
Non-pharmacological interventions for cancer-related fatigue in terminal cancer patients: a systematic review and meta-analysis
Introduction
Cancer-related fatigue (CRF) is a very distressing symptom in patients with cancer (1,2). The symptoms can occur regardless of the type or stage of cancer (3). The frequency of fatigue is particularly high (>50%) in patients with terminal cancer 1–4 weeks before death (4).
According to the Oncology Nursing Society, exercise is effective in reducing CRF (5), but the high physical burden is not appropriate for terminally ill cancer patients due to their declining condition and physical symptoms such as pain and weakness of the legs and feet and mental symptoms such as delirium (4). Chapman’s review of patients with terminal cancer reported that realistic and staged goals, tailored to the individual, are necessary to help avoid unachievable targets for physical activity that can increase psychological distress or exacerbate symptoms (6). A previous review on fatigue associated with progressive diseases recommended physical exercise, energy conservation, and psychoeducational therapy (7). However, this review included patients with amyotrophic lateral sclerosis, end-stage renal disease (ESRD), end-stage renal failure, and liver cirrhosis, and was not limited to cancer. There is also insufficient evidence on the effect of reducing fatigue due to the lack of data.
The National Comprehensive Cancer Network (NCCN) guidelines state that fatigue should be screened, assessed, and managed according to clinical practice guidelines (8,9). In the NCCN categories of evidence and consensus, category 1 is “Based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate”. However, there is currently no category 1 evidence for end-of-life non-pharmacological interventions in the NCCN guidelines.
In Japan, Clinical Evidence for Complementary and Alternative Therapies in Cancer Patients was published by the Japanese Society for Palliative Medicine (JSPM) (10) in 2016. The JSPM guidelines provide evidence for various complementary and alternative therapies such as health foods, yoga, and massage. In addition, the guidelines are not limited to certain carcinomas or disease stages.
In principle, treatment of the cause of cancer-related malaise (e.g., side effects of chemotherapy, nutritional disorders, anaemia, and electrolyte abnormalities) is the mainstay of treatment for alleviating cancer-related malaise. However, the causes of malaise are often unclear in terminally ill patients, and the effects of pharmacotherapy are often insufficient.
Therefore, it is necessary to review the literature on non-pharmacological interventions for fatigue in terminally ill cancer patients to clarify their effects. This will contribute to the more effective use of non-pharmacological therapies to reduce malaise in terminally ill cancer patients. This study aimed to review and clarify the effects of non-pharmacological interventions on fatigue in terminally ill patients with cancer. We present the following article in accordance with the PRISMA reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-655/rc).
Methods
This study was registered in PROSPERO (registration number, CRD42021240358).
Eligibility criteria
Studies were eligible if they were originally reported in English; used only human participants; included all participants aged 18 years or older, of either sex, with advanced cancer; evaluated and reported the effects of a non-pharmacological intervention on symptoms related to fatigue; made comparisons between groups receiving treatment versus no treatment, alternative treatment, or both, as well as pre and post-intervention comparisons; and measured fatigue (including measurements based on a subscale or symptom scale). Both randomised controlled trials (RCTs) and non-RCTs were included to assess the beneficial effects and adverse events associated with the interventions. We excluded studies with no descriptions of terminal or advanced cancer, no context of palliative care, participants under 18 years old, and those that did not measure fatigue on a scale. We also excluded case reports, those managing symptoms with medication, and those that included cancer patients undergoing chemotherapy, radiotherapy, or surgery.
The primary outcome was the change in fatigue from baseline to the last available follow-up using any scale measured by the original authors.
Information sources and search strategy
Four electronic databases were searched to identify relevant studies (PsycINFO, CINAHL, MEDLINE via EBSCOhost, and Web of Science) published between January 2015 and March 2021. Unpublished literature is not included in this study. These dates were selected because the search period used by the JSPM to develop the complementary and alternative therapy guidelines (10) was 1 January 2000 to 31 December 2014. We conducted the search on 27 May 2021.
