
Successful treatment with tigecycline of severe pandrug-resistant Acinetobacter baumannii pneumonia complicated with a diaphragmatic hernia: a case report
Introduction
Acinetobacter baumannii (A. baumannii) as an “opportunistic” pathogen is becoming a global epidemic. A. baumannii is one of the ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, A. baumannii, Pseudomonas aeruginosa, and Enterobacter spp) pathogens (1). The incidence of these strains continues to increase worldwide. A. baumannii is one of the important nosocomial pathogens, especially in intensive care units. The risk factors for clinical infection with A. baumannii are prolonged hospitalization, admission to the intensive care unit, mechanical ventilation, invasive operation, long-term constant use of antibiotics, and patient-specific underlying diseases (2). As a common pathogen of hospital-acquired pneumonia (HAP) and ventilator-acquired pneumonia (VAP), A. baumannii can readily acquire multidrug resistance or even pandrug resistance. Currently available options for treating A. baumannii infections include carbapenems, sulbactam, sulbactam-containing β-lactam formulation, polymyxins, glycylcyclines, tetracyclines, aminoglycosides, quinolones, and rifampin (3).
Antibiotic resistance is common for the A. baumannii infection due to the extensive use of antibiotics. Based on the extent and pattern of drug resistance, A. baumannii can be multidrug-resistant (MDR), extensive drug-resistant (XDR), or even pandrug-resistant (PDR) (4). MDR is defined as nonsusceptibility to at least 1 agent in 3 or more antimicrobial categories (5). XDR is defined as nonsusceptibility to at least 1 agent in all but 2 or fewer antimicrobial categories (5). PDR is defined as nonsusceptibility to all agents in all antimicrobial categories; that is, no agents tested are susceptible to that organism (5). Once MDR, XDR, or PDR occurs, clinical treatment is extremely difficult. Especially in severe cases, patients may have serious complications or even die.
Upon the emergence of pandrug-resistant isolates, the infection will be extremely challenging to treat, particularly if complicated with other diseases. Therefore, new approaches, methods, and strategies are urgently needed to treat and eradicate infections of PDR A. baumannii. Herein, we report a case in which we used intermediate antibiotic tigecycline to successfully treat an 8-year-old girl who had PDR A. baumannii pneumonia complicated with a diaphragmatic hernia. We present the following case in accordance with the CARE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-900/rc).
Case presentation
A girl aged 8 years and 11 months was admitted to Tongji Hospital for a cough that had lasted for over 2 weeks before admission despite her taking cold medicine and amoxicillin, a fever that lasted 8 days, and skin rashes that were present for 3 days. Eight days before admission, she visited a local doctor for a fever and was treated as an inpatient. Azithromycin, aztreonam, cefotaxime, and meropenem were administered successively for 7 days. However, the symptoms did not improve. Therefore, she visited our hospital for further diagnosis and therapy and was then admitted to the pediatric intensive care unit (PICU). She was previously healthy, with no family history of genetic diseases or exposure to infectious diseases.
When she was admitted to the PICU, a physical examination showed that her heart rate was 120 bpm, her respiratory rate was 38 bpm, and her blood pressure was 93/53 mm Hg. These indicated that her vitals were unstable. The patient was low-spirited, and her face and whole body were covered with dense red rashes. Coarse breath sounds and wet and dry rales could be heard in both lungs using auscultation. Laboratory tests were performed after admission. Routine blood tests showed her white blood cell count (WBC) was 14.87×109/L, her neutrophil count was 12.70×109/L, her hemoglobin level was 120 g/L, and her platelet count was 105×109/L. Her high-sensitivity C-reactive protein (hsCRP) level was 181.4 mg/L, and the level of procalcitonin (PCT) was 2.45 ng/mL. These results indicated that this girl was at high risk of bacterial infection and sepsis. Blood biochemistry showed that alanine transaminase (ALT) was 293.7 U/L, aspartate transaminase (AST) was 585.4 U/L, albumin (ALB) was 18.2 g/L, and lactate dehydrogenase (LDH) was as high as 1,312 U/L. These results indicated that the liver had been severely damaged. Ferritin was significantly elevated to 3,178 µg/L. The coagulation function showed a normal result, except that D-dimer was significantly elevated to 8.56 µg/mL. A chest X-ray showed infection of both lungs, pleural effusion on both sides, and a partially enveloped left lung (Figure 1A). Immunoglobulin levels and lymphocyte subsets were normal, indicating that she was immunocompetent. The serological results of common respiratory pathogens showed positive for Mycoplasma pneumoniae immunoglobin M (IgM). Blood and sputum cultures were negative, probably due to prior antibiotic use. The above results indicated that the patient was experiencing severe pneumonia caused by a bacterial infection and had complications, such as pleural effusion, liver damage, and a hypercoagulable state. Initially, we used teicoplanin, meropenem, and clarithromycin as antimicrobials and administered a high dose of methylprednisolone (20 mg/kg·d) for 4 days. Subsequently, we administered a regular dose of methylprednisolone (2 mg/kg·d) for maintenance as an anti-inflammatory, intravenous immune globulin (IVIG) 2 g/kg divided in 3 days as immunotherapy, and albumin supplementation.
