
Leukemia cutis in T-cell acute lymphoblastic leukemia: a 3-year follow-up case report
Introduction
Leukemia cutis (LC) is an extramedullary manifestation of leukemia which refers to the cutaneous infiltration of neoplastic leukocytes. LC can be observed in various types of leukemia including chronic lymphocytic leukemia, acute myeloid leukemia, and, occasionally, acute lymphoblastic leukemia (1,2). Herein, we report a case of a female patient with T-cell acute lymphoblastic leukemia (T-ALL) who presented with LC and was in excellent clinical condition during a 3-year follow-up after treatment with chemotherapy and allogeneic stem cell transplantation. We present the following case in accordance with the CARE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-665/rc).
Case presentation
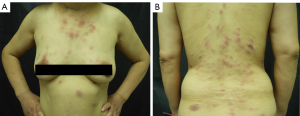
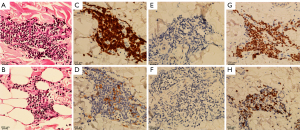
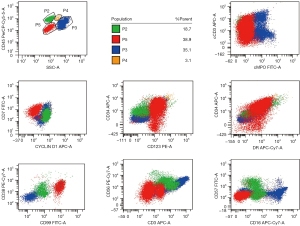
A 50-year-old Chinese woman presented with multiple infiltrated purplish plaques and tumors over the face, trunk, and lower extremities for more than 5 months (Figure 1), accompanied by recurrent fever and arthralgia. On general examination, we noticed bilateral palpable superficial lymph nodes in the axillary, cervical, and inguinal areas. The patient had a history of latent tuberculosis infection and had been taking the anti-tuberculosis drugs isoniazid and rifapentine. A skin biopsy from lesions in the sternal region showed a dense perivascular dermal and subcutaneous infiltrate composed of monomorphic cells (Figure 2A,2B). The medium-sized tumoral cells showed scant cytoplasm and a circular or irregular prominent nucleus with fine chromatin and inconspicuous nucleoli. The epidermis was uninvolved. Tumor lymphocytes were positive for CD3, partial CD4, partial CD10, CD7, LMO2, and terminal deoxynucleotidyl transferase (TdT), and negative for CD20, CD79a, CD1a, CD56, CD34, CD2, CD30, MPO, and CD117 (Figure 2C-2G). A high proliferative index (Ki67≈50%) was observed in the tumoral cells (Figure 2H), revealing the possible presence of lymphoblastic neoplasms. Anemia was notably discovered in the laboratory investigation, with a hemoglobin level of 10.5 g/Dl. Laboratory findings further revealed an increased percentage of lymphocytes in the peripheral blood (68.6%) and an elevated erythrocyte sedimentation rate (70 mm/h). Bone marrow smear examination indicated 85.5% abnormal lymphocytes, which were described as having dispersed chromatin, light blue-staining cytoplasm, and large nuclei in most cells. Flow cytometry revealed that these atypical cells expressed cCD3, CD7, CD123, CD34, CD38, HLA-DR, and CD99, and did not express CD56 and CD16 (Figure 3). Molecular studies were negative for BCR‐ABL fusion and MLL rearrangement. High CRLF2 expression and TERF2-Jak2 mutation were detected, which are usually identified in patients with high-risk T-ALL (3). Chromosomal analysis indicated 46, XX[9]. Positron emission tomography-computed tomography (PET-CT) results indicated glycolytic hypermetabolism in the cervical and bilateral supraclavicular lymph nodes, thymus gland, and spleen, as well as in the skin tumors of the trunk and lower extremities. The result of cerebrospinal fluid testing to investigate the involvement of the central nervous system was negative. The patient was ultimately diagnosed with T-ALL. She was subsequently referred to the department of hematology, where she was admitted for treatment with a VDLD regimen (vindesine, 4 mg/week ×4; daunorubicin 40 mg/m2/day ×3; L-asparaginase, 3,750 U/week ×2; dexamethasone, 10 mg/m2/day ×14) and allogeneic stem cell transplantation. No severe adverse events that could lead to the termination of treatment occurred. On the 10th day of chemotherapy, the skin lesions nearly disappeared. The patient was extremely satisfied with the treatment. She subsequently achieved a complete response and has not had disease recurrence in 3 years.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
LC involves the infiltration of neoplastic leukocytes in the skin. The sequence of the appearance of LC and the diagnosis of underlying leukemia varies. Most patients present with LC after leukemia has already been diagnosed or concurrently with systemic leukemia. On several occasions, skin involvement—which affects up to 7% of patients with leukemia—may precede bone marrow or peripheral blood involvement by several months or years (1). Importantly, LC may further signal the early relapse of leukemia (4).