The search terms (EBSCOhost) consisted of:
- Cancer OR tumor OR cancer patient* OR oncolog* patient* OR patient* with cancer;
- Fatigue OR exhaustion OR tiredness OR lethargy OR asthenia;
- Intervention* OR nursing OR treatment* OR management* OR therap*;
- End of life OR terminal OR hospice OR palliative OR advance* OR advance* ill*;
- Chemotherapy OR chemo* OR cancer treatment*;
- 1 AND 2 AND 3 AND 4 NOT 5.
Selection process and data collection process
Pairs of review authors (MH, AK, and KH) independently evaluated the titles and abstracts of articles retrieved using the search terms. The pairs of review authors then determined which studies met the eligibility criteria and independently extracted all data from each included study using a standard data extraction form developed by Cochrane (11). The reviewers discussed any disagreements regarding inclusion. A third reviewer (MM) was consulted to resolve any disagreements through discussion and consensus.
Data items
The following information was extracted from each included study: identification information (publication year, first author), study information (purpose, study design, duration of the study, ethical considerations), general information (country, sample size, study design), participants (type of cancer, age, gender), intervention details (type of non-pharmacological intervention, intervention frequency, duration), outcomes (data and time points for each measurement, safety), and results on the fatigue evaluation.
Study risk of bias assessment
Two reviewers (MH and MI) independently assessed all included studies for selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases using the Cochrane Collaboration tool for assessing the risk of bias (11). A third review author (MM) was consulted if any disagreements could not be resolved. We assessed reporting biases by visual inspection of funnel plots when ten or more studies were included (12).
Statistical analysis
A summary of the data extracted from each study was created. We also conducted a meta-analysis of RCTs using Review Manager 5.4 (RevMan 5.4) software (13). The standard mean difference (SMD) and corresponding 95% confidence interval (CI) were calculated for continuous outcome measures. We selected the final-time point from multiple time points, and as it was based on the intention-to-treat (ITT), a meta-analysis was conducted based on the number of participants at baseline. We used a random-effects model to account for clinical heterogeneity among the included studies and applied the principles of ITT analysis where possible. We used Cohen’s effect sizes as follows: 0.2 represented a small effect, 0.5 a moderate effect, and 0.8 a large effect (14). In addition, we used the standard deviations (SDs) for the endpoint scores when the SDs for the change scores were missing (15). We evaluated heterogeneity using the I2 statistic as follows: 0–40% was insignificant, 30–60% represented moderate heterogeneity, 50–90% represented substantial heterogeneity, and 75–100% represented considerable heterogeneity (15).
Results
Study selection
We screened 1,954 publications; eight were included in the final analysis (Figure 1).

The study characteristics are summarised in Table 1. Three RCTs and five non-RCTs that evaluated non-pharmacological treatments for fatigue in terminally ill cancer patients were extracted. Of the three RCTs, two focused on music therapy (16,17) and one on cognitive therapy (18). In addition, five non-RCTs, that compared the pre- and post-intervention periods, were included: one (19) on virtual reality, two (20,21) on art therapy, one (22) on cognitive therapy (acceptance and commitment therapy), and one on a healthcare team intervention (the Complex Cancer Management Service) (23). The method of measuring fatigue was different among all the included studies (Table 1).
Table 1
| Item | RCT (n=3) | Non-RCT (n=5) | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Type of cancer | |||||
| Any type of cancer | 2 | 66.7 | 5 | 100.0 | |
| No information | 1 | 33.3 | |||
| Outcome measure | |||||
| Edmonton Symptom Assessment System | 1 | 33.3 | 4 | 80.0 | |
| Brief Fatigue Inventory | 1 | 33.3 | |||
| EORTC QLQ-C15-PAL | 1 | 33.3 | |||
| PROMIS Fatigue Short Form | 1 | 20.0 | |||
| Number of eligible participants | |||||
| >101 | 1 | 33.3 | |||
| <100 | 2 | 66.7 | 5 | 100.0 | |
EORTC QLQ-C15-PAL, European Organisation for Research and Treatment of Cancer, Quality of Life Questionnaires Core-15 Palliative; PROMIS, Patient Reported Outcomes Measurement Information System; RCT, randomised controlled trial.
