Child’s symptom burden and depressive symptoms among caregivers of children with cancers: an argument for early integration of pediatric palliative care
Introduction
Childhood cancers have been linked with good prognosis as well as likelihood of full remission when treatment is early and optimum in general (1-3). Contrarily, such do not depict the situation in resource-restricted settings where childhood cancers are often characterized by late presentation, high symptom burden, compromised care and poorer survival. For instance, close to 160,000 new cases of childhood cancers occur in low and middle income countries annually; and despite its treatability, childhood cancers constitute the fourth leading contributory cancer to the number of years lost (4). This is not surprising, given that cancer diagnosis along with its care is still largely enmeshed in myths, stigma, denial and poor health resources across most of the developing contexts (5,6).
The high burden of symptoms experienced by children with cancers in resource-restricted settings points to the need for early integration of palliative care in pediatric oncology services. More especially because palliative care as recommended by World Health Organization involves the effective care of children with cancers using multidisciplinary approach to promote good quality of life while involving their families or caregivers and making use of community resources (6). As it is, palliative care in pediatric cancer treatment settings has extended benefits of addressing the concerns and well-being of caregivers of children with cancers (7,8). This is particularly important, given that caregivers play central roles across all aspects of cancer care ranging from identification of symptoms for prompt presentation and diagnosis to treatment related decisions. Such important roles of caregivers are understandable because children with cancers are ‘minors’ who are more likely to be living with caregivers that are either parents or relatives in most cases. In less developed regions of the world, poor availability of pediatric oncology services, poor access of such services when available and lack of resources due to widespread poverty do also tilt the care of cancer to home settings with more added physical, emotional and financial burden on caregivers (9).
Together with the above roles, the diagnosis along with care of children with cancers evokes various emotional reactions in caregivers that include depressive symptoms and are more often than not unattended (9,10). For these reasons, caregivers have been described as “hidden patients’’ and if depressed; both their wellbeing and caregiving roles are more likely to be impaired based on earlier findings in the west (11). Unfortunately, little is known about caregiving experience among children with cancers in developing country. It is also not clear which child cancer-related factors may constitute important identifiable correlates of depression in caregivers.
To this end, we aim to evaluate if there exist any relationship between child’s symptom burden and depressive symptoms in caregivers of children with cancers. We postulate that considerable number of caregivers of children with cancers would report morbid depressive symptoms and that child’s symptom would correlate with depressive symptoms among caregivers of children with cancers.
Methods
Study design and population
This is a cross-sectional descriptive study among 72 consenting caregivers and assenting children with cancers undergoing treatment in tertiary hospitals in Nigeria. In general, pediatric oncology services are rendered by a multidisciplinary team and the available treatment modalities include surgery, chemotherapy, radiotherapy and combination of any of these treatment modalities depending on individual child and cancer related factors. The sample size was estimated using the formula for sample size calculation (12,13). Eligibility criteria included children with cancers aged 7–12 years and their adult caregivers. The mean duration for cancer illness among the children seen in our study was 10.48±8.20 months and ranged from one to 48 months. The caregivers were primarily providing care for the child and not caring for another ill person. Again, both child and caregivers were psychologically fit to give consent or assent as well as complete questionnaire. Recruitment of all eligible participants was done consecutively for a period of twenty four months until the estimated sample size was completed.
Ethical approval
The study protocol was sent to the Health Research and Ethics committee of the Hospital and approval was obtained before commencement of the study. All participants gave informed consent while the children assented. Confidentiality was strictly maintained and voluntary refusal or disengagement was allowed at any point of the interview without any negative consequence on the participants or treatment of their children. Participants with significant depressive symptoms were counselled and referred for appropriate medical attention. Overall eight participants were excluded based on decline of consent.
Study instruments and data collection
Designed questionnaire
A designed questionnaire was administered to elicit socio-demographic and clinical profile of children with cancers and their caregivers. The socio-demographic data collected included caregivers age, gender, marital status, amount spent on treatment, family size and cause of illness. The clinical or disease related data collected include tumor types, modalities of treatment and treatment setting among others.
