Quality of life with Brain Symptom and Impact Questionnaire in patients with brain metastases
Introduction
Patients with brain metastases have complex symptom experiences, limited life expectancy and worsening quality of life (QOL) (1-3). Twenty to forty percent of cancer patients develop brain metastases. These patients often have some level of neurocognitive dysfunction and neurologic symptoms at baseline (4,5).
Management strategies include the use of corticosteroids to reduce edema and anticonvulsants to treat seizures. Whole brain radiation therapy (WBRT), stereotactic radiosurgery (SRS), neurosurgery, alone or in combination are used to treat metastatic disease to brain. Factors including the extent of intracranial and extracranial disease, age and functional status often influence the selection of treatment method (6-10).
WBRT may improve neurological symptoms and function (6,11). Median survival of three to six months has been suggested in the reported studies as most of these patients treated with WBRT have poor performance, multiple brain metastases or heavy burden of progressive systemic disease (6,11-13). WBRT alone has been reported to potentially contribute to symptom stabilization but not significantly improve most QOL domains (14-17). On the other hand, patients who do not belong to the above groups may be treated with SRS, neurosurgery with or without WBRT (18-33).
The management of brain metastases continues to evolve. Due to concerns regarding neurocognitive decline with the use of WBRT, a number of approaches including the use of SRS alone for selected patients have been advocated (34). The use of neurosurgery has shown benefit in terms of survival for selected patients with resectable single brain metastasis as compared to WBRT alone (35,36). WBRT when combined with SRS or neurosurgery improves overall brain control but at the possible detriment of neurocognitive decline (37) and worse QOL (38). In all the randomized trials, patients selected for SRS or neurosurgery had good performance status and the majority had controlled extracranial disease.
QOL is commonly assessed in brain metastases patients using multidimensional constructs encompassing both psychosocial and physical factors (39). The patient-reported QOL instruments, European Organization for Research and Treatment Cancer Quality of Life Questionnaire-Brain Neoplasm (EORTC QLQ BN20) (used alongside the QLQ-C30) and the Functional Assessment of Cancer Therapy-Brain (FACT-Br) (used alongside the FACT-General), were originally validated and intended for patients with primary brain tumours rather than those with brain metastases (9,40,41). Although brain metastases patients often have similar symptoms to patients with primary brain tumours, the prognoses and treatment regimens are different depending on primary cancer site, extent and volume of brain metastases and other sites of metastases. The two validated QOL instruments are quite lengthy (50 items for both questionnaires), and can be burdensome for advanced cancer patients (41-43).
The Brain Symptom and Impact Questionnaire (BASIQ) was developed as a novel brief QOL assessment tool for brain metastases patients. This instrument consists of 18 items that measures symptom severity (items 1–10) and impact (items 11–18) on daily functional activities. The questionnaire is scored from 0–10, with higher scores denoting worse symptom severity and lower QOL (41). The tool covers 12 domains relevant to patients with brain metastases: headaches, dizziness, nausea, numbness, energy, balance, vision, memory, cognition, physical activities and self-care. The relative brevity of this questionnaire reduces patient burden while also maintaining the breadth of coverage. Two studies have confirmed the reliability, validity and suitability of BASIQ in patients with brain metastases (41,43).
WBRT alone is usually recommended in patients with multiple brain metastases and/or poor performance status. SRS or neurosurgery is selected for patients with limited disease and better performance status. Some radiation oncologists may decide to follow with WBRT in some patients of the latter group. For those patients selected upfront for WBRT alone versus those for SRS or neurosurgery, the purpose of this study was to examine baseline QOL (as measured by BASIQ) and patient characteristics, among patients who underwent WBRT alone versus SRS or neurosurgery with or without WBRT.