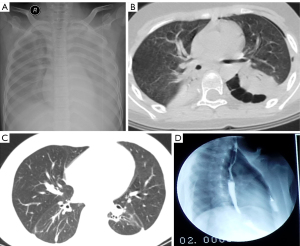
On the second day after admission, the patient had difficulty breathing and became unconscious. Since the breathing difficulties could not be relieved after use of a tight-fitting oxygen mask, a ventilator was used. On the fifth day of admission (i.e., the fourth day after being put on ventilator), the results of the sputum culture and drug sensitivity test indicated that A. baumannii was positive and was PDR (Table 1). We hypothesized that this PDR A. baumannii might have been hospital-acquired and/or ventilator-associated. Therefore, we replaced meropenem and teicoplanin with cefoperazone-sulbactam and moxifloxacin. After 14 days on the ventilator, her breathing improved, and she was taken off the ventilator. The next day after the withdrawal of the mechanical ventilation, chest computed tomography (CT) was conducted, showing a left diaphragmatic hernia (herniation of large bowel), small left-sided pneumothorax, and pulmonary infection with severe lesions in the bilateral lower lobes (Figure 1B). Therefore, we consulted with a pediatric surgeon, who determined that there was no indications for an immediate operation.
Table 1
| Antibiotics | A. baumannii 3+ | Reference ranges | Method | ||||
|---|---|---|---|---|---|---|---|
| Before the temporary withdrawal of all antibiotics | After the temporary withdrawal of all antibiotics | ||||||
