Olanzapine for the prophylaxis and rescue of chemotherapy-induced nausea and vomiting (CINV): a retrospective study
Introduction
Chemotherapy-induced nausea and vomiting (CINV) is a well-documented adverse effect for patients undergoing chemotherapy treatment (1-5). Left untreated, CINV may result in serious complications, thus potentially compromising outcome if dose reduction or discontinuation of therapy is required (6). At present, current antiemetic guidelines recommend that a three-drug combination of a 5-HT3 antagonist, dexamethasone, and neurokinin 1 (NK-1) inhibitors be used in the prophylaxis of acute CINV in the highly emetogenic chemotherapy (HEC) settings, while a two-drug combination of palonosetron and dexamethasone is recommended in the moderately emetogenic chemotherapy settings (MEC) (7,8). For delayed CINV, a combination of dexamethasone and aprepitant is recommended in the HEC settings, while dexamethasone or aprepitant can be used in MEC settings (7,8). However, despite prophylactic treatment, CINV may nevertheless arise. Failure to control CINV with the aid of prophylactic antiemetics is defined as breakthrough CINV (8), and at present, many medications are recommended by clinical guidelines but no specific agent for this type of CINV is preferred (8).
Olanzapine is an antipsychotic medication with clinically observed antiemetic effects (9). The Food and Drug Administration (FDA) approved drug blocks multiple neurotransmitters: dopamine at D1, D2, D3 and D4 brain receptors, serotonin at 5-HT2a, 5-HT2c, 5-HT3, and 5-HT6 receptors, catecholamines at alpha-1 adrenergic receptors, acetylcholine at muscarinic receptors, and histamine at H1 receptors (10).
Since 2000, when a case report first documented the efficacy of olanzapine in relieving the chronic nausea of a leukemia patient (11), several phase I and II studies (12-18), other randomized controlled trials (RCTs) (19-23), and recent systematic reviews and meta-analyses (24,25), have been published which document the effective use of olanzapine as a prophylactic antiemetic in controlling CINV. However, only two studies have documented the use of olanzapine for the rescue of breakthrough CINV (26,27).
With the relatively few number of studies in the literature documenting olanzapine in the breakthrough setting, the primary objective of this study was to determine the efficacy and safety of olanzapine when given as a rescue medication to patients who experience breakthrough CINV. The secondary objective was to determine the efficacy and safety of olanzapine as a prophylaxis treatment of CINV.
Methods
Study design and patient selection
Following approval from the Toronto Academic Health Sciences Network (TAHSN) Research Ethics Board, electronic medical records of adult patients aged >17 years receiving a prescription for olanzapine from the Odette Cancer Centre Pharmacy at Sunnybrook Hospital between January 2013 and June 2015 were reviewed retrospectively. Inclusion criteria required receiving one or more doses of olanzapine for the rescue or prophylaxis of CINV and documentation of the outcome. In the prophylactic setting, olanzapine was administered for the first three days after chemotherapy, starting on the day of chemotherapy, twice a day. In the breakthrough setting, each patient must have received some form of standard prophylactic antiemetic before the administration of a first-line rescue antiemetic. First-line rescue antiemetic is defined as the subsequent antiemetic medication given after clinical failure of prophylactic antiemetics. Olanzapine was administered as the first-line rescue antiemetic for the majority of patients. Other patients may have used prochlorperazine as first-line rescue medication, but switched to olanzapine because of clinical failure or the medication induced side effects severe enough to warrant discontinuation by the patient. Olanzapine administration for breakthrough CINV must have been given after clinical failure of prophylactic antiemetics and/or failure of non-olanzapine containing first-line rescue. In the rescue setting, each medication was always administered separately and sequentially. Patients were excluded if olanzapine was used before chemotherapy.
In both the rescue and prophylactic settings, the primary outcome measure was the percentage of patients who took olanzapine and found it to improve nausea or vomiting. In both the nausea and vomiting endpoints, failure was defined as the patient indicating CINV to be same or worse upon olanzapine use. Secondary outcome measures included the percentage of patients who took olanzapine and developed side effects.