A full summary of the characteristics of each study is shown in Tables 2,3. Most studies had relatively small numbers of participants. No serious adverse events following intervention were reported by Niki et al. (19); no other study addressed adverse events (16-18,20-23).
Table 2
| First author, year, country | Detail of intervention | Participant data | Outcome measure | Summary of results |
|---|---|---|---|---|
| Marco Warth et al., 2015, Germany | Music therapy: ❖ The music therapist began a short mindfulness exercise, accompanied by soft monochord sounds. Taking account of the patient’s breathing pattern, the volume, dynamics, and intensity of the monochord playing were then increased, and vocal improvisation began in Ionian or Mixolydian mode. Towards the end of the improvisation, which lasted approximately 15 min, the intensity was gradually reduced. In a five-minute discussion after the procedure, the patient had the opportunity to reflect on his or her experience of listening to the music. This intervention was repeated two days later; ❖ Patients in the control group underwent an intervention of the same duration but with no musical content or therapeutic relationship. This consisted of a 20 min excerpt from the Mindfulness-Based Stress Reduction Program, played through headphones |
Sample size: MT, 42; CG, 42 | Intervals in milliseconds between successive heartbeats | Music therapy was found to be superior in terms of the QoL fatigue subscale. |
| Age: MT, 63.8±14.1 years; CG, 62.2±12.8 years | Music therapy (n=42): before, mean =87.8±19.3; after, mean =80.4±23.2 | |||
| Other information: the most common primary diagnoses were breast cancer (n=17) | EORTC QLQ-C15-PAL | Control group (n=42): before, mean =93.7±13.5; after: mean =91.8±13.9 | ||
| Mean difference =−5.55, 95% CI: −13.4 to 2.33, P=0.03 | ||||
| Rafael Ramirez et al., 2018, Spain | Experimental group (music therapy): participants in the EG received an MT session of approximately 30 min. Each MT session consisted of a receptive song, an active song and a relaxation/imaginative receptive intervention | Sample size: EG, 20; CG, 20 | The ESAS | Of the symptom assessed using the ESAS, tiredness showed statistically significant differences between the pre- and post-intervention values in the EG (P=0.002, other statistical results can be found in this paper) |
| Control group: participants in the CG were accompanied by the same music therapists for approximately 30 min in which they conversed feely about music and their music preferences | Age: 69.0±15.0 years | |||
| EEG data was recorded before the MT session, during the session, and at the end of the session. In addition to EEG data, participants self-assessed several qualitative variables before and after the sessions by completing the ESAS pre- and post-intervention | Other information: advanced cancer in palliative care unit | |||
| Anja Mehnert et al., 2020, Germany | CALM: the CALM manual covers four domains: (I) SC: symptom management and communication with health care providers; (II) CSR: changes in self and relations with others; (III) SMP: spirituality, sense of meaning, and purpose; (IV) FHM: preparing for the future, sustaining hope, and manual on facing mortality | Sample size: CALM, 99; SPI, 107 | Brief Fatigue Inventory | CALM: baseline: n=93, mean =4.62±2.05; T2: n=67, mean =4.34±2.00 |
| SPI: SPI is a non-manualized psychotherapeutic counselling intervention; SPI helps patients dealing with distressing emotions by promoting adaptive coping with advanced cancer. Therapeutic techniques are derived from cognitive-behavioural techniques and/or psychodynamic treatment, including psychoeducation and regulation of emotions | Age: CALM, 59.5±12.1 years; SPI, 56.5±11.3 yeas | SPI: baseline: n=100, mean =4.56±2.10; T2: n=56, mean =4.00±2.1 | ||
| Within 6 months, CALM and SPI patients received up to six treatment sessions (50 min) and two optional booster sessions | Other information, patients had a malignant solid tumor (UICC III or IV) | Adj. effect: 0.13 | ||
| Evaluations were performed after 3 months (T1) and 6 months (T2) |
RCT, randomised controlled trial; MT, music therapy; CG, control group; EG, experimental group; EEG, electroencephalography; EORTC QLQ-C15-PAL, European Organisation for Research and Treatment of Cancer, Quality of Life Questionnaires Core-15 Palliative; CI, confidence interval; ESAS, Edmonton Symptom Assessment System; CALM, Managing Cancer and Living Meaningfully; SPI, Supportive psycho-oncological counselling intervention; UICC, Union for International Cancer Control; Adj. effect, adjusted effect; SC, symptom management and communication with health care providers; CSR, changes in self and relations with others; SMP, spirituality, sense of meaning and purpose; FHM, preparing for the future, sustaining hope and manual facing mortality.
