
Association of baseline blood glucose levels with 30-day mortality in patients with acute kidney injury: a retrospective cohort study
Introduction
Acute kidney injury (AKI) is a common organ dysfunction in critically ill patients. It has been reported that the incidence of AKI is 57.3% in the intensive care unit (ICU). AKI increases the risk of death; AKI inpatients have been reported to have a mortality rate of 26.9% (1), and a 90-day mortality rate as high as 35% (2). The AKI severity, duration and renal recovery, lower baseline renal function, male, older age, and comorbidities (diabetes, hypertension, cardiovascular disease, tumor) are all increase the risk of death (3). The incidence of AKI in diabetic patients has been reported to be 2.8 times that of non-diabetic patients (4). Diabetes is a metabolic disease characterized by hyperglycemia.
Glucose is the main carbon source for cell biosynthesis and energy production and plays a key role in cell growth. However, excessively high or low blood sugar levels can affect human health. Hyperglycemia (>11 mmol/L) increases the incidence and mortality of contrast-induced AKI (5), and hypoglycemia (<4.2mmol/L) increase the incidence and mortality of AKI after cardiac surgery (6). A retrospective cohort study of all hospitalized patients revealed that hyperglycemia on admission was closely associated with the incidence and 30-day mortality of AKI (7). These studies only analyzed the effects of simple hyperglycemia or hypoglycemia on the morbidity and mortality of AKI, but did not study the relationship between different blood glucose levels and mortality in patients with AKI, nor explored the optimal levels of glycemic control. To solve these problems, we investigated the relationship between the baseline blood glucose level and 30-day mortality in AKI patients, and analyzed the reasonable level of blood glucose control in patients with AKI. We present the following article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1049/rc).
Methods
Cohort
Adult patients from the Medical Information Mart for Intensive Care III (MIMIC-III) database (https://mimic.mit.edu) were included in this single-center, retrospective cohort study. The MIMIC-III database collected detailed information on the daily clinical procedures of over 60,000 ICU inpatients at Beth Israel Deaconess Medical Center (BIDMC) in Boston, Massachusetts, United States of America from 2001 to 2012. MIMIC-III was connected to the social security database to obtain information about out-of-hospital deaths (8). Data from all the patients in the MIMIC-III database have been divided into separate lists, are freely available for download by researchers, and have been widely used in the development of predictive models (9,10). An author of our current study had completed a series of courses offered by the National Institutes of Health, passed the examinations, and was thus authorized to use the relevant information from the MIMIC-III database (certification number: 40764077). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The use of the MIMIC database was approved by the Institutional Review Board of Massachusetts Institute of Technology and BIDMC, both of which waive the need for informed consent for studies related to the MIMIC-III database; thus, our current study did not need an approval from the ethics committee of our own center.
Subject screening
To be eligible for inclusion in this study, patients had to meet the following inclusion criteria: (I) had been admitted to the ICU as per the MIMIC-III database from June 2001 to October 2012; and (II) had experienced AKI within 48 hours of admission. AKI was diagnosed according to the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines as follows: an increase in serum creatinine by ≥0.3 mg/dL within 48 h, or an increase in serum creatinine to ≥1.5 times the baseline level, or a urine volume <0.5 mL/(kg·h) for ≥6 h (11). Patients were excluded from the study if they met any of the following exclusion criteria: (I) had not been admitted to the ICU for the first time; (II) were aged <18 years; (III) had a previous history of chronic kidney disease; (IV) had not completed blood glucose testing within the 1st day of ICU admission or had an extremely abnormal blood glucose value on the 1st measurement; and/or (V) had an inaccurately recorded time of death.
Variables and data
The baseline variables extracted from the MIMIC-III database included gender, age, weight, service unit, vital signs, Oxford Acute Severity of Illness Score (OASIS) score, laboratory test results (within the first day after admission), comorbidities, and life-support measures. The service units included the medical ICU (MICU), surgical/trauma surgical ICU (SICU/TSICU), and coronary care unit (CCU)/cardiac surgery recovery unit (CSRU). The vital signs included heart rate, mean arterial pressure, respiratory rate, body temperature, and oxygen saturation. The comorbidities included congestive heart failure (CHF), hypertension, chronic obstructive pulmonary disease (COPD), diabetes mellitus, liver disease, and malignancy. Life-support measures (within 24 hours) included vasopressor use, renal replacement therapy (RRT), and mechanical ventilation (MV). The laboratory tests included measurements of the white blood cell (WBC) count, hemoglobin, the platelet count, prothrombin time, activated partial thromboplastin time, blood potassium, blood sodium, anion gap, creatinine, and glucose levels.