Methods
Eligibility criteria and data collection
Patients referred for treatment of brain metastases to the Odette Cancer Centre at Sunnybrook Health Sciences Centre in Toronto, Ontario, Canada were approached for entry into this study. Informed consent was obtained. Baseline BASIQ results were obtained before patients were treated with WBRT, neurosurgery, or SRS. Demographic information, including age, gender, primary cancer site, years from primary cancer to brain metastases, Karnofsky Performance Status (KPS) score, and Eastern Cooperative Oncology Group Performance Status (ECOG PS) score were collected. Eligible patients were stratified based on their treatment: WBRT alone (group A) versus SRS or neurosurgery with or without WBRT (group B).
Statistical methods
Demographics were summarized in all patients and in patients from group A or B, using median and range for age, KPS, years from primary cancer to brain cancer, and proportions for categorical variables. To compare demographics between group A and group B, Wilcoxon rank-sum nonparametric test or Fisher exact test was applied for continuous or categorical variables. Descriptive analysis on 18 BASIQ items and 2 summary scores were conducted using mean, standard deviation (SD), median and ranges in total patients and in patients from group A or B. Wilcoxon rank-sum nonparametric test was also used to compare BASIQ items and summary scores between group A and B. Two-sided P value <0.05 was considered statistically significant. All analyses were performed using Statistical Analysis Software (SAS version 9.4 for Windows).
Results
Patient demographics
A total of 172 patients were accrued, with 76 (44%) and 96 patients (56%) grouped to A and B, respectively. Detailed demographics of the collective group are listed in Table 1. The median age was 62 years old (range, 24–90). Ninety-eight patients (57%) were female, and seventy-three (43%) were male. The time frame between primary cancer and development of brain metastases ranged from 0 to 28 years, with the median being 1 year. The median KPS score was 80 (range, 40–100). Eighty-four patients (55%) had KPS scores less than or equal to 80, and 68 patients (45%) had scores greater than 80. With respect to ECOG PS scores, 68 patients (45%) had a score of 0, 67 (44%) had a score of 1, 15 (10%) had a score of 2 and 2 (1%) had a score of 4. Lung, breast, melanoma and renal cancer were the most prevalent primary cancer sites among the study population, with 91 (53%), 25 (15%), 17 (10%) and 15 (9%) patients, respectively. Sixty-one patients (36%) had one brain metastasis, five had two brain metastases, thirty-eight (23%) had three brain metastases, and sixty-five (39%) had more than three brain metastases. Bone, lung, liver and lymph were among other sites of metastases observed in patients, with 32 (19%), 34 (20%), 18 (11%) and 18 (11%) patients, respectively, while 98 patients (57%) reported no other metastases (Table 1).
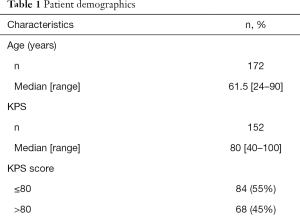
Full table
There was significant difference in age between the two groups (P=0.04); group A had a median age of 61 years old whereas group B had a median age of 66 years old. Baseline KPS scores were generally lower for group A (median =80) as opposed to group B (median =90) (P=0.02). The largest proportion of patients in group B had a KPS score of 100 (28%) compared to group A (10%) (P=0.001). Group B had significantly more ECOG PS scores of 0 and 1, with 46 patients (51%) compared to 22 patients (36%) and 41 patients (45%) in comparison to 26 patients (43%), respectively. A higher percentage of patients in group A had more than 3 brain metastases in 38 patients (50%) compared to 27 patients (29%), and a lower proportion had one brain metastasis in 23 patients (30%) compared to 38 patients (41%). There were more patients in group A with extra-cranial metastases when compared to group B (P=0.0003) (Table 2).