Table 3
| First author, year, country | Detail of intervention | Participant data | Outcome measure | Summary of results |
|---|---|---|---|---|
| Cedric Lefèvre et al., 2016, France | Art therapy: ❖ Patients took part in at least one session facilitated by a qualified art therapist. Different art techniques were employed including painting, drawing, photography, modeling, and sculpture. The session could take place in the patient’s room or in a studio outside the unit, depending on the patient’s wishes and their physical abilities. Depending on the project and the length of the stay of the patient, 1–10 hour-long session(s) were offered; ❖ Patients were invited to complete the modified ESAS five minutes before and five minutes after each session |
Sample size: 22 patients | The ESAS | The hour-long art therapy session had a significant effect on tiredness. n=22 |
| Age: 56.5±10.7 (range, 32–85) years | Average score reduced: −36%, P<0.0001 (other statistical results can be found in this paper) | |||
| Other information: diagnosed with advanced cancer in an acute palliative care unit | ||||
| Andrea Feldstain et al., 2018, Canada | The CCMS: ❖ At the clinic, patients met with a physician, pharmacist, and psychosocial clinician. All patients had completed SFD measures, discussed in the following section, when meeting any of their healthcare teams as part of routine care; ❖ CCMS medicine follow-up was performed via telephone by one of the team’s pharmacists |
Sample size: 114 patients | The ESAS | Low group (n=32): T0 (referral), mean =2.1±1.1; T2 (discharge), mean =3.8±2.3; P≤0.01 |
| Age: 59.8±12.8 (range, 25–84) years | Moderate group (n=44): T0 (referral), mean =5.1±0.8; T2 (discharge), mean =5.5±2.5 | |||
| Other information: experiencing complex cancer symptoms, including physical symptoms or psychosocial-spiritual concerns | High group (n=38): T0 (referral), mean =8.1±0.8; T2 (discharge), mean =5.6±2.8; P≤0.01 | |||
| Salimah H. Meghani et al., 2018, USA | Mindfulness-based art therapy: participants were invited to tear the 2-sided sheet used for this exploration and combine it with origami paper, colored tissue, and a diverse selection of high-quality magazine photos to construct a collage prior to expanding the field of creative practice to photography in session 2. Using digital cameras during mindful walkabouts away from the medical facility in 4 to 5 of the 8 weeks of the program, each participant gained a personal library of photos, which they printed for collage making in the following weeks. Repeated-measures data were gathered at baseline (T1), week 4 (T2), and week 8 (T3) | Sample size: 18 patients; | The ESAS-R | The change in tiredness was not statistically significant. n=16 |
| Age: 30–49 years: n=5 (27.7%), 50–59 years: n=6 (33.3%), 60+ years: n=7 (38.9%) | Mean =−0.56±2.78 | |||
| Effect size =0.20, 95% CI: −2.04 to 0.92, P=0.431 | ||||
| Other information: diagnosed with early or recurrent cancer with any cancer diagnosis, except brain cancer | ||||
| Kazuyuki Niki et al., 2019, Japan | Virtual reality: the participants experienced one VR travel session that lasted 30 min in principle. Shortly before and immediately after VR travel, participants’ symptoms were evaluated | Sample size: 20 patients | The ESAS Japanese version | Virtual reality caused a significant reduction in the tiredness score in the ESAS. n=20 |
| Age: 72.3±11.9 years | Before, mean =2.90±2.71; after, mean =1.35±1.90; P=0.004; Cohen’s d=0.679 | |||
| Other information: terminal cancer in two palliative care wards | ||||
| Jennifer C. Plumb Vilardaga et al., 2020, USA | Acceptance and commitment therapy: | Sample size: 21 patients | The PROMIS Fatigue Short Form | Fatigue assessed with PROMIS showed no significant reduction. n=20 |