The data were queried and extracted using Structured Query Language (SQL), and the software used was the open-source PostgreSQL (v9.6) and its GUI software Navicat (v.12.1.11 [64-bit] Premium).
Statistical analysis
The normally distributed baseline measurement data are expressed as the mean ± standard deviation (), and the non-normally distributed data are presented as the median (interquartile). The count data are expressed as the frequency with percentage (%). Multiple interpolation was applied for missing data. The patients were divided equally into four groups according to the 25th, 50th, and 75th percentiles of blood glucose. For the analysis of the baseline features, the statistical differences in the continuous variables among the four groups were analyzed using a 1-way analysis of variance or Kruskal-Wallis H test, while the categorical variables were analyzed using a chi-square test. Hazard ratios (HR) and 95% confidence intervals (CIs) for deaths in the different blood glucose groups were calculated using a multivariate Cox regression analysis. To control for confounding factors, we input the covariates into the Cox regression model one by one in the basic model or eliminated the covariates one by one in the complete model. The regression coefficients were compared, and the covariates that changed the regression coefficient by 10% were included in the Cox regression analysis for adjustment. Eventually, weight, service unit, respiratory rate, pulse oxygen saturation (SpO2), Oxford Acute Severity of Illness Score (OASIS) score, activated partial thromboplastin time (APTT), prothrombin time (PT), Anion gap, and diabetes mellitus were included in the adjusted model. Gender and age were usually mandatory adjustment covariates. The Kaplan-Meier method (log-rank test) was used to compare survival among patients in these four groups. A generalized additive model was used to identify the non-linear relationship between blood glucose and the 30-day mortality rate. A 2-segment linear regression model was created based on the smoothed curves. The inflection point of blood glucose was determined using a recursion algorithm, including the selection of the inflection point along a predefined interval to generate the maximum likelihood value. The comparison of the 2-segment linear regression model and the 1-segment linear model was performed using the log-likelihood ratio test in the segmented package of R Language (12). All the statistical analyses were performed in the R Language (https://www.r-project.org, The R Foundation) and Free Statistics software. All the P values reported are 2-tailed, and P values <0.05 were considered statistically significant.
Sensitivity analysis
To further analyze the effects of comorbidities and major treatments on the study outcomes, subgroup analyses were performed in terms of comorbidities (including CHF, hypertension, and COPD) and RRT to assess the stability of the research results. At the same time, the stability of Cox regression models before and after interpolation data was analyzed.
Results
Subjects and baseline features
The data of 23,119 AKI patients admitted to the ICU within 48 hours were retrieved from the MIMIC-III database. After the exclusion of 4,886 cases of non-first-time ICU admissions, 795 cases of patients aged <18 years, 2,716 cases of patients with chronic kidney disease before admission, 70 cases of patients with inaccurately recorded times of death, and 203 cases of patients for whom blood glucose measurements were not taken on the 1st day of ICU admission or whose 1st measurement value was extremely abnormal, 14,449 patients were included in the final analysis. All the patients completed 30-day follow-up (see Figure 1).
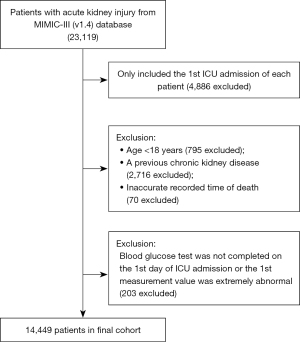
Patients were divided into the following four groups according to their blood glucose levels: (I) Q1 group (blood glucose ≤6.36 mmol/L); (II) Q2 group (blood glucose: 6.36–7.35 mmol/L); (III) Q3 group (blood glucose: 7.35–8.89 mmol/L); and (IV) Q4 group (blood glucose ≥8.89 mmol/L). The demographic features (gender, age, and weight), the service unit, vital signs, OASIS score, laboratory examination results, comorbidities, and life support measures are summarized in Table 1.