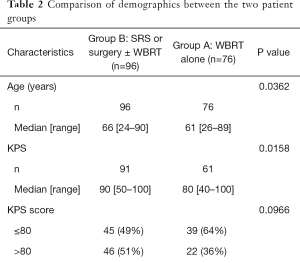
Full table
BASIQ items/summary scores
Baseline BASIQ scores for both groups are summarized in Table 3. Group A had significantly higher mean symptom scores pertaining to headache (4.7>2.3; P=0.0001), dizziness (4.7>1.9; P<0.0001), nausea (4.4>1.7; P<0.0001), numbness (4.6>2.0; P<0.0001), fatigue (6.0>4.6; P=0.005), physical strength (5.6>3.5; P=0.0003), balance (5.1>2.1; P<0.0001), vision (4.6>1.8; P<0.0001), and memory (5.5>2.7; P<0.0001). Only the symptom score of energy (6.2 and 5.4 for group A and B, respectively) was not significantly higher in group A (P=0.1).
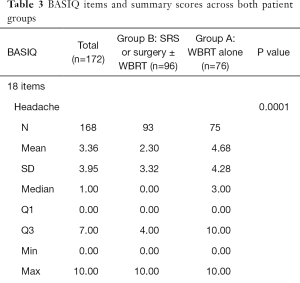
Full table
Group B had lower mean function scores across all eight evaluated items (P<0.0001): putting ideas into words (2.1<4.7), staying focused (1.9<4.8), following a story (1.8<4.6), reading (1.7<4.7), walking (2.6<5.5), housework (3.0<5.6), bathing (1.7<4.8) and getting dressed (1.6<4.6). Symptom and function summary scores also favoured group B (P<0.0001), with group A registering higher mean symptom (51.4 vs. 27.9) and function (38.9 vs. 15.9) scores.
Discussion
Studies (6,7,16,18,44-46) on brain metastases have evaluated overall survival, local brain tumor control, intracranial progression-free duration, neurologic function, dexamethasone dose and duration, and adverse effects between treatment options. There are also several studies (6,7,17,47,48) investigating the choice between WBRT or SRS alone, or a combination of the two treatments. All of the published randomized trials have included good prognosis patients (good performance status, controlled extracranial disease) and up to four small brain metastases for the use of radiosurgery alone, with some benefit in terms of neurocognitive sparing and QOL as compared to WBRT (37,38). Patients who are eligible for neurosurgery in the randomized trials were also those who also have good prognosis (good performance status and controlled extracranial disease) and resectable single brain metastasis. These patients who underwent neurosurgery had better survival as compared to patients who did not have neurosurgery but had WBRT alone (35,36).
Patients with multiple brain metastases and poor prognostic features (poor performance status and active extracranial disease) may be managed with palliative WBRT or comfort measures (49,50). Consistent with the inclusion of patients in the above reported trials, we also found poorer prognostic patients managed with WBRT alone and better prognostic patients managed with SRS or neurosurgery with or without WBRT. Furthermore, patients managed with WBRT alone had worse baseline QOL scores, as measured by BASIQ as compared to those managed by SRS or neurosurgery with or without WBRT.
In this study, the median KPS score was lower in group A, and the distribution of ECOG scores (more patients in group B who had ECOG PS scores of 0 or 1 than group A) reaffirms that group B had better performance status than group A. Group A patients also had a higher proportion with multiple brain metastases and active uncontrolled systemic disease. Baseline BASIQ scores for patients in group A showed significantly more difficulty with vision, memory, staying focused, following a story, and putting ideas into words when compared with patients in group B (P<0.001). Group A also had substantially higher BASIQ scores (worse QOL) in 17 of the 18 evaluated items.
The baseline QOL results from this study may not represent the entire population of patients who undergo WBRT, SRS, neurosurgery as only English speaking patients were enrolled and patients who declined entry into this study may have different QOL compared to patients who entered this study. Only baseline QOL as assessed by BASIQ was evaluated. We did not evaluate serial QOL outcomes after treatment. Although we reported statistically significant differences in QOL scores, we did not use methods to determine clinical meaningful differences.