Memorial Symptom Assessment Scale (MSAS) [7–12] (14-16)
The child’s symptoms were profiled with questions adapted from the MSAS [7–12] (14). The MSAS [7–12] (14) is an 8-item revised version of the MSAS [10–18] (15) done to address the distressing symptoms that are prevalent among young children. It assesses symptoms burden with respect to frequency, intensity and distress. The scores range for each item in terms of frequency, intensity and distress include 1, 2, and 3 depending on the severity of the symptom been assessed. MSAS [7–12] is a valid, reliable and widely used instrument (14-16).
Center for Epidemiologic Studies Depression Scale-Revised (CES-DR)
Depressive symptoms in caregivers were elicited using CESD-R (17,18). The CES-DR (17,18) is 20-item scale frequently used in the assessment of depression. It has proven cross cultural validity, reliability and has been well used in many languages and contexts. The cut-off score of 16 has been shown to have high specificity and sensitivity (17-20). Each item was rated using four points Likert scale (0, 1, 2 and 3) which is indicative of the severity of the symptoms over the last one week. The possible scores ranged from 0 to 60 and higher scores indicating greater impairment.
Overall, questionnaires were serially numbered as well as linked to hospital number in order to ensure that participants were seen only once. Questionnaires were filled by participants and interviewer administered where indicated.
Data analyses
The analyses of the data were conducted with the Statistical Package for Social Sciences (SPSS) version 16 (21). Preliminary Shapiro-Wilk tests for normality of data were done findings on CES-DR (W=0.99; df=72; P=0.34) and MSAS [7–12] (W=0.92; df=72; P=0.31). A confidence interval of 95% which allows for 5% sampling error at significant level less than or equals to 0.05 was used. Percentages, frequencies and means were used to describe variables. Scores on MSAS [7–12] were inputted and grouped to reflect burden based on intensity, frequency and distress. Comparative relationship between scores on CESD-R and MSAS [7–12] was done using Pearson correlation.
Results
Socio-demographic and clinical profile of children and caregivers
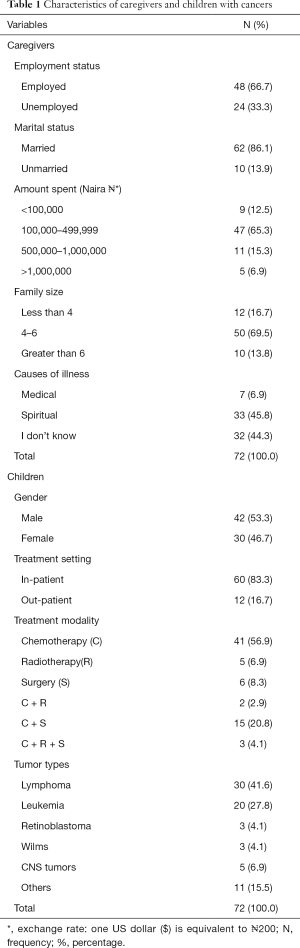
As shown in Table 1; a total of 72 caregivers were included in the study. All the caregivers in this study were made up of parents, with majority (83.7%) being mothers. The mean ages of the children and their caregivers were 10±2 and 39±2 years respectively. Two-thirds 48 (66.7%) of the caregivers were employed; and majority of them 62 (86.1%) were married. The largest proportion of the caregivers 47 (65.3%) reported that between ₦100,000 ($500) to ₦499,999 ($2,500) had been spent on cancer care. Overall, 33 (45.8%) caregivers attributed spiritual causations to the cancer while similar proportion of the caregivers (44.3%) reported virtual lack of knowledge of the cause of the cancer. With respect to the child, almost equal proportions were male 42 (53.3%) and female. Most of the children 60 (83.3%) were receiving in-patient care; lymphomas 30 (41.6%) and chemotherapy 41 (56.9%) were the commonest type of cancer and treatment modalities respectively. See Table 1.