Table 1
| Variables | All patients (n=14,449) | Baseline blood glucose (mmol/L) | P value | |||
|---|---|---|---|---|---|---|
| Q1 (n=3,611) | Q2 (n=3,606) | Q3 (n=3,609) | Q4 (n=3,623) | |||
| Gender, n (%) | <0.001 | |||||
| Female | 6,102 (42.2) | 1,517 (42.0) | 1,416 (39.3) | 1,535 (42.5) | 1,634 (45.1) | |
| Male | 8,347 (57.8) | 2,094 (58.0) | 2,190 (60.7) | 2,074 (57.5) | 1,989 (54.9) | |
| Age (years), mean ± SD | 73.0±50.8 | 71.2±52.0 | 73.2±49.5 | 75.2±52.8 | 72.5±48.8 | 0.008 |
| Weight (kg), M (IQR) | 78.8 (66.0, 93.0) | 75.0 (63.2, 88.8) | 78.8 (66.0, 92.2) | 80.0 (67.0, 94.4) | 80.5 (68.0, 96.1) | <0.001 |
| Service unit, n (%) | <0.001 | |||||
| MICU | 5,648 (39.1) | 1,720 (47.6) | 1,050 (29.1) | 1,191 (33.0) | 1,687 (46.6) | |
| SICU/TSICU | 3,919 (27.1) | 882 (24.4) | 837 (23.2) | 1,203 (33.3) | 997 (27.5) | |
| CCU/CSRU | 4,882 (33.8) | 1,009 (27.9) | 1,719 (47.7) | 1,215 (33.7) | 939 (25.9) | |
| Vital signs | ||||||
| Heart rate (bpm), mean ± SD | 87.6±16.0 | 86.7±16.5 | 86.7±14.9 | 87.8±16.0 | 89.3±16.5 | <0.001 |
| MAP (mmHg), mean ± SD | 77.3±11.1 | 76.5±11.8 | 76.8±10.2 | 77.5±10.8 | 78.3±11.5 | <0.001 |
| Respiratory rate (bpm), mean ± SD | 19.0±4.1 | 19.0±4.2 | 18.5±3.9 | 19.0±4.0 | 19.6±4.3 | <0.001 |
| Temperature (℃), mean ± SD | 36.9±0.7 | 36.9±0.6 | 36.9±0.6 | 36.9±0.7 | 36.8±0.8 | 0.002 |
| SpO2 (%), M (IQR) | 97.7 (96.3, 98.8) | 97.5 (96.2, 98.7) | 97.8 (96.6, 98.8) | 97.7 (96.4, 98.8) | 97.6 (96.0, 98.8) | <0.001 |
| OASIS score, mean ± SD | 33.5±9.0 | 32.5±8.8 | 32.6±8.2 | 34.1±8.9 | 34.7±9.7 | <0.001 |
| Laboratory tests | ||||||
| WBC (×109/L), M (IQR) | 9.7 (6.9, 13.3) | 8.7 (6.2, 12.1) | 9.7 (7.1, 12.9) | 10.1 (7.2, 13.6) | 10.5 (7.4, 14.3) | <0.001 |
| Hemoglobin (g/dL), mean ± SD | 9.8±2.2 | 9.9±2.2 | 9.5±2.2 | 9.8±2.2 | 10.1±2.2 | <0.001 |
| Platelets (×109/L), mean ± SD | 244.4±125.9 | 242.2±131.6 | 233.2±118.1 | 248.5±123.9 | 253.7±128.6 | <0.001 |
| PT (seconds), M (IQR) | 29.1 (25.4, 34.3) | 29.6 (26.0, 35.3) | 29.8 (26.1, 34.4) | 28.4 (25.0, 33.2) | 28.5 (24.8, 34.0) | <0.001 |
| APTT (seconds), M (IQR) | 13.8 (12.8, 15.2) | 13.8 (12.8, 15.6) | 13.8 (12.9, 15.1) | 13.7 (12.8, 14.9) | 13.7 (12.8, 15.2) | <0.001 |
| Sodium (mmol/L), mean ± SD | 136.0±5.3 | 136.1±5.2 | 135.9±4.7 | 136.2±5.0 | 135.7±6.2 | 0.001 |
| Potassium (mmol/L), mean ± SD | 3.7±0.6 | 3.7±0.6 | 3.7±0.5 | 3.7±0.6 | 3.7±0.6 | <0.001 |
| Anion gap (mmol/L), mean ± SD | 13.2±3.5 | 13.1±3.7 | 12.4±3.2 | 13.1±3.4 | 14.0±3.7 | <0.001 |