| Diameter | Results | Diameter | Results | ||||
| Tigecycline | 12 | R | 13 | I | 13–15 | KB | |
| Meropenem | 6 | R | 6 | R | 14–15 | KB | |
| Piperacillin-tazobactam | 6 | R | 13 | R | 18–20 | KB | |
| Gentamicin | 6 | R | 6 | R | 13–14 | KB | |
| Piperacillin | 6 | R | 11 | R | 18–20 | KB | |
| Aztreonam | 6 | R | 6 | R | 16–21 | KB | |
| Levofloxacin | 6 | R | 6 | R | 14–16 | KB | |
| Minocycline | 11 | R | 10 | R | 13–15 | KB | |
| Sulfamethoxazole-trimethoprim | 6 | R | 6 | R | 11–15 | KB | |
| Imipenem | 6 | R | 6 | R | 14–15 | KB | |
| Ampicillin-salbactam | 6 | R | 6 | R | 12–14 | KB | |
| Amikacin | 6 | R | 6 | R | 15–16 | KB | |
| Tobramycin | 6 | R | 6 | R | 13–14 | KB | |
| Cefoperazone-sulbactam | 8 | R | 15 | R | 16–20 | KB | |
| Ciprofloxacin | 6 | R | 6 | R | 16–20 | KB | |
| Ceftazidime | 6 | R | 6 | R | 15–17 | KB | |
| Cefepime | 10 | R | 14 | R | 15–17 | KB | |
A. baumannii, Acinetobacter baumannii; R, resistance; I, intermediate; KB, Kirby-Bauer paper disk diffusion method.
On the 20th day in the PICU, the patient’s condition improved. The results of laboratory reexamination showed that the WBC was 22.5×109/L, the neutrophil ratio was 93%, hsCRP was 22 mg/L, PCT was 0.05 ng/mL, ALT was 40 U/L, AST was 23 U/L, and ALB was 32.2 g/L. These indicated that the girl’s condition had improved and that she could leave the PICU. She was subsequently transferred to the pediatric infection ward. However, the girl was still listless and pale. She felt short of breath and could not lie down in a supine position. She also had mild cyanosis even with an oxygen supply, as well as fever and cough, with pale yellow purulent sputum. During the first 3 days in the pediatric infection ward, multiantibiotics, including amoxicillin-sulbactam, ornidazole, and oral fluconazole, were given to the patient. The results of the sputum culture and drug sensitivity tests were the same as before: positive for PDR A. baumannii. Consequently, all antibiotics were stopped for 8 days, and on days 3 and 8 without antibiotics, a positive sputum culture was still observed. However, antibiotics sensitivity tests demonstrated an intermediate response to tigecycline, as shown in Table 1. According to the US Food and Drug Administration (FDA), tigecycline is not approved for use in children, and it should not be used in pediatric patients unless no alternative antibacterial drugs are available. The girl’s condition was consistent with this indication. Tigecycline treatment was initiated with the consent of the girl’s parents. The first dose of tigecycline was 100 mg, which was followed by a dose of 50 mg applied once every 12 hours. After being treated with tigecycline for 1 week, the patient’s condition did not improve and her body temperature was still up to 39.7 °C. After 2 weeks of tigecycline treatment, the levels of hsCRP decreased and the frequency of fever decreased to 1 to 2 times per day. During the third week, the patient’s body temperature dropped below 38 °C, and only a mild fever occurred once a day. After tigecycline treatment for 22 days, the patient gradually recovered and was discharged to return home. She subsequently continued receiving tigecycline treatment in the outpatient clinic. The liver function reexamination before discharge showed normal transaminases. The clinical follow-up after discharge showed that on the eighth day after discharge, the patient was walking and playing happily although small dry rales could be heard in her lungs. The sputum culture showed detectable A. baumannii. Nearly 2 weeks after discharge, tigecycline was discontinued. In total, tigecycline was administered for a total of 5 weeks. No adverse reactions to tigecycline were observed. Two months after discharge, chest CT and upper gastrointestinal examination with iodinated contrast showed that most lung lesions had been absorbed and that the diaphragmatic hernia had disappeared (Figure 1C,1D). Throughout the treatment course, sputum cultures were performed 30 times, 25 of which were positive for the A. baumannii infection. Of the 5 negative cultures, 2 were tested on the first 2 days after the girl was hospitalized. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s legal guardian for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. The case report was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. TJ-IRB20220818)
Discussion
A. baumannii is of low pathogenic potential. However, with the widespread use of antibiotics, an increasing trend in incidence, and the emergence of resistant strains, it has become a threat to human health (6). A. baumannii can cause various infections, such as pneumonia, urinary tract infections, meningitis, bacteremia, and gastrointestinal and numerous skin diseases (7). A meta-analysis study showed that the overall prevalence of MDR among A. baumannii causing HAP and VAP is 79.9%, and the overall mortality is 42.6% (8). Although many antibiotics are available to treat A. baumannii infection, the emergence of resistant strains, especially PDR strains, has largely limited the treatment options for this opportunistic pathogen.
A. baumannii may acquire antibiotic resistance through several distinct mechanisms: by altering the antibiotic target site, by controlling the passage of antibiotics through its membranes, and by neutralizing antibiotics via enzymatic modification (2). Secondary to innate mechanisms of antibiotic resistance that are conferred by genes, A. baumannii may facilitate antibiotic resistance through various mechanisms linked with its virulence (2).