Full table
Prevalence and burden of symptoms among children with cancers
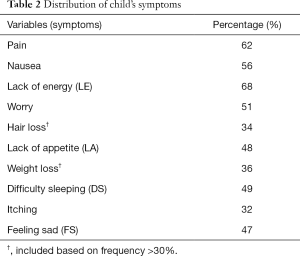
Table 2 shows the distribution of symptoms based on their frequency. The common symptoms with prevalence >50% in the children included pain, nausea, worry, and lack of energy (LE) and symptoms prevalence ranged from LE (68%) to itching (32%). It is also notable that about one-thirds each of the children reported hair loss (34%) and weight loss (36%) in spite of their consideration as “unusual” in the existing instrument.
Full table
The symptom character showed variability in frequency, intensity and distress. Over all, more than half (>50%) the children reported pain, lack of appetite (LA) and feeling sad (FS) to be burdensome when moderate to severe [‘medium amount’ (MA) to ‘almost always’] frequency, moderate to high [‘MA’ to ‘a lot’ (AL)] intensity and moderate to high (‘MA’ to ‘very much’) distress were considered. See Tables 2 and 3.

Full table
Correlation between caregivers CESDR scores and child’s symptoms score on MSAS [7–12]
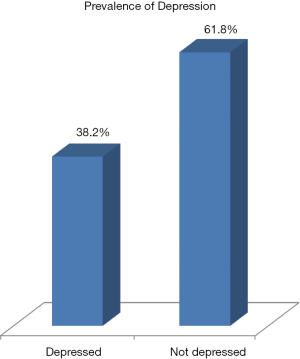
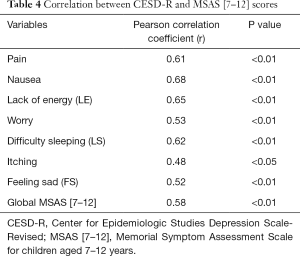
Figure 1 showed that 38.2% of caregivers had depression based on CESDR cut off score of 16 and above. Table 4 showed the relationship between symptoms in the children with cancer and significant depressive symptoms in their caregivers. The global symptom burden (r=0.58; P<0.01) and individual child symptoms in children were correlated positively with depressive symptoms in caregivers (P<0.05). Considering the correlation coefficients (r), nausea (r=0.68), LE (r=0.65), sleep difficulty (r=0.62) and pain (r=0.61) were highly correlated with depressive symptoms in caregivers (see Table 4).
Full table
Discussion
The current state of things with childhood cancers and their care across the less developed countries are worrisome; albeit pockets of improvement have been noted in certain areas. For instance, there is increasing effort at creating awareness in order to drive early presentation for diagnoses and treatment. Notwithstanding, pediatric palliative care service is still poorly developed and little is known about caregiving despite pointers to their central roles in the care of childhood cancers. Findings from our work depict an extension of knowledge by validating the prevalence of high symptoms burden among children with cancers in resource restricted settings, and unlike earlier works profiled the pattern of symptoms experienced using a structured instrument. Furthermore, we were able to establish the frequent occurrence of significant depressive symptoms among caregivers of children with cancers, and importantly, the child’s symptoms burden taking into consideration their frequency, intensity and distress was positively correlated with caregivers’ depressive symptoms.
In our study, caregivers of children with cancers were all parents and largely made up of mothers. Not surprisingly so because earlier works have documented the special roles of mothers in the caregiving of sick children and they often share closer relationship with children in general (22). Implicitly, our finding also brings to fore the significance of informal caregiving among children with cancers. Particularly in resource-restricted settings where the care of children with cancers are more likely to be tilted to home setting, and parents more possibly saddled with caregiving roles due to a number of reasons. Such reasons include the virtual lack of formal caregiving, inadequate pediatric oncology services which are poorly accessed where available, poverty, and out-of-pocket payment due to poor health insurance coverage (23). Majority of caregivers were in their third decade of life, married and employed; thereby showing the involvement of women in their reproductive as well as productive age bracket in similarity to previous works on childhood cancers (24,25). This is important considering the implications of the indirect costs incurred and other negative impacts on other roles as these mothers provide care to their sick children.