Patients were stratified by chemotherapy emetogenicity level and type, number of prophylactic antiemetics received, dosage of olanzapine used, treatment intent, as well as by risk factors such as age, gender, and alcohol consumption. Chemotherapy emetogenicity levels were separated into high, medium and low potentials, prophylactic anti-emetics divided into groups of single, double, or triple agents, dosage of olanzapine was examined in either 2.5 or 5 mg, and treatment intent into adjuvant, neoadjuvant, palliative, or curative. Risk factors were separated into age younger than 55 versus age 55 years or older and alcohol use less than 5 drinks/week versus use of 5 drinks/week or more.
Assessment procedures
At the Odette Cancer Centre, patients are followed up with a phone call by a pharmacist or pharmacy research assistant 72 hours after every cycle of chemotherapy. Any outcomes of CINV for the initial cycle after this 72-hour period would be made in the next treatment cycle in the hospital or within the 72 hour period follow-up in the next cycle. An assessment of CINV was documented in the patient’s electronic medical records. In both the prophylactic and breakthrough setting, patients were asked whether they experienced “improved”, “worse”, or “same” CINV after taking olanzapine.
Statistical methods
Descriptive analysis was conducted using median and ranges for continuous variables and proportions for categorical variables. To compare olanzapine outcomes on clinical factors, Wilcoxon rank-sum (for two categories of olanzapine outcomes) or Kruskal-Wallis (for >2 categories of olanzapine outcomes) nonparametric test was used for continuous variables and Chi-squared test or Fisher exact test was used as appropriate for categorical variables. Two-sided P value <0.05 was considered statistically significant. All analyses were performed by Statistical Analysis Software (SAS version 9.4 for Windows).
Results
Patient characteristics
A total of 170 patients and 213 treatment cycles were included: 142 women and 28 men with a mean age of 50.9 years (range, 19–77) (Table 1). The most common primary cancer site was breast cancer (n=97, 57%) (Table 1). Other demographic information, including baseline risk factors such as alcohol and caffeine consumption, can be found in Table 1.
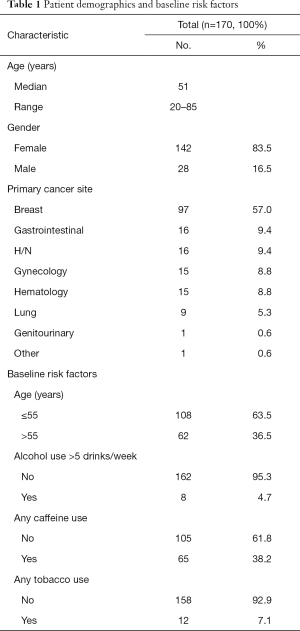
Full table
Efficacy and safety parameters in the breakthrough setting
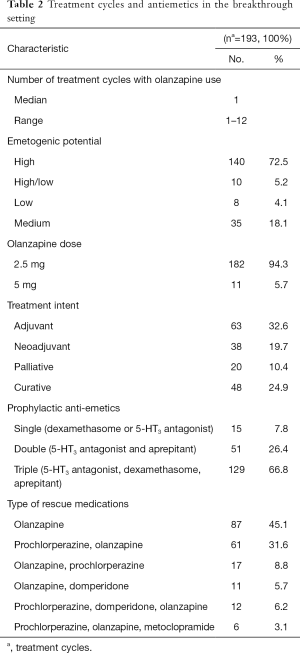
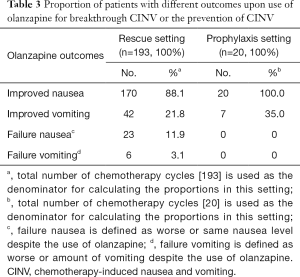
A total of 154 patients and 193 treatment cycles were included in the breakthrough setting (Table 2). Table 3 documents the proportion of patients with different olanzapine outcomes for nausea and vomiting in the breakthrough setting.
Full table
Full table
Subgroup analysis revealed that the effectiveness of olanzapine for breakthrough CINV was not dependent on the cycle it starts on (P=0.3), nor was it dependent on the emetogenic potential (P=0.1), the treatment intent (P=0.2), or the type (P=0.3) of the chemotherapy regimen. In addition, there is no significant relationship between having a single, double, or triple prophylactic antiemetic regimen and subsequent olanzapine outcomes (P=0.4). In addition, there was no significant difference on side effects or outcomes between the use of a 2.5 or 5 mg dose of olanzapine. No significant relationship between baseline demographics and different olanzapine outcomes were found either.