| Session 1: biopsychosocial-spiritual model of health | Age: 66.0±10.8 (range, 35–82) years | Pre, mean =54.47±9.07; post, mean =53.73±9.18 | ||
| Session 2: value-guided activity planning | Other information: a primary diagnosis of advanced cancer | Effect size =0.08, 95% CI: −4.84 to 3.37, P=0.71 | ||
| Session 3: coping with negative thoughts | ||||
| Session 4: skills integration |
RCT, randomised controlled trial; ESAS, Edmonton Symptom Assessment System; ESAS-R, Edmonton Symptom Assessment Scale-R; CCMS, Complex Cancer Management Service; SFD, screening for distress; CI, confidence interval; VR, virtual reality; RROMIS, Patient-Reported Outcomes Measurement Information System.
Risk of bias in studies
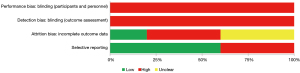
Figures 2,3 present graphs of the risk of bias and Figures S1,S2 present summaries of the risk of bias assessment. RCTs using computerised sequence generation (18) or block randomisation (16,17) were at low risk of bias. Regarding allocation concealment, two studies were at low risk of bias because of the use of a central randomisation method (18) and sequentially numbered envelopes (17). The detection bias was high because all the studies used self-administered questionnaires to assess fatigue. Regarding attrition bias, one study was performed on the ITT population (18), and one study did not analyse missing data (17).

Results of syntheses
We conducted a meta-analysis on the primary outcomes of only two of the three RCTs because detailed results could not be obtained from one study; the results are presented in Table 2 and Figure 4. Due to the insufficient number of studies and 95% CI, the evidence on the effects of the intervention compared to the controls was inconclusive (SMD −0.05; 95% CI: −0.48 to 0.37; two studies; 290 participants; I2=65%). In addition, the heterogeneity index indicated substantial heterogeneity.
Discussion
This study has two main findings. First, few studies have been conducted on terminally ill cancer patients (excluding those undergoing anti-cancer treatment). Second, there was insufficient evidence to determine differences in fatigue among the interventions.
Interventions to reduce CRF in terminally ill cancer patients
This study was a systematic review of non-pharmacological interventions for terminally ill cancer patients who did not receive anti-cancer treatment. A high rate of malaise has been reportedly associated with anti-cancer treatment (24-27). In this study, studies of patients in the treatment phase were excluded, because of the influence of anti-cancer treatment on the effects of malaise. Therefore, 28 studies that included patients undergoing treatment were excluded. Thus, this review is specific to non-pharmacological interventions for fatigue in terminally ill cancer patients. Based on the results of this study, three interventions might be considered to reduce fatigue in terminally ill cancer patients: music therapy, cognitive therapy, and virtual reality.
First, we found that few reports exist on non-pharmacological interventions for reducing fatigue, which is similar to the results of reviews on the effects of physical exercise (7) on fatigue. Furthermore, of the ten interventions listed in the JSPM “Clinical Evidence for Complementary and Alternative Therapies in Cancer Patients 2016”, four have not undergone a systematic review. Exercise interventions were reported in 20 cases. Among the remaining five interventions, two systematic reviews reported on massage, one on relaxation, two on music therapy, four on acupuncture, and three on yoga (10). Therefore, the lack of RCTs on non-pharmacological interventions for reducing fatigue in terminal care patients should be addressed. Numerous reviews have reported evidence for non-pharmacological treatments for anxiety (28,29), dyspnoea (30,31), etc., in terminally ill cancer patients.