| Creatinine (mg/dL), mean ± SD | 1.5±1.7 | 1.8±2.1 | 1.3±1.5 | 1.4±1.6 | 1.6±1.6 | <0.001 |
| Glucose (mmol/L), mean ± SD | 7.9±2.4 | 5.6±0.6 | 6.9±0.3 | 8.0±0.4 | 11.2±2.2 | <0.001 |
| Patients, n (%) | ||||||
| CHF | 3,979 (27.6) | 952 (26.4) | 961 (26.7) | 1,003 (27.8) | 1,063 (29.4) | 0.018 |
| Hypertension | 7,641 (52.9) | 1,774 (49.1) | 2,031 (56.3) | 1,942 (53.8) | 1,894 (52.3) | <0.001 |
| COPD | 2,898 (20.1) | 714 (19.8) | 690 (19.2) | 760 (21.1) | 734 (20.3) | 0.221 |
| Diabetes mellitus | 3,938 (27.3) | 521 (14.4) | 756 (21.0) | 945 (26.2) | 1,716 (47.4) | <0.001 |
| Liver disease | 1,835 (12.7) | 535 (14.8) | 359 (10.0) | 363 (10.1) | 578 (16.0) | <0.001 |
| Malignancy | 1,352 (9.4) | 383 (10.6) | 317 (8.8) | 310 (8.6) | 342 (9.5) | 0.014 |
| Vasopressor use (1st 24 h), n (%) | 3,521 (24.4) | 672 (18.6) | 811 (22.5) | 953 (26.5) | 1,085 (30.0) | <0.001 |
| ELF, n (%) | ||||||
| RRT use (1st 24 h) | 931 (6.4) | 323 (8.9) | 155 (4.3) | 207 (5.7) | 246 (6.8) | <0.001 |
| MV use (1st 24 h) | 7,894 (54.6) | 1,641 (45.4) | 2,233 (61.9) | 2,116 (58.6) | 1,904 (52.6) | <0.001 |
Q1: ≤6.36 mmol/L; Q2: 6.36–7.35 mmol/L; Q3: 7.35–8.89 mmol/L; Q4: ≥8.89 mmol/L. MICU, medical intensive care; SICU, surgical intensive care unit; TSICU, trauma surgical intensive care unit; CCU, coronary care unit; CSRU, cardiac surgery recovery unit; MAP, mean arterial pressure; SpO2, pulse oxygen saturation; M (IQR), median (interquartile); OASIS, Oxford Acute Severity of Illness Score; WBC, white blood cell; PT, prothrombin time; APTT, activated partial thromboplastin time; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; ELF, extracorporeal life support; RRT, renal replacement therapy; MV, mechanical ventilation.
Mortality risk of AKI patients with different blood glucose levels
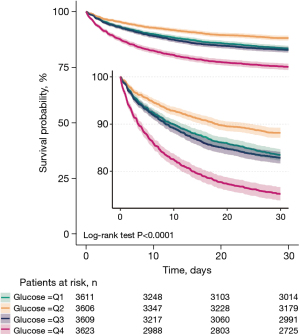
Up to 2,540 of 14,449 patients died within 30 days of ICU admission, yielding a mortality rate of 17.6%. Calculations of the HR and 95% CI for blood glucose and 30-day death in the 4 unadjusted and stepwise adjusted models showed that the risk of death was lowest in the Q2 group and highest in the Q4 group, but comparable between the Q3 group and the Q1 group. All the P values were <0.001 in the trend tests (see Table 2). The Kaplan-Meier survival curves and log-rank tests showed a significant difference in survival among the Q1, Q2, Q3, and Q4 groups (P<0.001), with the shortest survival time in the Q4 group and the longest survival time in the Q2 group (see Figure 2).