Treatment options for PDR A. baumannii strains are limited. Antimicrobial combinations are the major treatment option for PDR A. baumannii. However, a meta-analysis showed that antibiotic combination therapy has no effect on the mortality rate or length of hospitalization, although the microbiological eradication rate may be more effective than that of monotherapy (9). In addition, antibiotic combination therapy may further increase the risk of antibiotic resistance. Tigecycline is a semi\synthetic derivative of glycylcyclines, which exerts its antibacterial activity by inhibiting bacterial protein synthesis via high-affinity binding to the bacterial 30S ribosome (10). The most frequent side effects of tigecycline are nausea and vomiting, with less liver and kidney damage than tetracycline (11). Tigecycline was approved by the US FDA in June 2005 for skin and soft tissue infections and complicated intra-abdominal infections in adults, and it was approved in 2008 for community-acquired pneumonia in adults (12), but its use in children is limited. Tigecycline was shown to be active in vitro and clinically against some MDR strains of A. baumannii, and the European Medicines Agency (EMA) has approved it for patients above the age of 8 years (13). Therefore, it is frequently used in the treatment of drug-resistant A. baumannii and achieves a good therapeutic effect. A study reported a case of PDR A. baumannii ventriculoperitoneal shunt infection and ventriculitis successfully treated with systemic ampicillin-sulbactam administration in a 6-year-old boy (14). Tekçe et al. (15) used tigecycline to successfully treat a case of PDR A. baumannii mediastinitis with hypertension, diabetes, and chronic obstructive pulmonary disease in a 58-year-old woman, which was the first case of A. baumannii mediastinitis treated with tigecycline successfully. Tigecycline alone was also used successfully to treat PDR in children. Kanık-Yüksek et al. (16) successfully used tigecycline to treat 2 cases of PDR A. baumannii infections in 2 pediatric burn patients.
The patient in our case had underlying diseases, such as severe pneumonia, and was subject to the long-term use of multiple broad-spectrum or ultra-broad-spectrum antibiotics, invasive procedures (support with mechanical ventilation for 14 days), and an extended stay in the PICU (up to 20 days), all of which are risk factors for resistant A. baumannii infection. On the fifth day after hospitalization, a positive A. baumannii infection was detectable in the sputum culture, and susceptibility test results demonstrated it was PDR. Several subsequent sputum cultures and sensitivity testing results further confirmed the A. baumannii infection and PDR. After the discontinuation of all antibiotics for 3 and 8 days, the sputum culture and drug sensitivity testing were positive for A. baumannii infection and tigecycline intermediate. These results suggested that the discontinuation of all antibiotics induced the PDR A. baumannii to become sensitive to tigecycline again, which may be an effective strategy to treating MDR bacterial infections in the future.
Doubts exist regarding the role of tigecycline in monotherapy for PDR A. baumannii infections. In our case, we used a high dose (first dose of 100 mg followed by 50 mg Q12h) and a long course of treatment (5 weeks). There are recommendations for bacterial infection with MDR, and pediatric doses of tigecycline include up to 4 mg/kg/dose×1 (max 200 mg/dose) followed by a 2 to 3.2 mg/kg/dose Q12h (max 100 mg/dose) administration (17). However, considering the adverse reactions, we only used the recommended doses for adults. Although the recommended duration of tigecycline is 5–14 days (18), we extended the duration for better clinical efficacy. After receiving the treatment of 5 weeks with tigecycline, the patient showed progressively improved clinical conditions, and she finally recovered, suggesting that even an intermediate antibiotic can achieve the expected results if a high dose and prolonged course of treatment are applied.
Reports show that high doses of tigecycline work well in treating resistant A. baumannii (19,20). Considering the severity of the disease and the treatment difficulty, we chose a long course and high dose of tigecycline treatment and closely monitored liver and kidney function. Although the patient experienced a transient increase in ALT and AST after being discharged, the common side effects of nausea and vomiting of the antibiotic were not observed. Therefore, tigecycline may be used as a therapeutic option for A. baumannii infection in children.
To the best of our knowledge, the complication of severe PDR A. baumannii pneumonia with a diaphragmatic hernia is rarely encountered and has not been reported previously. In this case, we did not treat the hernia but focused on treating the bacterial infection and undertook regular follow-ups. We found that upon controlling lung infection, the diaphragmatic hernia improved and finally disappeared, suggesting that the effective controlling of A. baumannii infection resulted in the improvement of the diaphragmatic hernia.