Second, there was insufficient evidence to determine differences in effects on fatigue among the interventions, although our meta-analysis indicated a change in fatigue in all interventions. In this study, the number of studies included in the analysis is small and the evaluation period since the intervention is varied. We believe this is one of the reasons for the heterogeneity between studies. Therefore, we could not show any effect of music therapy and symptom management on fatigue, although previous reviews on fatigue and physical exercise (7) and music interventions (27) indicated a small effect. Bradt et al. reviewed ten studies of participants receiving cancer treatment (e.g., chemotherapy, radiotherapy, surgery, and blood and marrow transplantation), and Mochamat et al. reviewed studies on advanced diseases not limited to cancer, which included cancer (10 studies), amyotrophic lateral sclerosis (two studies), ESRD (one study), and cirrhosis (one study) (7).
The substantial heterogeneity identified in our data may suggest that our comparison was not specific to usual care alone. For example, “managing cancer and living meaningfully” was compared with “supportive psycho-oncological counselling”. Specifically, this case differed regarding the usual care patients received.
Difficulties of research on interventions for terminally ill cancer patients
We consider that one of the reasons for the paucity of intervention studies on end-stage cancer patients is the difficulty of assessment due to the decline in physical and cognitive functions. For example, Seyama et al. investigated impairments in activities of daily living experienced by terminally ill cancer patients before death and reported that approximately 60% of patients had difficulty communicating, and 65% had difficulty responding to questions the day before death (32).
In addition, as CRF is a subjective symptom, assessments are based on patient-reported outcomes, which are difficult to assess in terminally ill cancer patients at the end of life. A study showed that 4 weeks before death, fatigue and pain appeared in >50% of the patients, in addition to general weakness (43%), sleepiness (24%), and mental haziness/confusion (24%) (4). Based on the studies described above, we consider it very difficult to conduct and subjectively evaluate intervention studies in terminally ill cancer patients with various physical and psychiatric symptoms.
Limitations of the present study
Our study had some limitations. First, the statistical power was low because the number of RCTs was insufficient. Further research is required to resolve the effects of non-pharmacological interventions. However, we deemed it important to show the results of a few studies in a systematic review. In this study, we conducted a meta-analysis, although there were only two studies. We believe that our results represent the available data, and we aimed to discuss the few studies in great detail. In addition, one of the studies had two-time points, however, the meta-analysis for this review was conducted using the final-time point; thus, the results may have differed if the meta-analysis had incorporated another time point. Second, the control groups in the extracted literature were not included for the same interventions. Third, we were unable to ask questions of some of the original authors, although we may update this review with the new data when we obtain the answers.
Implications for future research
In this study, we reviewed research on fatigue over the last decade, although the target patients were limited to those with terminal cancer. Furthermore, we investigated the adverse events in the included studies.
In terminal cancer patients, with a variety of physical and psychological symptoms, interventions used to reduce fatigue should avoid burdening the patient physically and assess the patient's mental state independently of their condition. One study on virtual reality was reviewed in the present study. Although it had limitations, such as not including a control group and not considering the influence of interpersonal bias, the study reported results that are expected to have a mitigating effect on CRF (19). Although the patient-reported complaints of “claustrophobia” at the time of first treatment (33), Niki et al. reported no adverse events due to virtual reality (19). Virtual reality does not require a specific location for the intervention, as long as the equipment is available. It can be used by patients, as no special qualifications are required for its implementation. Therefore, interventions can be performed at home.
Intervention methods that can be implemented in any location, such as virtual reality, and that do not impose a physical burden on patients, should be tested further. Additionally, although fatigue is assessed subjectively, future studies would benefit from incorporating objective assessments such as physiological indices. Moreover, it is necessary to evaluate the effects of single non-pharmacological interventions and to build evidence from a series of RCT studies on the combined effects of non-pharmacological interventions on fatigue in terminally ill cancer patients.