In our group of brain metastases patients, those treated with WBRT alone had significantly higher baseline BASIQ scores (in 17 or 18 items), indicating worse QOL as compared to those patients treated with SRS, neurosurgery with or without WBRT. Consistent with the published randomized trials, those patients managed with SRS, neurosurgery (with or without WBRT) had generally good prognostic features including good performance status (all ECOG performance status 0–2) and less extracranial disease (70% of patients did not have extracranial disease).
Our study was limited by the fact that a significant proportion of the patients were referred from other hospitals, therefore we did not have complete data on the use of systemic therapy including chemotherapy and target therapy which also have an impact on the patient reported QOL.
This new BASIQ scale will be another alternative to the brain module with EORTC and FACT (51). Other scales such as Spitzer index (52), Functional living index-cancer (53) can be used in cancer patients or such MDASI (54) for the evaluation of neurological symptoms. For patients with advanced disease and in the late stages of the disease, the QLQ C15Pal scale (42) can be used and consists in a limited number of items. The BASIQ was developed from patients with brain metastases and can be used in conjunction.
Acknowledgements
We thank the sponsor of AbbVie and the survey participants.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Sunnybrook Research Ethics Board (REB), and has REB number of: 073-2013.
References
- Wong J, Hird A, Kirou-Mauro A, et al. Quality of life in brain metastases radiation trials: a literature review. Curr Oncol 2008;15:25-45. [PubMed]
- Chamberlain MC. Brain metastases: a medical neurooncology perspective. Expert Rev Neurother 2010;10:563-73. [Crossref] [PubMed]
- Sperduto PW, Chao S, Sneed P, et al. Diagnosis-specific prognostic factors, indexes and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys 2010;77:655-61. [Crossref] [PubMed]
- Doyle M, Bradley NM, Li K, et al. Quality of life in patients with brain metastases treated with a palliative course of whole-brain radiotherapy. J Palliat Med 2007;10:367-74. [Crossref] [PubMed]
- Thomas SS, Dunbar EM. Modern multidisciplinary management of brain metastases. Curr Oncol Rep 2010;12:34-40. [Crossref] [PubMed]
- Sahgal A, Larson D, Knisely J. Stereotactic radiosurgery alone for brain metastases. Lancet Oncol 2015;16:249-50. [Crossref] [PubMed]
- Sahgal A, Aoyama H, Kocher M, et al. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 2015;91:710-7. [Crossref] [PubMed]
- Thavarajah N, Bedard G, Zhang L, et al. The Functional Assessment of Cancer Therapy – Brain (FACT-Br) for assessing quality of life in patients with brain metastases: a comparison of recall periods. J Pain Manage 2013;6:223-34.
- Eichler AF, Loeffler JS. Multidisciplinary management of brain metastases. Oncologist 2007;12:884-98. [Crossref] [PubMed]
- Movsas B. Quality of life in oncology trials: a clinical guide. Semin Radiat Oncol 2003;13:235-47. [Crossref] [PubMed]
- Chao JH, Philips R, Nickson JJ. Roentgen ray therapy for cerebral metastases. Cancer 1954;7:682-9. [Crossref] [PubMed]
- Katz HR. The relative effectiveness of radiation therapy, corticosteroids and surgery in the management of melanoma metastatic to the central nervous system. Int J Radiat Oncol Biol Phys 1981;7:897-906. [Crossref] [PubMed]
- Zimm S, Wampler GL, Stablein D. Intracerebral metastases in solid tumor patients: natural history and results of treatment. Cancer 1981;48:384-94. [Crossref] [PubMed]