Efficacy and safety parameters in the prophylactic setting
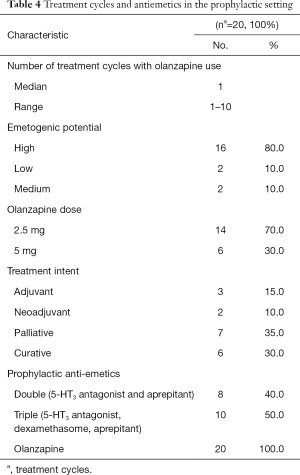
A total of 16 patients and 20 treatment cycles were included in the prophylaxis setting (Table 4). In all 16 patients and 20 cases, olanzapine use was indicated by patients to have improved nausea in the prophylactic setting, while 7 cases (35%) achieved improved vomiting (Table 3). A total of 65% of the cases indicated the feeling of sedation after olanzapine use, while 35% of the cases indicated experiencing constipation (Table 5).
Full table

Full table
As in the breakthrough setting, subgroup analysis revealed that the effectiveness of olanzapine for the prevention of CINV was not dependent on the cycle it starts on (P=0.98), the emetogenic potential (P=0.6), the treatment intent (P=0.9), or the type (P=0.4) of the chemotherapy regimen. Moreover, there was no significant relationship between having a single, double, or triple prophylactic antiemetic regimen and subsequent olanzapine outcomes (P=0.5). Although there was no significant difference between the use of a 2.5 or 5 mg dose of olanzapine with respect to the antiemetic outcome or the incidence of constipation, there was a significant difference between the two doses with regards to the incidence of sedation. The use of a 5 mg olanzapine dose was associated with significantly higher proportions of sedation compared to a 2.5 mg dose (P=0.03).
Discussion
The efficacy of olanzapine in the prophylaxis of CINV has been documented (12-23). However, literature on the use of olanzapine as a rescue medication for breakthrough CINV has been scarce, with only two studies investigating the use of olanzapine for this purpose (26,27). As such, we focus on the two articles by Chanthawong et al. (26) and Navari et al. (27) as a framework for our discussion.
The current study retrospectively details the efficacy and safety of olanzapine for the treatment of breakthrough CINV. It is the largest study of this nature to date, with a total of 154 patients and 193 treatment cycles in the breakthrough setting. With approximately 88% of cases indicating that olanzapine improved breakthrough nausea, the proportion in this study is, as expected, higher than the 68% of patients in the Navari study that indicated no nausea (27), and the complete response in nausea rate of 50% reported by Chanthawong et al. (26). The proportion of improved vomiting (22%) in our study is considerably lower than the no emesis (70%) and complete response in emesis (60.9%) rates reported by Navari et al. and Chanthawong et al., respectively. In the Chanthawong study, complete response in nausea was as no emetic episodes, no rescue therapy and no nausea, while the complete response in emesis was defined as no emetic episodes and no rescue therapy. It should be noted that the doses used by Navari et al. and Chanthawong et al. were 10 mg daily and 5 mg twice a day, respectively. Given that the methodology used in this study is different from the ones used by Chanthawong et al. and Navari et al., the results are not comparable. Specifically, the study by Chanthawong et al. is a phase II prospective open label clinical trial that measured the rates of complete response for breakthrough emesis, retching and nausea using the Index of Nausea, Vomiting and Retching: INVR tool. The study by Navari et al. is a randomized phase III trial that measured rates of complete response using a visual analog scale from 0 to 10, with 0 indicating no nausea and 10 indicating a maximal level of nausea. On the other hand, the retrospective nature of this study and the limited information available from the electronic medical records limited this study’s ability to report on the more commonly used endpoints in antiemetic clinical trials such as no emesis and no nausea. Moreover, the proportions of improvement for nausea and vomiting may be under-estimated. As documentation is predicated on phone-call follow-up, improvement of CINV may not always be communicated by patients.
This study is the first to report the proportion of patients that experienced side effects in the breakthrough setting, with 42% of cases experiencing sedation and 32% experiencing constipation. With 65% of patients experiencing sedation in the prophylactic setting, this study reports the incidence of sedation to be lower than the 73% indicated by a study by Tan et al. (19). It should be noted that in our study, olanzapine was administered in smaller 2.5 or 5 mg doses than the 10 mg dose used by Tan et al. Our findings that a 2.5 mg dose of olanzapine in the prophylactic setting is associated with similar CINV outcomes, but a smaller incidence of sedation than a 5 mg dose further support the recommendation of dosage reduction.