In agreement with earlier work from sub-Saharan region of the world, the commonest type of childhood cancers in this study was lymphoma; and chemotherapy was the commonest type of treatment modality (26). One specific public health gap that deserves concerted effort from all tiers of stakeholders is our finding that up to nine in every ten caregivers were either not aware of the cause of their children cancers or attributed spiritual causation. Regrettably, this can be adjudged as one of the socio-cultural explanations for late presentations for cancer care and the preference of alternative care in the analyses of existing pathway to care in many less developed countries (20).
High burden of symptoms was the rule rather than exception among the children with cancers in our work. The most distressful symptoms reported by more than half of the children included pain, nausea, worry, and LE. While this is consistent with earlier findings among children with cancers in terms of range of prevalent symptoms; however differs with regards to variability in distress, frequency intensity and overall burden (14,15). It is also notable that up to one-third each of the children reported hair and weight loss which were not considered typical symptoms to be included in the design of MSAS [7–12]. The high symptom burden in our work may be understandable given the background of late presentation as it relates to cancer-related factors and the side-effects of cancer treatments; it is however unacceptable that the available pediatric oncology services seem limited in dealing with this array of symptoms. Moreover, the development of palliative care is still at an ‘infantile’ stage despite more than half of the children reporting pain, LA and FS to be burdensome when moderate to severe frequency, intensity and distress are considered. Our work demonstrates the frustrations and gruesome experience of children with cancers and their families with regards to symptoms (14,15). Again, the report of other ‘unusual’ symptoms depicts the unique experience of children in resource-restricted settings that warrants further attention.
More than two-thirds of caregivers in our work screened positive for depression. This prevalence of depression among caregivers of children with cancer in our work is not just high but up to seven-fold what has been fielded among the general population in the same context (27). A general comparison between findings on prevalence of depression in our work to other related studies among caregivers of children seems difficult. It may be better to be more specific, moreover because while our finding is comparable to findings among certain caregivers of chronically ill children populations (28,29) on one hand; it is much higher when compared to caregivers of children with asthma (30), and the prevalence range of 4–24% for depression among caregivers of children with developmental disabilities (31). The differences in the cited studies and our study may partly be due to methodological issues that include study instruments, design and population among others. That said it is important to emphasis that our finding illustrates the significance of the potential psychosocial crisis likely to be encountered by caregivers of children with cancers. For example, our study suggests that caregivers in our study were not shielded from the protective effect of marriage against depression despite majority being married as seen in other settings (32).
Another important finding in our work borders on the positive correlation between child’s symptom burden and depressive symptoms among caregivers. Such that the higher the symptom burden score of the children; the higher the caregivers’ score on the depressive symptom scale. Unlike earlier studies, our work illustrates the potential negative relationship that cancer or treatment-related factors may have on caregiver’s wellbeing (33). The import of our finding can be viewed from different viewpoints; but largely important is the demonstration of the potential increased of vulnerability for mental crisis among parent caregivers in parallel to the experience of diverse symptoms in their children. This is particularly important in resource-restricted settings because it is possible that such parent caregivers may interpret the incidence of these symptoms as indicative of worsening disease progression, failed treatment and perhaps bad or fatal prognosis. If such symptoms were due to side-effects of effective treatment, then patient-caregivers’ education and navigations may be beneficial and should be promoted to alleviate their distress (34).
Despite the extension of existing knowledge on caregiving among children with cancers by the findings in our study, a number of limitations constrain the direct extrapolation of our findings to all caregivers. For example, the study design was cross sectional in a clinical setting, thus generalizability need be done cautiously. There is need for future works to use larger with robust sample size and longitudinal study design so as to provide better insight into the relationship between childhood cancer-related factors and depression among caregivers.
