGPS.
Multiple prognostic models have been developed for patients with advanced cancer. GPS was initially developed from patients with inoperable NSCLC with an expected survival of approximately one year (12,13). It is the length of survival of the population that differentiated GPS from most of the prognostic models that have been derived from terminally ill patients with weeks of survival. Our study investigated ambulatory patients with expected survival of less than a year, which was similar to the original population of the GPS. Other prognostic indices such as mGPS, PNI, NLR, and CAR have been studied in various stages of cancer encompassing operable, inoperable, and terminally ill patients (14,15,19-21,25-31). All these indices showed comparable performance in our study in terms of AUROCs for estimating 12- and 24-week survival. It is surprising when we consider that these indices were originally developed from patients with different survival times other than one year. MUAC also showed high AUROCs of 0.71 and 0.72 for estimating 12- and 24-week survival, respectively. Notably, GPS showed the highest sensitivity for estimating 12-week survival, while MUAC had the highest specificity for estimating 12-week survival. Clinicians can select indices with high sensitivity for screening patients for palliative care referral. Thus, GPS can be used as prognostic information in advance care planning conversations. Higher specificity of MUAC would be useful for clinicians to communicate with patients and families with more evidence. For instance, patients with higher MUAC levels may live longer than 12 weeks. This information will be helpful for patients to reallocate their resources, such as business and leave, in the end-of-life period.
It was reported decades ago that elevated resting energy expenditure and weight loss in advanced cancer are associated with the presence of a systemic inflammatory response (36,37). CRP is the most commonly used marker to measure the magnitude of the systemic inflammatory response, because of its sensitivity, specificity, and reproducibility. Decreased albumin concentrations were found to be related to decreased body cell mass and the systemic inflammatory response (15). GPS/mGPS was developed based on the observation of this inverse relationship between CRP concentrations and albumin concentrations across various tumor types (38). A high mGPS was independently associated with a low skeletal muscle density and hand grip strength test failure in a recent study that enrolled 523 patients with advanced cancer (39). Previously, our group studied MUAC as a surrogate marker of muscle depletion, and demonstrated the prognostic significance of MUAC in advanced cancer patients (32). In that study, we proved that MUAC is a significant prognostic factor to estimate 3-month survival in medical oncology outpatients. In the present study, the MUAC showed comparable performance with GPS/mGPS. MUAC is simple and objective, making it easy for clinicians to use it in everyday clinical practice. It neither requires equipment nor invasive blood sampling. With a tapeline, caregivers at home can report the MUAC of patients by simple training. It would be helpful to monitor patients with advanced cancer when their functional status declines rapidly. We believe that MUAC is a useful alternative to GPS/mGPS in medical oncology outpatients, with special advantages in terms of simplicity and non-invasiveness.
In the present study, the performance of MUAC was compared with GPS, mGPS, PNI, NLR, and CAR by use of AUROC. The AUROC of CAR for estimating 12- and 24-week survival was numerically the highest among the indices examined in our study. However, all 6 indices were similarly accurate statistically. We compared MUAC with GPS/mGPS because they have distinct cutoff points among the 5 indices. In addition, GPS/mGPS are the most widely investigated inflammation and nutrition-based prognostic indices (15). However, the cutoff point of CRP was rather arbitrarily defined in an earlier study testing prognostic factors in 91 advanced cancer patients with weight loss (40). The cutoff point of albumin was also described as ‘standard thresholds’ (12). GPS, mGPS and CAR utilize CRP and albumin in common for scoring. Thus, we still have to explore the best way to combine and explain the results. Further studies are needed to define optimal cutoff values of CRP, albumin, and MUAC by adopting artificial intelligence and machine learning in a larger population.
Major limitations of our study include that MUAC is not a widely validated prognostic factor in patients with advanced cancer. Because MUAC is measured by humans, there is a possibility of variability depending on the rater and timing of measurements. It also requires a cutoff point, which might be different across sex, age, ethnicity, and other relevant parameters (41-43). We could not standardize MUAC using an accepted criterion, since there is no nationally standardized data for MUAC in South Korea. In addition, the predictive capacity of MUAC for survival may be directly linked to malnutrition. However, independent nutritional assessment was not performed in this study. Another limitation of our study is that our patient population may be biased, as all patients were medical oncology outpatients with relatively good performance status. Finally, our study was conducted in a single tertiary hospital in South Korea with a convenient sample approach, which might be related to selection bias. Therefore, further studies are required to validate our findings and define optimal cutoff values.