Table 2
| Exposure | Non-adjusted | Adjust I | Adjust II | Adjust III | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||||
| Glucose (mmol/L) | 1.09 (1.08–1.11) | <0.001 | 1.04 (1.01–1.07) | 0.004 | 1.02 (0.99–1.05) | 0.182 | 1.01 (0.98–1.04) | 0.480 | |||
| Glucose quintiles | |||||||||||
| Q1 | Reference | Reference | Reference | Reference | |||||||
| Q2 | 0.70 (0.62–0.79) | <0.001 | 0.70 (0.61–0.79) | <0.001 | 0.82 (0.72–0.93) | 0.002 | 0.89 (0.78–1) | 0.058 | |||
| Q3 | 1.04 (0.93–1.17) | 0.471 | 1.02 (0.92–1.15) | 0.667 | 1.09 (0.98–1.23) | 0.125 | 1.03 (0.92–1.16) | 0.594 | |||
| Q4 | 1.60 (1.44–1.78) | <0.001 | 1.60 (1.44–1.77) | <0.001 | 1.60 (1.44–1.78) | <0.001 | 1.41 (1.26–1.57) | <0.001 | |||
| P for trend | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
Q1: ≤6.36 mmol/L; Q2: 6.36–7.35 mmol/L; Q3: 7.35–8.89 mmol/L; Q4: ≥8.89 mmol/L. Non-adjusted: no variables were adjusted; Adjust I model: adjusted for age and gender; Adjust II model: adjusted for adjusts I + weight, service unit, respiratory rate, SpO2; Adjust III model: adjusted for adjusts II + OASIS, PT, APTT, anion gap, and diabetes mellitus. SpO2, pulse oxygen saturation; OASIS, Oxford Acute Severity of Illness Score; PT prothrombin time; APTT, activated partial thromboplastin time; HR, hazard ratio; CI, confidence interval.
Non-linear relationship between blood glucose and 30-day mortality in AKI patients
The multivariate Cox regression model and smoothed curve fitting revealed that blood glucose level had a U-shaped relationship with 30-day mortality, and the blood glucose inflection point was 5.52 mmol/L (see Figure 3). We fitted 2 different slopes with the segmented multivariate Cox regression models, and found that the P value of the likelihood ratio test was 0.001 (see Table 3). Thus, we used 2 segmented models to fit the association between the blood glucose levels and 30-day mortality. The effect value was 0.773 (HR =0.773; 95% CI: 0.614–0.975, P=0.030) for blood glucose <5.52 mmol/L; however, when blood glucose was ≥5.52 mmol/L, the effect value was 1.077 (HR =1.077; 95% CI: 1.059–1.097, P<0.001) (see Table 3).
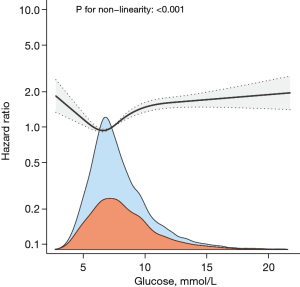
Table 3
| Threshold of driving pressure | HR | 95% CI | P value |
|---|---|---|---|
| <5.52 | 0.773 | (0.614, 0.975) | 0.030 |
| ≥5.52 | 1.077 | (1.059, 1.097) | <0.001 |
| Likelihood ratio test | – | – | 0.001 |
Adjusted for age, gender, weight, service unit, respiratory rate, SpO2, OASIS, PT, APTT, anion gap, and diabetes mellitus. SpO2, pulse oxygen saturation; OASIS, Oxford Acute Severity of Illness Score; PT, prothrombin time; APTT, activated partial thromboplastin time; HR, hazard ratio; CI, confidence interval.
Sensitivity analysis
Table S1 provides the proportion of missing data for all variables in Table 1. Table S2 shows the Cox model of the original data, which is consistent with the direction of the effect value after multiple interpolation data (see Table 2).
In the subgroup analysis, regardless of whether there was concomitant CHF, hypertension, and/or COPD, the Q4 group had the highest risk of death, and the Q2 group had the lowest risk of death. The risk of death in the Q1 group and the Q3 group was similar and between the former two groups. All the P values were <0.001 in the trend tests. The subgroup analysis also revealed a correlation between RRT and 30-day mortality (P=0.018; see Table 4).