Our findings illustrate the prevalence of high symptoms burden among children with cancers in resource-restricted setting and the frequent encounter of threshold depressive symptoms among their caregivers. There was preponderance of ignorance or attribution of spiritual causations of cancer among these parent caregivers. Given the aforementioned, our findings suggest the need for sustained efforts in the promotion of health education, early presentation for diagnosis and improved development of pediatric oncology services. Such development should consider the role of early integration of palliative care in pediatric oncology services as this may enhance active symptom management as well as extended potential benefits on the emotional wellbeing of caregivers. Again, the ‘‘unusual’’ pattern of certain symptoms in children with cancer in our work suggests the need to design or modify existing instrument in order to put into consideration ‘novel’ symptoms which were not captured previously. Further research on supportive care in pediatric oncology is warranted.
Acknowledgements
We will like to acknowledge the International Society of Pediatric Oncology (SIOP) for selecting our work for the young investigator award and all those who made encouraging contributions in the course of our presentation at 2015 SIOP congress in Cape Town, South Africa. We also want to recognize the American Society of Clinical Oncology and the European Association of Palliative Care Research for their support of development of clinical oncology with palliative care services in resource-restricted settings as well as making online resources available for this work.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was sent to the Health Research and Ethics committee of the Hospital and approval was obtained before commencement of the study. All participants gave informed consent while the children assented.
References
- Bryce J, Boschi-Pinto C, Shibuya K, et al. WHO estimates of the causes of death in children. Lancet 2005;365:1147-52. [Crossref] [PubMed]
- Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008 v2.0, Cancer incidence and mortality worldwide: IARC Cancer Base No. 10. Lyon, France: International Agency for Research on Cancer; 2010. Available online: . Accessed August 15, 2015.http://globocan.iarc.fr
- Centers for Disease Control and Prevention (CDC). Cancer survivorship--United States, 1971-2001. MMWR Morb Mortal Wkly Rep 2004;53:526-9. [PubMed]
- Craft AW. Childhood cancer--mainly curable so where next? Acta Paediatr 2000;89:386-92. [Crossref] [PubMed]
- Yaris N, Mandiracioglu A, Büyükpamukcu M. Childhood cancer in developing countries. Pediatr Hematol Oncol 2004;21:237-53. [Crossref] [PubMed]
- World Health Organization (WHO). Palliative care is an essential part of cancer control. Available online: . Accessed on 09/11/2015.http://www.who.int/cancer/palliative/en/
- Wilimas JA, Ribeiro RC. Pediatric hematology-oncology outreach for developing countries. Hematol Oncol Clin North Am 2001;15:775-87. x. [Crossref] [PubMed]
- Jemal A, Bray F, Forman D, et al. Cancer burden in Africa and opportunities for prevention. Cancer 2012;118:4372-84. [Crossref] [PubMed]
- Dolgin MJ, Phipps S, Fairclough DL, et al. Trajectories of adjustment in mothers of children with newly diagnosed cancer: a natural history investigation. J Pediatr Psychol 2007;32:771-82. [Crossref] [PubMed]
- Cohen S, Doyle WJ, Skoner DP. Psychological stress, cytokine production, and severity of upper respiratory illness. Psychosom Med 1999;61:175-80. [Crossref] [PubMed]
- Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA 1999;282:2215-9. [Crossref] [PubMed]
- Fleiss JL, editor. Statistical Methods for Rates and Proportions, 2nd ed. New York: John Wiley and Sons, 1981:17.
- Araoye MO, editor. Research Methodology with Statistics for Health and Social Sciences. Ilorin, Nigeria: Nathadex Publishers, 2004:117-20.