- Wong J, Hird A, Zhang L, et al. Symptoms and quality of life in cancer patients with brain metastases following palliative radiotherapy. Int J Radiat Oncol Biol Phys 2009;75:1125-31. [Crossref] [PubMed]
- Bezjak A, Adams J, Barton R, et al. Symptom response after palliative radiotherapy for patients with brain metastases. Eur J Cancer 2002;38:487-96. [Crossref] [PubMed]
- Andrews DW, Scott S, Sperduto P, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665-72. [Crossref] [PubMed]
- Li B, Yu J, Suntharalingam M, et al. Comparison of three treatment options for single brain metastases from lung cancer. Int J Cancer 2000;90:37-45. [Crossref] [PubMed]
- Kondziolka D, Patel A, Lundsford LD, et al. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys 1999;45:427-34. [Crossref] [PubMed]
- Noordijk EM, Vecht CJ, Haaxman-Reiche H, et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys 1994;29:711-7. [Crossref] [PubMed]
- Mintz AH, Kestle J, Rathbone MP, et al. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer 1996;78:1470-6. [Crossref] [PubMed]
- Patchell RA, Tibbs P, Walsh J, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990;322:494-500. [Crossref] [PubMed]
- Alexander E, Moriarty TM, Davis RB, et al. Stereotactic radiosurgery for the definitive noninvasive treatment of brain metastases. J Natl Cancer Inst 1995;87:34-40. [Crossref] [PubMed]
- Coffey RJ, Flickinger JC, Bissonette DJ, et al. Radiosurgery for brain metastases using the Cobalt-60 gamma unit: Methods and results in 24 patients. Int J Radiat Oncol Biol Phys 1991;20:1287-95. [Crossref] [PubMed]
- Loeffler JS, Shrieve DC. What is appropriate therapy for a patient with a single brain metastasis? Int J Radiat Oncol Biol Phys 1994;29:915-7. [Crossref] [PubMed]
- Wu A, Lindner G, Maitz AM, et al. Physics of gamma knife approach on convergent beams in stereotactic radiosurgery. Int J Radiat Oncol Biol Phys 1990;18:941-9. [Crossref] [PubMed]
- Auchter RM, Lamond J, Alexander E, et al. A multi-institutional outcome and prognostic factor analysis of radiosurgery for resectable single brain metastasis. Int J Radiat Oncol Biol Phys 1996;35:27-35. [Crossref] [PubMed]
- Flickinger JC, Kondziolka D, Lunsford LD, et al. A multi-institutional experience with stereotactic radiosurgery for solitary brain metastasis. Int J Radiat Oncol Biol Phys 1994;28:797-802. [Crossref] [PubMed]
- Fuller BG, Kaplan I, Adler J, et al. Stereotactic radiosurgery for brain metastases: the importance of adjuvant whole brain irradiation. Int J Radiat Oncol Biol Phys 1992;23:413-8. [Crossref] [PubMed]
- Adler JR, Cox R, Kaplan I, et al. Stereotactic radiosurgical treatment of brain metastases. J Neurosurg 1992;76:444-9. [Crossref] [PubMed]
- Engenhart R, Kimmig BN, Hover KH, et al. Long term follow-up for brain metastases treated by percutaneous stereo- tactic single high-dose irradiation. Cancer 1993;71:1353-61. [Crossref] [PubMed]
- Flickinger JC, Lunsford LD, Somaza S, et al. Radiosurgery: Its role in brain metastasis management. Neurosurg Clin N Am 1996;7:497-504. [PubMed]
- Loeffler JS, Kooy AM, Wen PY, et al. The treatment of recurrent brain metastases with stereotactic radiosurgery. J Clin Oncol 1990;8:576-82. [PubMed]
- Deutsch M, Parsons JA, Mercado R. Radiotherapy for intracranial metastases. Cancer 1974;34:1607-11. [Crossref] [PubMed]
- Mehta MP, Ahluwalia MS. Whole-brain radiotherapy and stereotactic radiosurgery in brain metastases: what is the evidence? Am Soc Clin Oncol Educ Book 2015;35:e99-104. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990;322:494-500. [Crossref] [PubMed]