Table 4
| Confounding factor category | Blood glucose quintiles (mmol/L) | P for trend | P for interaction | |||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| CHF | 0.887 | |||||
| No | 1 (Ref) | 0.91 (0.77–1.06) | 1.01 (0.87–1.17) | 1.40 (1.21–1.60) | <0.001 | |
| Yes | 1 (Ref) | 0.87 (0.69–1.09) | 1.12 (0.91–1.38) | 1.56 (1.28–1.90) | <0.001 | |
| Hypertension | 0.315 | |||||
| No | 1 (Ref) | 0.95 (0.79–1.13) | 1.02 (0.87–1.20) | 1.43 (1.22–1.67) | <0.001 | |
| Yes | 1 (Ref) | 0.85 (0.70–1.03) | 1.06 (0.89–1.27) | 1.44 (1.22–1.70) | <0.001 | |
| COPD | 0.316 | |||||
| No | 1 (Ref) | 0.96 (0.83–1.11) | 1.08 (0.95–1.24) | 1.46 (1.29–1.66) | <0.001 | |
| Yes | 1 (Ref) | 0.71 (0.53–0.96) | 0.92 (0.72–1.19) | 1.38 (1.08–1.77) | <0.001 | |
| RRT use | 0.018 | |||||
| No | 1 (Ref) | 0.90 (0.78–1.03) | 1.05 (0.93–1.19) | 1.50 (1.33–1.69) | <0.001 | |
| Yes | 1 (Ref) | 0.89 (0.58–1.36) | 0.91 (0.63–1.33) | 0.91 (0.64–1.31) | 0.64 | |
Q1: ≤6.36 mmol/L; Q2: 6.36–7.35 mmol/L; Q3: 7.35–8.89 mmol/L; Q4: ≥8.89 mmol/L. Adjusted for age, gender, weight, service unit, respiratory rate, SpO2, OASIS, PT, APTT, anion gap, and diabetes mellitus. SpO2, pulse oxygen saturation; OASIS, Oxford Acute Severity of Illness Score; PT, prothrombin time; APTT, activated partial thromboplastin time; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; RRT, renal replacement therapy.
Discussion
In this observational retrospective cohort study, we found a U-shaped relationship between the blood glucose levels and 30-day mortality in patients with AKI, with an inflection point of 5.52 mmol/L. When the blood glucose level was <5.52 mmol/L, the 30-day mortality rate of AKI patients decreased by 22.7% for every 1-mmol/L increase in blood glucose (HR =0.773; 95% CI: 0.614–0.975, P=0.030). Conversely, when the blood glucose level was ≥5.52 mmol/L, the 30-day mortality rate increased by 7.7% for every 1-mmol/L increase in blood glucose (HR =1.077; 95% CI: 1.059–1.097, P<0.001). Thus, blood sugar must be maintained at a reasonable level as both hyperglycemia and hypoglycemia increase the risk of death in AKI patients. These findings support those of the Normoglycemia in Intensive Care Evaluation-Survival Using Glucose Algorithm Regulation (NICE-SUGAR) study (13).
According to the Kidney Disease Improving Global Outcomes (KDIGO) guidelines (11), AKI can be divided into stages 1, 2, and 3, corresponding to the increasing severity and increasing risk of death (1,14). AKI has a variety of etiologies, and its outcome is also related to multiple factors. Dysglycemia is closely related to the case-fatality rate of AKI after contrast agent administration (5) or cardiac surgery (6). These two studies involved special populations. Gorelik et al. (7) analyzed the data of 6,170 AKI inpatients at their center from 2012 to 2021 and found that hyperglycemia (>10 mmol/L) on admission was positively correlated with the incidence of AKI, the recovery rate of AKI, and the 30-day mortality rate (7). However, none of the above studies researched the relationship between different blood glucose levels and mortality in patients with AKI and proposed an optimal range for glycemic control. The subjects in our current study were ICU inpatients, and the results showed that the baseline blood glucose level in AKI patients had a U-shaped relationship with the 30-day mortality rate, and the Q2 group (6.36–7.35 mmol/L) had the lowest mortality risk.