Conclusions
In conclusion, inflammation and nutrition-based prognostic indices including GPS, mGPS, PNI, NLR, CAR, and MUAC showed similar performances in estimating the 12- and 24-week survival of medical oncology outpatients. In particular, the AUROCs of MUAC were comparable to those of GPS/mGPS. Considering the special advantages of MUAC in terms of simplicity and non-invasiveness, it may serve as a useful alternative measure to GPS/mGPS in the care for patients with advanced cancer.
Acknowledgments
We thank the participating patients and clinical research nurses, Jin Suk Kim and Esther Jeon, for supporting this study.
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Science, Informatics, Communication and Technology; No. 2015R1C1A2A01053357).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-481/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-481/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-481/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki as revised in 2013. Written informed consent was obtained from each patient before enrollment. The study was approved by the IRB of Seoul National University Bundang Hospital (No. B-1601/332-302).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489-95. [Crossref] [PubMed]
- Meza-Valderrama D, Marco E, Dávalos-Yerovi V, et al. Sarcopenia, Malnutrition, and Cachexia: Adapting Definitions and Terminology of Nutritional Disorders in Older People with Cancer. Nutrients 2021;13:761. [Crossref] [PubMed]
- Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539-47. [Crossref] [PubMed]
- Costamagna D, Costelli P, Sampaolesi M, et al. Role of Inflammation in Muscle Homeostasis and Myogenesis. Mediators Inflamm 2015;2015:805172. [Crossref] [PubMed]
- McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care 2009;12:223-6. [Crossref] [PubMed]
- Tan CS, Read JA, Phan VH, et al. The relationship between nutritional status, inflammatory markers and survival in patients with advanced cancer: a prospective cohort study. Support Care Cancer 2015;23:385-91. [Crossref] [PubMed]
- Souza Cunha M, Wiegert EVM, Calixto-Lima L, et al. Relationship of nutritional status and inflammation with survival in patients with advanced cancer in palliative care. Nutrition 2018;51-52:98-103. [Crossref] [PubMed]
- Cordeiro LAF, Silva TH, de Oliveira LC, et al. Systemic Inflammation and Nutritional Status in Patients on Palliative Cancer Care: A Systematic Review of Observational Studies. Am J Hosp Palliat Care 2020;37:565-71. [Crossref] [PubMed]
- Falconer JS, Fearon KC, Ross JA, et al. Acute-phase protein response and survival duration of patients with pancreatic cancer. Cancer 1995;75:2077-82. [Crossref] [PubMed]
- Suh SY, Ahn HY. A prospective study on C-reactive protein as a prognostic factor for survival time of terminally ill cancer patients. Support Care Cancer 2007;15:613. [Crossref] [PubMed]
- Deme D, Kovacs S, Telekes A. Overall Survival Prediction of Advanced Cancer Patients by Selection of the Most Significant Baseline Serum Biomarker Combination. Pathol Oncol Res 2022;28:1610004. Erratum in: Pathol Oncol Res 2022;28:1610517. [Crossref] [PubMed]
- Forrest LM, McMillan DC, McArdle CS, et al. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer 2003;89:1028-30. [Crossref] [PubMed]
- Forrest LM, McMillan DC, McArdle CS, et al. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br J Cancer 2004;90:1704-6. [Crossref] [PubMed]
- McMillan DC, Crozier JE, Canna K, et al. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis 2007;22:881-6. [Crossref] [PubMed]
- McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 2013;39:534-40. [Crossref] [PubMed]
- Laird BJ, Kaasa S, McMillan DC, et al. Prognostic factors in patients with advanced cancer: a comparison of clinicopathological factors and the development of an inflammation-based prognostic system. Clin Cancer Res 2013;19:5456-64. [Crossref] [PubMed]
- Simmons C, McMillan DC, Tuck S, et al. "How Long Have I Got?"-A Prospective Cohort Study Comparing Validated Prognostic Factors for Use in Patients with Advanced Cancer. Oncologist 2019;24:e960-7. [Crossref] [PubMed]
- Fan H, Shao ZY, Xiao YY, et al. Comparison of the Glasgow Prognostic Score (GPS) and the modified Glasgow Prognostic Score (mGPS) in evaluating the prognosis of patients with operable and inoperable non-small cell lung cancer. J Cancer Res Clin Oncol 2016;142:1285-97. [Crossref] [PubMed]