- Collins JJ, Devine TD, Dick GS, et al. The measurement of symptoms in young children with cancer: the validation of the Memorial Symptom Assessment Scale in children aged 7-12. J Pain Symptom Manage 2002;23:10-6. [Crossref] [PubMed]
- Collins JJ, Byrnes ME, Dunkel IJ, et al. The measurement of symptoms in children with cancer. J Pain Symptom Manage 2000;19:363-77. [Crossref] [PubMed]
- Portenoy RK, Thaler HT, Kornblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer 1994;30A:1326-36. [Crossref] [PubMed]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement 1977;1:385-401. [Crossref]
- Eaton WW, Muntaner C, Smith C, et al. Center for Epidemiologic Studies Depression Scale: Review and revision (CESD and CESD-R). In: Maruish ME, editor. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment. 3rd ed. Mahwah, NJ: Lawrence Erlbaum, 2004:363-77.
- Olagunju AT, Aina OF, Fadipe B. Screening for depression with Centre for Epidemiological Studies Depression Scale Revised and its implication for consultation-liaison psychiatry practice among cancer subjects: a perspective from a developing country. Psychooncology 2013;22:1901-6. [Crossref] [PubMed]
- Olagunju AT, Aina OF. A controlled study of depression among attendees of an oncology clinic in West Africa. Int J Psychiatry Med 2011;42:339-52. [Crossref] [PubMed]
- Statistical Package for Social Sciences (SPSS) Statistics for Windows, Version 20.0. Chicago: SPSS Inc., 2011.
- Gillespie JF, Primavera J, editors. Diverse families, competent families: innovations in research and preventive intervention practice. New York: Haworth Press, 2000.
- National Health Insurance Scheme. 2014. Available online: . Accessed 01/09/2015.www.nhis.gov.ng
- Kazak AE, Boeving CA, Alderfer MA, et al. Posttraumatic stress symptoms during treatment in parents of children with cancer. J Clin Oncol 2005;23:7405-10. [Crossref] [PubMed]
- Akpan-Idiok PA, Anarado AN. Perceptions of burden of caregiving by informal caregivers of cancer patients attending University of Calabar Teaching Hospital, Calabar, Nigeria. Pan Afr Med J 2014;18:159. [Crossref] [PubMed]
- Shehu UA, Adegoke SA, Abdulsalam U, et al. Pattern of childhood malignant tumours in two tertiary teaching hospitals in Nigeria: comparative study. Niger J Paed 2013;40:175-8.
- Amoran O, Lawoyin T, Lasebikan V. Prevalence of depression among adults in Oyo State, Nigeria: a comparative study of rural and urban communities. Aust J Rural Health 2007;15:211-5. [Crossref] [PubMed]
- Iseri PK, Ozten E, Aker AT. Posttraumatic stress disorder and major depressive disorder is common in parents of children with epilepsy. Epilepsy Behav 2006;8:250-5. [Crossref] [PubMed]
- Fauman KR, Pituch KJ, Han YY, et al. Predictors of depressive symptoms in parents of chronically ill children admitted to the pediatric intensive care unit. Am J Hosp Palliat Care 2011;28:556-63. [Crossref] [PubMed]
- Fagnano M, Berkman E, Wiesenthal E, et al. Depression among caregivers of children with asthma and its impact on communication with health care providers. Public Health 2012;126:1051-7. [Crossref] [PubMed]
- Singer GH. Meta-analysis of comparative studies of depression in mothers of children with and without developmental disabilities. Am J Ment Retard 2006;111:155-69. [Crossref] [PubMed]
- Semple D, Symth R, Burns J, et al, editors. Oxoford handbook of psychiatry, 2nd ed. Oxford: Oxford University Press, 2005.
- Mandac C, Battista V. Contributions of palliative care to pediatric patient care. Semin Oncol Nurs 2014;30:212-26. [Crossref] [PubMed]
- Hannon B, Zimmermann C, Knaul FM, et al. Provision of Palliative Care in Low- and Middle-Income Countries: Overcoming Obstacles for Effective Treatment Delivery. J Clin Oncol 2016;34:62-8. [Crossref] [PubMed]