While it is well known that the emetogenicity of chemotherapy administered, gender, age, as well as a history of low prior alcohol intake, can affect patients’ risk factors for CINV (28), it is relatively unknown whether the before mentioned baseline risk factors can affect the efficacy of olanzapine use. Our study found no significant differences between patients with baseline risk factors and those without, in both the prophylaxis and breakthrough settings. Our study also found that the effectiveness of olanzapine in both the prophylaxis and breakthrough settings is not dependent on the cycle it started on.
This study is limited by its retrospective design. Specifically, information on the intensity of breakthrough nausea or the number of breakthrough vomiting episodes before and after the use of olanzapine was not recorded, and thus, more standard definitions of failure in the treatment of breakthrough CINV could not be used. In addition, patients received different combinations of prophylactic anti-emetics that may have confounded the efficacy of olanzapine for breakthrough CINV. However, measures have been taken to minimize its limitations. For example, our analysis showed that there was no significant difference between receiving a single agent prophylactic anti-emetic (dexamethasone or a 5-HT3 antagonist), a double agent antiemetic (a 5-HT3 antagonist coupled with a NK-1 inhibitor), or a triple agent anti-emetic (a 5-HT3 antagonist, dexamethasone, and a NK-1 inhibitor) with respect to the efficacy of olanzapine as rescue medication for breakthrough CINV.
In conclusion, this study found olanzapine to be effective in improving CINV in both the prophylactic and breakthrough settings. The incidence of sedation in both settings was found to be lower than the only other reported incidence of sedation of 73%, though it should be noted that our study used lower doses. Given the similar benefit afforded by a 2.5 and 5 mg dose, we recommend the use of lower doses of olanzapine in both the prophylactic and breakthrough settings. The safety, efficacy, and appropriate dosage of olanzapine for the rescue of breakthrough CINV should be prospectively evaluated in a RCT.
Acknowledgements
We thank the generous support of Bratty Family Fund, Michael and Karyn Goldstein Cancer Research Fund, Joey and Mary Furfari Cancer Research Fund, Pulenzas Cancer Research Fund, Joseph and Silvana Melara Cancer Research Fund, and Ofelia Cancer Research Fund.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Toronto Academic Health Sciences Network (TAHSN) Research Ethics Board.
References
- Coates A, Abraham S, Kaye SB, et al. On the receiving end--patient perception of the side-effects of cancer chemotherapy. Eur J Cancer Clin Oncol 1983;19:203-8. [Crossref] [PubMed]
- Laszlo J. Nausea and vomiting as major complications of cancer chemotherapy. Drugs 1983;25 Suppl 1:1-7. [Crossref] [PubMed]
- Levine AM, Richardson JL, Marks G, et al. Compliance with oral drug therapy in patients with hematologic malignancy. J Clin Oncol 1987;5:1469-76. [PubMed]
- Lindley CM, Bernard S, Fields SM. Incidence and duration of chemotherapy-induced nausea and vomiting in the outpatient oncology population. J Clin Oncol 1989;7:1142-9. [PubMed]
- Richardson JL, Marks G, Levine A. The influence of symptoms of disease and side effects of treatment on compliance with cancer therapy. J Clin Oncol 1988;6:1746-52. [PubMed]
- Osoba D, Zee B, Warr D, et al. Effect of postchemotherapy nausea and vomiting on health-related quality of life. The Quality of Life and Symptom Control Committees of the National Cancer Institute of Canada Clinical Trials Group. Support Care Cancer 1997;5:307-13. [Crossref] [PubMed]
- Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2011;29:4189-98. [Crossref] [PubMed]
- Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 2010;21 Suppl 5:v232-43. [Crossref] [PubMed]
- Kast RE, Foley KF. Cancer chemotherapy and cachexia: mirtazapine and olanzapine are 5-HT3 antagonists with good antinausea effects. Eur J Cancer Care (Engl) 2007;16:351-4. [Crossref] [PubMed]