Both hypoglycemia and hyperglycemia increase the risk of death in patients, but hypoglycemia is more harmful (15). Similar results were obtained in the current study. Kidneys play an important role in blood glucose metabolism, mainly through glucose reabsorption, gluconeogenesis, and kidney utilization of glucose (16). Research suggests that there are 2 etiologies for kidney injuries caused by elevated blood sugar: (I) during hyperglycemia emergencies (e.g., ketoacidosis and the hyperglycemic hyperosmolar state), the glucose filtered through the glomerulus exceeds the reabsorption capability of renal tubules, leading to osmotic diuresis, which in turn results in severe dehydration and rhabdomyolysis, thereby aggravating renal injury (17); or (II) hyperglycemia induces the apoptosis of renal tubular epithelial cells (18). Additionally, fluctuations in blood glucose also aggravate inflammatory lesions and the apoptosis of mouse mesangial cells (19). Hypoglycemia occurs when blood glucose falls <3.9 mmol/L, and patients may experience tachycardia, hunger, tremors, irritability, coma, and even death (15). For patients with hypoglycemia at hospital admission, the gluconeogenesis of the kidneys is notably weakened after AKI, and hypoglycemia is more likely to occur during hospitalization (20) and after discharge (21), thereby increasing the risk of death. Serraino et al. (6) showed that hypoglycemia (blood glucose <4.2 mmol/L) increased the morbidity and mortality rates of AKI in patients undergoing cardiac surgery. However, the question of whether hypoglycemia itself causes direct damage to the kidneys requires further investigation.
The target of glycemic control in AKI patients differs in different guidelines. The 2012 KDIGO guidelines recommended a glycemic control target of 6.1–8.3 mmol/L in AKI patients (11). The 2022 American Diabetes Association guidelines recommend a target blood glucose range of 7.8–10.0 mmol/L for most critically ill patients (22). In our current study, the Q2 group (6.36–7.35 mmol/L) had the lowest risk of death, which was similar to the target blood glucose range in the 2012 KDIGO guidelines but was quite different to that in the 2022 American Diabetes Association guidelines. Notably, since critically ill patients often have stress hyperglycemia (23), the baseline blood glucose in our current study was based on pooled data from both pre-hospital glycemic control and impact of critical illness and thus cannot be used as a direct reference value for post-hospital glycemic control.
In the subgroup analysis, the risk of death from high to low in patients without renal replacement therapy (RRT) was Q4 group, Q3 group, Q1 group and Q2 group. There was no significant difference in 30-day mortality among the four groups patients treated with RRT, regardless of baseline blood glucose values. It is suggested that RRT treatment reduces the risk of death in patients with hyperglycemia, which may be related to the maintenance of blood glucose balance by RRT (especially continuous renal replacement therapy).
Our present study had two advantages. First, it was a real-world study with a large sample size; all the data were naturally generated in daily patient care and thus are highly generalizable. Second, the baseline blood glucose in this study was the patient’s average blood glucose level on the 1st day after admission, which minimized the effect of immediate blood glucose fluctuations on the outcome. These two advantages also allow the good extrapolation of our findings.
However, our study also had some limitations. First, it had multiple confounding factors due to its retrospective cohort design. Second, some data were missing, resulting in incomplete sample inclusion; for example, in 203 patients, blood glucose tests were not performed within 24 hours after ICU admission or the results of the 1st test were extremely abnormal, and in 70 patients, the recorded time of death was incorrect. Third, the measurements were not strictly standardized in some cases. For example, finger prick or venous blood tests were performed in different cases, which might have led to measurement errors. Finally, only AKI patients were included in this analysis, and the association between blood glucose and mortality may be different in non-AKI patients.
Conclusions
There is a U-shaped relationship between the baseline blood glucose levels and the 30-day mortality rate in AKI patients. The blood glucose of patients with AKI should be controlled at a reasonable level and should not be lower than 5.52 mmol/L, and the optimal blood sugar control range warrants further investigations.