In addition, this review only included studies conducted in Europe, the USA, and Japan. It is desirable to conduct intervention studies in a variety of countries without bias towards Europe and the USA. To ensure continued implementation of interventions to treat CRF, it is also necessary to consider future costs.
Conclusions
There are few reports on non-pharmacological interventions for terminal cancer patients, and we found insufficient evidence to determine the differences in their effects on fatigue.
This highlights the lack of RCTs for some procedures and therapies used in non-pharmacological interventions for reducing fatigue.
Acknowledgments
We would like to express our warm appreciation to all the participants in the analysed studies and to those who published all the eligible studies included in the current systematic review and meta-analysis.
Funding: This work was supported by a JSPS KAKENHI Grant-in-Aid for Scientific Research (No. JP20K10715).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-655/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-655/coif). MM serves as an unpaid editorial board member of Annals of Palliative Medicine from January 2013 to January 2024. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Carlson LE, Angen M, Cullum J, et al. High levels of untreated distress and fatigue in cancer patients. Br J Cancer 2004;90:2297-304. [Crossref] [PubMed]
- Storey DJ, Waters RA, Hibberd CJ, et al. Clinically relevant fatigue in cancer outpatients: the Edinburgh Cancer Centre symptom study. Ann Oncol 2007;18:1861-9. [Crossref] [PubMed]
- Portenoy RK, Thaler HT, Kornblith AB, et al. Symptom prevalence, characteristics and distress in a cancer population. Qual Life Res 1994;3:183-9. [Crossref] [PubMed]
- Coyle N, Adelhardt J, Foley KM, et al. Character of terminal illness in the advanced cancer patient: pain and other symptoms during the last four weeks of life. J Pain Symptom Manage 1990;5:83-93. [Crossref] [PubMed]
- Oncology Nursing Society [Internet]. PA; ONS Guidelines™ Symptom Interventions and guidelines [Fatigue]; c2022 [cited 2022 May 18]. Available online: https://www.ons.org/pep/fatigue?display=pepnavigator&sort_by=created&items_per_page=50
- Chapman EJ, Martino ED, Edwards Z, et al. Practice review: Evidence-based and effective management of fatigue in patients with advanced cancer. Palliat Med 2022;36:7-14. [Crossref] [PubMed]
- Mochamat Cuhls H. Fatigue in advanced disease associated with palliative care: A systematic review of non-pharmacological treatments. Palliat Med 2021;35:697-709. [Crossref] [PubMed]
- National Comprehensive Cancer Network (NCCN) [Internet]. PA: NCCN Guidelines; c2022 [cited 2022 May 11]. NCCN Clinical Practice Guidelines in Oncology Cancer-Related Fatigue Version 2.2022-February 9, 2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf
- National Comprehensive Cancer Network (NCCN) [Internet]. PA; NCCN Guidelines; c2022 [cited 2022 May 15]. Available online: https://www.nccn.org/
- Japanese Society for Palliative Medicine. Clinical Evidence for Complementary and Alternative Therapies in Cancer Patients 2016. Tokyo, Japan: Kanehara & Co., Ltd., 2016:7.
- Cochrane Developmental, Psychosocial and Learning Problems (2022): Data extraction forms. Available online: https://dplp.cochrane.org/data-extraction-forms
- Lin L, Chu H, Murad MH, et al. Empirical Comparison of Publication Bias Tests in Meta-Analysis. J Gen Intern Med 2018;33:1260-7. [Crossref] [PubMed]
-
Training Cochrane - Jacob Cohen. Statistical Power Analysis in the Behavioral Sciences. 2nd edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc., 1988.