The most common clinical lesions in LC present as papules, nodules, infiltrated plaques, and larger tumors that may be distributed over the scalp, face, trunk, and extremities (5). Nearly all skin lesions present with a purple or red appearance without obvious accompanying subjective symptoms such as pruritus and pain. Despite the characteristic clinical manifestations of LC, its diagnosis predominantly depends on histopathological features including the pattern of distribution, morphology, and immunophenotype of tumor cells. In T-ALL, a dense and diffuse monotonous infiltrate of medium-sized lymphoid cells appears in the dermis and even in the subcutaneous tissue. The epidermis is uninvolved, potentially differentiating primary cutaneous T-cell lymphoma. Tumor cells typically display T lineage-specific markers such as CD3, CD4, or CD8 and an immature T-cell phenotype including positive staining for one or more precursor markers such as TdT, CD99, CD34, and CD1a (1,4,6). Most notably, TdT shows higher sensitivity than CD99, CD34, and CD1a. Before making the ultimate diagnosis, both hematologic findings from the peripheral blood and bone marrow and the results of PET-CT should be considered. Additionally, as our patient presented with skin lesions as the initial symptom and the lymph nodes and bone marrow were subsequently found to be involved, other tumors with similar manifestations—such as blastic plasmacytoid dendritic cell neoplasm—should be excluded. The tumor cells in blastic plasmacytoid dendritic cell neoplasm are usually monomorphic, poorly differentiated, and intermediate-sized blasts with fine chromatin, which most importantly express CD4, CD56, and CD123 (7).
Although a few cases of LC in T-ALL were reported in the literature from 1999 to 2021 (4,6,8-11), only one neonate has been described with a follow-up of 2 years after treatment, and no detailed records of treatment and follow-up in adult patients have been found (11). This case is the first described with a favorable evolution after a 3-year follow-up. LC is considered an unfavorable prognostic factor in most situations. A recent study from the United States has shown that more than 80% of patients with LC patients die within one 1 year of diagnosis (12), and another international study indicated that the median survival time of patients with LC is 7.2 months (5). Furthermore, elevated CRLF2 expression is proven to be associated with poor outcomes in children and adults (3). Despite the evidence suggesting a poor prognosis, we describe a rare case where the patient with LC maintained good health for 3 years after receiving the combination of chemotherapy and allogeneic stem cell transplantation.
In summary, although uncommon, LC can be the first indication of the presence of T-ALL. Clinicians should consider the possibility of LC when skin lesions appear as multiple purplish red nodules, infiltrated plaques, or larger tumors without additional symptoms. Early identification of LC may allow for more rapid initiation of treatment and thus improved prognosis.
Acknowledgments
We would like to thank Anahid Pinchis and Kelly Zammit for their help in polishing our paper.
Funding: This work was supported by the National High Level Hospital Clinical Research Funding (No. 2022-PUMCH-A-164 to TW), the Beijing Natural Science Foundation (No. Z210017) and Peking Union Medical College Hospital (No. ZC201911051 to TW).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-665/rc
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-665/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-665/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wagner G, Fenchel K, Back W, et al. Leukemia cutis – epidemiology, clinical presentation, and differential diagnoses. J Dtsch Dermatol Ges 2012;10:27-36. [Crossref] [PubMed]
- Gupta L, Levoska MA, Sharma T, et al. Bilateral periorbital leukemia cutis presenting as suspected cellulitis. Orbit 2022;41:506-8. [Crossref] [PubMed]
- Palmi C, Savino AM, Silvestri D, et al. CRLF2 over-expression is a poor prognostic marker in children with high risk T-cell acute lymphoblastic leukemia. Oncotarget 2016;7:59260-72. [Crossref] [PubMed]
- Lee E, Park HJ, Cho BK, et al. Leukemia cutis as early relapse of T-cell acute lymphoblastic leukemia. Int J Dermatol 2010;49:335-7. [Crossref] [PubMed]
- Chang YW, Lee CH, Tseng HC. Leukemia cutis in a medical center in southern Taiwan: A retrospective study of 42 patients. J Formos Med Assoc 2021;120:226-33. [Crossref] [PubMed]
- Arora P, Sinha N, Malhotra P, et al. T-cell acute lymphoblastic leukemia with a rare chromosomal translocation presenting as leukemia cutis. Int J Dermatol 2022;61:e120-3. [Crossref] [PubMed]
- Adimora IJ, Wilson NR, Pemmaraju N. Blastic plasmacytoid dendritic cell neoplasm (BPDCN): A promising future in the era of targeted therapeutics. Cancer 2022;128:3019-26. [Crossref] [PubMed]
- Nohria A, Criscito MC, Weston GK, et al. Profound leukemia cutis in a patient with relapsed T-cell acute lymphoblastic leukemia. JAAD Case Rep 2021;18:51-3. [Crossref] [PubMed]
- Najem N, Zadeh VB, Badawi M, et al. Aleukemic leukemia cutis in a child preceding T-cell acute lymphoblastic leukemia. Pediatr Dermatol 2011;28:535-7. [Crossref] [PubMed]
- Chao SC, Lee JY, Tsao CJ. Leukemia cutis in acute lymphocytic leukemia masquerading as viral exanthem. J Dermatol 1999;26:216-9. [Crossref] [PubMed]
- Schlegel S, Hamm H, Reichel A, et al. Neonatal Acute Lymphoblastic Leukemia with t(9;11) Translocation Presenting as Blueberry Muffin Baby: Successful Treatment by ALL-BFM Induction Therapy, Allogeneic Stem Cell Transplantation from an Unrelated Donor, and PCR-MRD-Guided Post-Transplant Follow-Up. Am J Case Rep 2020;21:e927153. [Crossref] [PubMed]
- Su WP, Buechner SA, Li CY. Clinicopathologic correlations in leukemia cutis. J Am Acad Dermatol 1984;11:121-8. [Crossref] [PubMed]