- Buzby GP, Mullen JL, Matthews DC, et al. Prognostic nutritional index in gastrointestinal surgery. Am J Surg 1980;139:160-7. [Crossref] [PubMed]
- Walsh SR, Cook EJ, Goulder F, et al. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 2005;91:181-4. [Crossref] [PubMed]
- Guthrie GJ, Charles KA, Roxburgh CS, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013;88:218-30. [Crossref] [PubMed]
- Kim YJ, Kim SJ, Lee JK, et al. Prediction of survival in terminally ill cancer patients at the time of terminal cancer diagnosis. J Cancer Res Clin Oncol 2014;140:1567-74. [Crossref] [PubMed]
- Smith RA, Bosonnet L, Raraty M, et al. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg 2009;197:466-72. [Crossref] [PubMed]
- Kasymjanova G, MacDonald N, Agulnik JS, et al. The predictive value of pre-treatment inflammatory markers in advanced non-small-cell lung cancer. Curr Oncol 2010;17:52-8. [PubMed]
- Proctor MJ, Morrison DS, Talwar D, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer 2011;47:2633-41. [Crossref] [PubMed]
- Kinoshita A, Onoda H, Imai N, et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol 2015;22:803-10. [Crossref] [PubMed]
- Wei XL, Wang FH, Zhang DS, et al. A novel inflammation-based prognostic score in esophageal squamous cell carcinoma: the C-reactive protein/albumin ratio. BMC Cancer 2015;15:350. [Crossref] [PubMed]
- Zhou T, Zhan J, Hong S, et al. Ratio of C-Reactive Protein/Albumin is An Inflammatory Prognostic Score for Predicting Overall Survival of Patients with Small-cell Lung Cancer. Sci Rep 2015;5:10481. [Crossref] [PubMed]
- Pan Y, Lou Y, Wang L. Prognostic value of C-reactive protein to albumin ratio in metastatic colorectal cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2021;100:e27783. [Crossref] [PubMed]
- Zhang J, Zhang C, Li Q, et al. C-Reactive Protein/Albumin Ratio Is an Independent Prognostic Predictor of Survival in Advanced Cancer Patients Receiving Palliative Care. J Palliat Med 2019;22:1536-45. [Crossref] [PubMed]
- Cunha GDC, Rosa KSDC, Wiegert EVM, et al. Clinical Relevance and Prognostic Value of Inflammatory Biomarkers: A prospective Study in Terminal Cancer Patients Receiving Palliative Care. J Pain Symptom Manage 2021;62:978-86. [Crossref] [PubMed]
- Kim YJ, Hiratsuka Y, Suh SY, et al. A Prognostic Model to Facilitate Palliative Care Referral in Oncology Outpatients. Cancer Res Treat 2022;54:621-9. [Crossref] [PubMed]
- Sala A, Rossi E, Antillon F, et al. Nutritional status at diagnosis is related to clinical outcomes in children and adolescents with cancer: a perspective from Central America. Eur J Cancer 2012;48:243-52. [Crossref] [PubMed]
- Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361-87. [Crossref] [PubMed]
- Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32-5. [Crossref] [PubMed]
- Falconer JS, Fearon KC, Plester CE, et al. Cytokines, the acute-phase response, and resting energy expenditure in cachectic patients with pancreatic cancer. Ann Surg 1994;219:325-31. [Crossref] [PubMed]
- Staal-van den Brekel AJ, Dentener MA, Schols AM, et al. Increased resting energy expenditure and weight loss are related to a systemic inflammatory response in lung cancer patients. J Clin Oncol 1995;13:2600-5. [Crossref] [PubMed]
- McMillan DC, Elahi MM, Sattar N, et al. Measurement of the systemic inflammatory response predicts cancer-specific and non-cancer survival in patients with cancer. Nutr Cancer 2001;41:64-9. [Crossref] [PubMed]
- Dolan RD, Daly LE, Simmons CP, et al. The Relationship between ECOG-PS, mGPS, BMI/WL Grade and Body Composition and Physical Function in Patients with Advanced Cancer. Cancers (Basel) 2020;12:1187. [Crossref] [PubMed]
- O'Gorman P, McMillan DC, McArdle CS. Prognostic factors in advanced gastrointestinal cancer patients with weight loss. Nutr Cancer 2000;37:36-40. [Crossref] [PubMed]
- Yang US, Cho JH. Standard values for nutritional assessment by anthropometry in healthy Korean adults. Korean J Intern Med 1999;56:560-8.
- Schwekendiek D. Biosocial comparison of mid-upper arm circumference in the two Koreas. J Biosoc Sci 2013;45:615-25. [Crossref] [PubMed]
- Khadilkar AV, Khadilkar VV, Gondhalekar KM, et al. Reference centile curves for mid-upper arm circumference for assessment of under- and overnutrition in school-aged Indian children and adolescents. Nutrition 2021;91-92:111401. [Crossref] [PubMed]