- Srivastava M, Brito-Dellan N, Davis MP, et al. Olanzapine as an antiemetic in refractory nausea and vomiting in advanced cancer. J Pain Symptom Manage 2003;25:578-82. [Crossref] [PubMed]
- Pirl WF, Roth AJ. Remission of chemotherapy-induced emesis with concurrent olanzapine treatment: a case report. Psychooncology 2000;9:84-7. [Crossref] [PubMed]
- Navari RM, Einhorn LH, Passik SD, et al. A phase II trial of olanzapine for the prevention of chemotherapy-induced nausea and vomiting: a Hoosier Oncology Group study. Support Care Cancer 2005;13:529-34. [Crossref] [PubMed]
- Passik SD, Navari RM, Jung SH, et al. A phase I trial of olanzapine (Zyprexa) for the prevention of delayed emesis in cancer patients: a Hoosier Oncology Group study. Cancer Invest 2004;22:383-8. [Crossref] [PubMed]
- Passik SD, Kirsh KL, Theobald DE, et al. A retrospective chart review of the use of olanzapine for the prevention of delayed emesis in cancer patients. J Pain Symptom Manage 2003;25:485-8. [Crossref] [PubMed]
- Passik SD, Lundberg J, Kirsh KL, et al. A pilot exploration of the antiemetic activity of olanzapine for the relief of nausea in patients with advanced cancer and pain. J Pain Symptom Manage 2002;23:526-32. [Crossref] [PubMed]
- Navari RM, Einhorn LH, Loehrer PJ Sr, et al. A phase II trial of olanzapine, dexamethasone, and palonosetron for the prevention of chemotherapy-induced nausea and vomiting: a Hoosier oncology group study. Support Care Cancer 2007;15:1285-91. [Crossref] [PubMed]
- Abe M, Kasamatsu Y, Kado N, et al. Efficacy of Olanzapine Combined Therapy for Patients Receiving Highly Emetogenic Chemotherapy Resistant to Standard Antiemetic Therapy. Biomed Res Int 2015;2015:956785.
- Abe M, Hirashima Y, Kasamatsu Y, et al. Efficacy and safety of olanzapine combined with aprepitant, palonosetron, and dexamethasone for preventing nausea and vomiting induced by cisplatin-based chemotherapy in gynecological cancer: KCOG-G1301 phase II trial. Support Care Cancer 2016;24:675-82. [Crossref] [PubMed]
- Tan L, Liu J, Liu X, et al. Clinical research of Olanzapine for prevention of chemotherapy-induced nausea and vomiting. J Exp Clin Cancer Res 2009;28:131. [Crossref] [PubMed]
- Mizukami N, Yamauchi M, Koike K, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting in patients receiving highly or moderately emetogenic chemotherapy: a randomized, double-blind, placebo-controlled study. J Pain Symptom Manage 2014;47:542-50. [Crossref] [PubMed]
- Wang X, Wang L, Wang H, et al. Effectiveness of Olanzapine Combined with Ondansetron in Prevention of Chemotherapy-Induced Nausea and Vomiting of Non-small Cell Lung Cancer. Cell Biochem Biophys 2015. [Epub ahead of print]. [PubMed]
- Mao WK, Peng L. Clinical observation of Olanzapine combined with Granisetrom and Hexadecadrol prevent nausea vomit induced by chemoradiontherapy. Chinese Journal of Medicine Guide 2011;13:452-4.
- Lu YL, Liu W, Du YJ, et al. Antiemetic effect of low dose olanzapine in solid tumor chemotherapy. Clin J Cancer Prev Treat 2013;20:544-54.
- Chow R, Chiu L, Navari R, et al. Efficacy and safety of olanzapine for the prophylaxis of chemotherapy-induced nausea and vomiting (CINV) as reported in phase I and II studies: a systematic review. Support Care Cancer 2016;24:1001-8. [Crossref] [PubMed]
- Chiu L, Chow R, Popovic M, et al. Efficacy of olanzapine for the prophylaxis and rescue of chemotherapy-induced nausea and vomiting (CINV): a systematic review and meta-analysis. Support Care Cancer 2016;24:2381-92. [Crossref] [PubMed]
- Chanthawong S, Subongkot S, Sookprasert A. Effectiveness of olanzapine for the treatment of breakthrough chemotherapy induced nausea and vomiting. J Med Assoc Thai 2014;97:349-55. [PubMed]
- Navari RM, Nagy CK, Gray SE. The use of olanzapine versus metoclopramide for the treatment of breakthrough chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy. Support Care Cancer 2013;21:1655-63. [Crossref] [PubMed]
- Navari RM. Management of chemotherapy-induced nausea and vomiting: focus on newer agents and new uses for older agents. Drugs 2013;73:249-62. [Crossref] [PubMed]