- Cochrane Handbook for Systematic Reviews of Interventions version 6.2; 2021. Available online: https://training.cochrane.org/handbook/archive/v6.2
- Ramirez R, Planas J, Escude N, et al. EEG-Based Analysis of the Emotional Effect of Music Therapy on Palliative Care Cancer Patients. Front Psychol 2018;9:254. [Crossref] [PubMed]
- Warth M, Keßler J, Hillecke TK, et al. Music Therapy in Palliative Care. Dtsch Arztebl Int 2015;112:788-94. [PubMed]
- Mehnert A, Koranyi S, Philipp R, et al. Efficacy of the Managing Cancer and Living Meaningfully (CALM) individual psychotherapy for patients with advanced cancer: A single-blind randomized controlled trial. Psychooncology 2020;29:1895-904. [Crossref] [PubMed]
- Niki K, Okamoto Y, Maeda I, et al. A Novel Palliative Care Approach Using Virtual Reality for Improving Various Symptoms of Terminal Cancer Patients: A Preliminary Prospective, Multicenter Study. J Palliat Med 2019;22:702-7. [Crossref] [PubMed]
- Meghani SH, Peterson C, Kaiser DH, et al. A Pilot Study of a Mindfulness-Based Art Therapy Intervention in Outpatients With Cancer. Am J Hosp Palliat Care 2018;35:1195-200. [Crossref] [PubMed]
- Lefèvre C, Ledoux M, Filbet M. Art therapy among palliative cancer patients: Aesthetic dimensions and impacts on symptoms. Palliat Support Care 2016;14:376-80. [Crossref] [PubMed]
- Plumb Vilardaga JC, Winger JG, Teo I, et al. Coping Skills Training and Acceptance and Commitment Therapy for Symptom Management: Feasibility and Acceptability of a Brief Telephone-Delivered Protocol for Patients With Advanced Cancer. J Pain Symptom Manage 2020;59:270-8. [Crossref] [PubMed]
- Feldstain A, Bultz BD, de Groot J, et al. Outcomes From a Patient-Centered, Interprofessional, Palliative Consult Team in Oncology. J Natl Compr Canc Netw 2018;16:719-26. [Crossref] [PubMed]
- Portenoy RK, Itri LM. Cancer-related fatigue: guidelines for evaluation and management. Oncologist 1999;4:1-10. [Crossref] [PubMed]
- Kiss I, Kuhn M, Hrusak K, et al. Incidence of fatigue associated with immune checkpoint inhibitors in patients with cancer: a meta-analysis. ESMO Open 2022;7:100474. [Crossref] [PubMed]
- André L, Antherieu G, Boinet A, et al. Oncological Treatment-Related Fatigue in Oncogeriatrics: A Scoping Review. Cancers (Basel) 2022;14:2470. [Crossref] [PubMed]
- Bradt J, Dileo C, Myers-Coffman K, et al. Music interventions for improving psychological and physical outcomes in people with cancer. Cochrane Database Syst Rev 2021;10:CD006911. [PubMed]
- Oncology Nursing Society [Internet]. PA; ONS Guidelines™ Symptom Interventions and guidelines [Anxiety]; c2022 [cited 2022 May 18]. Available online: https://www.ons.org/pep/anxiety?display=pepnavigator&sort_by=created&items_per_page=50
- Andersen BL, DeRubeis RJ, Berman BS, et al. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol 2014;32:1605-19. [Crossref] [PubMed]
- Hui D, Bohlke K, Bao T, et al. Management of Dyspnea in Advanced Cancer: ASCO Guideline. J Clin Oncol 2021;39:1389-411. [Crossref] [PubMed]
- Bausewein C, Booth S, Gysels M, et al. Effectiveness of a hand-held fan for breathlessness: a randomised phase II trial. BMC Palliat Care 2010;9:22. [Crossref] [PubMed]
- Seyama R, Ishida K, Nakajima Y, et al. Examination of an obstacle of Activities of Daily Living that a cancer patient has for the end period in the university hospital, and consideration of nursing support. Ann Gunma Health Sci 2008;29:31-8.
- Reynolds LM, Cavadino A, Chin S, et al. The benefits and acceptability of virtual reality interventions for women with metastatic breast cancer in their homes; a pilot randomised trial. BMC Cancer 2022;22:360. [Crossref] [PubMed]