- Vecht CJ, Haaxma-Reiche H, Noordijk EM, et al. Treatment of single brain metastasis radiotherapy alone or combined with neurosurgery? Ann Neurol 1993;33:583-90. [Crossref] [PubMed]
- Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009;10:1037-44. [Crossref] [PubMed]
- Soffietti R, Kocher M, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol 2013;31:65-72. [Crossref] [PubMed]
- Chen E, Cella D, Zeng L, et al. Content validation of the FACT-Br with patients and health-care professionals to assess quality of life in patients with brain metastases. J Radiat Oncol 2012;3:105-13. [Crossref]
- Caissie A, Nguyen J, Chen E, et al. Quality of life in patients with brain metastases using the EORTC QLQ-BN20+2 and C15-PAL. Int J Radiat Oncol Biol Phys 2012;83:1238-45. [Crossref] [PubMed]
- Thavarajah N, Ray S, Bedard G, et al. Psychometric validation of the Brain Symptom and Impact Questionnaire (BASIQ) version 1.0 to assess quality of life in patients with brain metastases. CNS Oncol 2015;4:11-23. [Crossref] [PubMed]
- Caissie A, Culleton S, Nguyen J, et al. EORTC QLQ-C15 PAL quality of life scores in patients with advanced cancer referred for palliative radiotherapy. Support Care Cancer 2012;20:841-8. [Crossref] [PubMed]
- Bedard G, Ray S, Zhang L, et al. Validation of the Brain Symptom and Impact Questionnaire (BASIQ) to assess symptom and quality of life in brain metastases. CNS Oncol 2014;3:275-85. [Crossref] [PubMed]
- Chongule PB, Burton-Williams M, Saris S, et al. Randomized treatment of brain metastasis with gamma knife radiosurgery, whole brain radiotherapy or both. Int J Radiat Oncol Biol Phys 2000;48:114. [Crossref]
- Jawahar A, Ampil F, Wielbaecher C, et al. Management strategies for patients with brain metastases: has radiosurgery made a different? South Med J 2004;97:254-8. [Crossref] [PubMed]
- Sneed PK, Lamborn KR, Forstner JM, et al. Radiosurgery for brain metastases: is whole brain radiotherapy necessary? Int J Radiat Oncol Biol Phys 1999;43:549-58. [Crossref] [PubMed]
- Tsao MN, Lloyd N, Wong R, et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database of Systematic Reviews 2012;4:CD003869. [PubMed]
- Stafinski T, Jhangri GS, Yan E, et al. Effectiveness of stereotactic radiosurgery alone or in combination with whole brain radiotherapy compared to conventional surgery and/or whole brain radiotherapy for the treatment of one or more brain metastases: a systematic review and meta-analysis. Cancer Treat Rev 2006;32:203-13. [Crossref] [PubMed]
- Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis (es): an American society for radiation oncology evidence-based guidelines. Pract Radiat Oncol 2012;2:210-25. [Crossref] [PubMed]
- Jones JA, Simone CB. Whole brain radiotherapy for patients with poor prognosis: possibilities for the impact of the QUARTZ trial. Ann Palliat Med 2015;4:58-60. [PubMed]
- Chow R, Lao N, Popovic M, et al. Comparison of the EORTC QLQ-BN20 and the FACT-Br quality of life questionnaires for patients with primary brain cancers: a literature review. Support Care Cancer 2014;22:2593-8. [Crossref] [PubMed]
- Spitzer WO, Dobson A, Hall J, et al. Measuring the quality of life of cancer patients: A concise QL-Index for use by physicians. J Chronic Dis 1981;34:585-97. [Crossref] [PubMed]
- Schipper H, Clinch J, McMurray A, et al. Measuring the quality of life of cancer patients: the Functional Living Index-Cancer: development and validation. J Clin Oncol 1984;2:472-83. [PubMed]
- Armstrong TS, Mendoza T, Gring I, et al. Validation of the MD Anderson symptom inventory brain tumor module (MDASI-BT). J. Neurooncol 2006;80:27-35. [Crossref] [PubMed]