Acknowledgments
Funding: This article was supported by the Eighth Division of the Xinjiang Production and Construction Corps Shihezi Municipal Science and Technology Program (No. 2022RC05).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1049/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1049/coif). All authors report that this article was fully funded by the Eighth Division of the Xinjiang Production and Construction Corps Shihezi Municipal Science and Technology Program (No. 2022RC05) and the payments were made to Shihezi City People’s Hospital. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 2015;41:1411-23. [Crossref] [PubMed]
- Kellum JA, Romagnani P, Ashuntantang G, et al. Acute kidney injury. Nat Rev Dis Primers 2021;7:52. [Crossref] [PubMed]
- Gameiro J, Marques F, Lopes JA. Long-term consequences of acute kidney injury: a narrative review. Clin Kidney J 2020;14:789-804. [Crossref] [PubMed]
- Hapca S, Siddiqui MK, Kwan RSY, et al. The Relationship between AKI and CKD in Patients with Type 2 Diabetes: An Observational Cohort Study. J Am Soc Nephrol 2021;32:138-50. [Crossref] [PubMed]
- Lin KY, Shang XL, Guo YS, et al. Association of Preprocedural Hyperglycemia With Contrast-Induced Acute Kidney Injury and Poor Outcomes After Emergency Percutaneous Coronary Intervention. Angiology 2018;69:770-8. [Crossref] [PubMed]
- Serraino GF, Provenzano M, Jiritano F, et al. Risk factors for acute kidney injury and mortality in high risk patients undergoing cardiac surgery. PLoS One 2021;16:e0252209. [Crossref] [PubMed]
- Gorelik Y, Bloch-Isenberg N, Hashoul S, et al. Hyperglycemia on Admission Predicts Acute Kidney Failure and Renal Functional Recovery among Inpatients. J Clin Med 2021;11:54. [Crossref] [PubMed]
- Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data 2016;3:160035. [Crossref] [PubMed]
- Li X, Zheng R, Zhang T, et al. Association between blood urea nitrogen and 30-day mortality in patients with sepsis: a retrospective analysis. Ann Palliat Med 2021;10:11653-63. [Crossref] [PubMed]
- Cao B, Chen Q, Tang T, et al. Non-linear relationship between baseline mean arterial pressure and 30-day mortality in patients with sepsis: a retrospective cohort study based on the MIMIC-III database. Ann Transl Med 2022;10:872. [Crossref] [PubMed]
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179-84. [Crossref] [PubMed]
- Muggeo VMR. Segmented: an R package to fit regression models with broken-line relationships. R News 2008;8:20-5.
- NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009;360:1283-97. [Crossref] [PubMed]
- Poston JT, Koyner JL. Sepsis associated acute kidney injury. BMJ 2019;364:k4891. [Crossref] [PubMed]
- American Diabetes Association Professional Practice Committee. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022;45:S83-96. [Crossref] [PubMed]
- Wen L, Li Y, Li S, et al. Glucose Metabolism in Acute Kidney Injury and Kidney Repair. Front Med (Lausanne) 2021;8:744122. [Crossref] [PubMed]
- Advani A. Acute Kidney Injury: A Bona Fide Complication of Diabetes. Diabetes 2020;69:2229-37. [Crossref] [PubMed]
- Peng J, Li X, Zhang D, et al. Hyperglycemia, p53, and mitochondrial pathway of apoptosis are involved in the susceptibility of diabetic models to ischemic acute kidney injury. Kidney Int 2015;87:137-50. [Crossref] [PubMed]
- Xu WL, Liu S, Li N, et al. Quercetin Antagonizes Glucose Fluctuation Induced Renal Injury by Inhibiting Aerobic Glycolysis via HIF-1α/miR-210/ISCU/FeS Pathway. Front Med (Lausanne) 2021;8:656086. [Crossref] [PubMed]
- Khanimov I, Shimonov M, Wainstein J, et al. Hypoglycemia, Malnutrition and Body Composition. Adv Exp Med Biol 2021;1307:71-84. [Crossref] [PubMed]
- Hung AM, Siew ED, Wilson OD, et al. Risk of Hypoglycemia Following Hospital Discharge in Patients With Diabetes and Acute Kidney Injury. Diabetes Care 2018;41:503-12. [Crossref] [PubMed]
- American Diabetes Association Professional Practice Committee. 16. Diabetes Care in the Hospital: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022;45:S244-53. [Crossref] [PubMed]
- Olariu E, Pooley N, Danel A, et al. A systematic scoping review on the consequences of stress-related hyperglycaemia. PLoS One 2018;13:e0194952. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