Based on our overall experience from the case, we suggest that antibiotic treatment for PDR A. baumannii infection should be stopped for a period of time, if the patient’s condition permits it, to help restore bacterial sensitivity to antibiotics. Intermediate antibiotics may still achieve better results if adequate doses and a long course of treatment are used. In addition, it is more important to control the infection and improve lesion absorption than it is to eliminate resistant A. baumannii.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-900/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-900/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s legal guardian for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. The case report was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. TJ-IRB20220818).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Patil A, Banerji R, Kanojiya P, et al. Bacteriophages for ESKAPE: role in pathogenicity and measures of control. Expert Rev Anti Infect Ther 2021;19:845-65. [Crossref] [PubMed]
- Munoz-Price LS, Weinstein RA. Acinetobacter infection. N Engl J Med 2008;358:1271-81. [Crossref] [PubMed]
- Karageorgopoulos DE, Falagas ME. Current control and treatment of multidrug-resistant Acinetobacter baumannii infections. Lancet Infect Dis 2008;8:751-62. [Crossref] [PubMed]
- Falagas ME, Koletsi PK, Bliziotis IA. The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa. J Med Microbiol 2006;55:1619-29. [Crossref] [PubMed]
- Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012;18:268-81. [Crossref] [PubMed]
- Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 2008;21:538-82. [Crossref] [PubMed]
- Kyriakidis I, Vasileiou E, Pana ZD, et al. Acinetobacter baumannii Antibiotic Resistance Mechanisms. Pathogens 2021;10:373. [Crossref] [PubMed]
- Mohd Sazlly Lim S, Zainal Abidin A, Liew SM, et al. The global prevalence of multidrug-resistance among Acinetobacter baumannii causing hospital-acquired and ventilator-associated pneumonia and its associated mortality: A systematic review and meta-analysis. J Infect 2019;79:593-600. [Crossref] [PubMed]
- Mohammadi M, Khayat H, Sayehmiri K, et al. Synergistic Effect of Colistin and Rifampin Against Multidrug Resistant Acinetobacter baumannii: A Systematic Review and Meta-Analysis. Open Microbiol J 2017;11:63-71. [Crossref] [PubMed]
- Rose WE, Rybak MJ. Tigecycline: first of a new class of antimicrobial agents. Pharmacotherapy 2006;26:1099-110. [Crossref] [PubMed]
- Bauer G, Berens C, Projan SJ, et al. Comparison of tetracycline and tigecycline binding to ribosomes mapped by dimethylsulphate and drug-directed Fe2+ cleavage of 16S rRNA. J Antimicrob Chemother 2004;53:592-9. [Crossref] [PubMed]
- Stein GE, Babinchak T. Tigecycline: an update. Diagn Microbiol Infect Dis 2013;75:331-6. [Crossref] [PubMed]
- Leng B, Yan G, Wang C, et al. Dose optimisation based on pharmacokinetic/pharmacodynamic target of tigecycline. J Glob Antimicrob Resist 2021;25:315-22. [Crossref] [PubMed]
- Demoz GT, Alebachew M, Legesse Y, et al. Treatment of ventriculoperitoneal shunt infection and ventriculitis caused by Acinetobacter baumannii: a case report. J Med Case Rep 2018;12:141. [Crossref] [PubMed]
- Tekçe AY, Erbay A, Çabadak H, et al. Pan-resistant Acinetobacter baumannii mediastinitis treated successfully with tigecycline: a case report. Surg Infect (Larchmt) 2011;12:141-3. [Crossref] [PubMed]
- Kanık-Yüksek S, Tezer H, Ozkaya-Parlakay A, et al. Multidrug-resistant Acinetobacter baumannii bacteremia treated with tigecycline in two pediatric burn patients. Pediatr Infect Dis J 2015;34:677. [Crossref] [PubMed]
- Chiotos K, Hayes M, Gerber JS, et al. Treatment of Carbapenem-Resistant Enterobacteriaceae Infections in Children. J Pediatric Infect Dis Soc 2020;9:56-66. [Crossref] [PubMed]
- Mastrolia MV, Galli L, De Martino M, et al. Use of tigecycline in pediatric clinical practice. Expert Rev Anti Infect Ther 2017;15:605-12. [Crossref] [PubMed]
- Falagas ME, Vardakas KZ, Tsiveriotis KP, et al. Effectiveness and safety of high-dose tigecycline-containing regimens for the treatment of severe bacterial infections. Int J Antimicrob Agents 2014;44:1-7. [Crossref] [PubMed]
- De Pascale G, Montini L, Pennisi M, et al. High dose tigecycline in critically ill patients with severe infections due to multidrug-resistant bacteria. Crit Care 2014;18:R90. [Crossref] [PubMed]


