
Localization diagnosis of adrenocorticotrophic hormone-dependent Cushing’s syndrome: two case reports and literature review
Highlight box
Key findings
• The differentiation between CD and EAS should be evaluated in combination with the medical history, function tests, pituitary MRI, and other tests. Complete BIPSS if necessary. And neck-to-pelvis CT scan is helpful to localize the lesions of EAS.
What is known and what is new?
• The current case reports on CD and EAS are usually limited to one of the two diseases. Rare case reports describe the two diseases at the same time, and intuitively compare their clinical manifestations, differential diagnosis and lesion location;
• This article reports the localization diagnosis, treatment, and follow-up results of two patients with ACTH-dependent CS with different causes.
What is the implication, and what should change now?
• Surgical resection of the lesion is the first-line treatment, and good management of perioperative hypercortisolemia related complications is conducive to the success of surgery. Regular postoperative follow-up is essential.
Introduction
Cushing’s syndrome (CS) includes adrenocorticotrophic hormone (ACTH)-dependent CS and ACTH-independent CS. According to the literature, the annual incidence rate of CS is between 1.8 and 3.2 cases per million people (1). A recent Swedish study found the annual incidence rate of endogenous CS was 3.2 cases/million/year, of which Cushing’s disease (CD) was 1.5, ectopic ACTH syndrome (EAS) was 0.8, benign adrenal CS was 0.7, and adrenocortical carcinoma was 0.2, respectively (2). A Korean study suggested the annual incidence and prevalence rate of CD from 2013 to 2017 were 2.3 cases per million per year and 9.8 cases per million, respectively (3). Although both the incidence and prevalence rate of CS are not high, the impact on patients is enormous. The reported mortality rate of active CS is higher than that of the general population, and cardiovascular disease is considered the leading cause of death (1). Moreover, a nationwide study in Sweden demonstrated that even after remission, the incidence of stroke, thromboembolism, and sepsis was still elevated in CD patients (4). One study even found an elevated risk of overall mortality for CD patients who were in remission for more than 10 years, and only those who underwent pituitary surgery alone had an average life expectation (5). Given this, early diagnosis and timely treatment of CS are particularly important. In clinical work, the differential diagnosis and lesion localization of ACTH-dependent CS are often difficult, and only by accurately locating the lesions can we clarify the direction for subsequent treatment. However, there is no single, optimal test for patients with ACTH-dependent CS. Several commonly used endocrine dynamic tests have their own pros and cons (6). The differentiation between CD and EAS requires a comprehensive consideration of clinical manifestations, endocrine function tests, bilateral inferior petrosal sinus sampling (BIPSS), and various imaging examinations to make the final diagnosis (7). At present, most of the case reports on CD and EAS are only limited to one of the two. Rare case reports show the two diseases at the same time, and directly compare their clinical manifestations, differential diagnosis and lesion location. This article introduces the clinical feature, diagnosis, treatment and follow-up of two cases of ACTH-dependent CS with different etiologies, with emphasis on the localization diagnosis of the two cases and reviews the literature. It is expected to provide ideas for clinicians in the differential diagnosis of the etiology of ACTH-dependent CS, so as to correctly locate the lesion, and then provide appropriate treatment as early as possible to improve the prognosis of these patients. We present the following article in accordance with the CARE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1177/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Case 1
A 29-year-old female patient was admitted to the hospital in July 2020 due to irregular menstruation for 7 months, weight gain for 6 months, and violaceous striae for 3 months. Seven months ago, the patient went to the gynecology department of another hospital because of a delay of menstruation for 1 month. After completing the relevant examinations, she was diagnosed with polycystic ovary syndrome and started oral ethinylestradiol and cyproterone acetate tablets (Diane-35) treatment. During the medication period, her menstruation was regular, with a cycle of 30 days and a menstrual period of 6–7 days, and she started to gain weight gradually 6 months ago, with a total weight gain of 10 kg. Three months ago, violaceous striae appeared on the patient’s inner thigh and gradually spread to the inner calf skin. She became hirsute and felt fatigued and weak. Ethinylestradiol and cyproterone acetate tablets were discontinued 6 weeks prior to her admission for follow-up. The oral glucose tolerance test (OGTT) results showed blood glucose levels at 0, 1, 2, and 3 h were 5.14, 12.78, 14.84, and 8.09 mmol/L, respectively, and insulin levels were 10.1, 51.3, 86.4, and 54.8 µU/mL respectively. Cortisol at 3 pm was 508.1 nmol/L and ACTH was 11.91 pmol/L. A plain and enhanced computed tomography (CT) scan of the adrenal gland showed the left gland was slightly thickened, while the size, shape, and density of the right adrenal gland were not significantly abnormal. Chest X-ray and bone mineral density were unremarkable. For further diagnosis and treatment, the patient was hospitalized in the Department of Endocrinology and Metabolism. Her past history included a total thyroidectomy for papillary thyroid cancer in 2014, and she was currently undergoing levothyroxine sodium 100 µg qd replacement therapy. Her personal history was unremarkable, and her menstrual history included menarche at age 12, with a menstrual period of 5–6 days and cycle of 28–30 days, with a 1-month delay in menstruation 7 months ago. Her last menstruation was May 21st, 2020. She was married and had a daughter, and both her spouse and daughter were healthy. Her father suffered from diabetes.
On physical examination, her blood pressure was 104/72 mmHg, height 168 cm, weight 71.9 kg, body mass index (BMI) 25.47 kg/m2, waist circumference 91 cm, hip circumference 104 cm, and waist-to-hip ratio 0.88. A full moon face and buffalo back could be seen without obvious pigmentation. Facial hair and fine hair on the limbs and back were obvious, and vellus hair could be seen on her forehead. Scattered acne could be seen on the front chest, and violaceous striae could be seen on the skin of the bilateral armpits, inner thighs, and inner knee joints. Surgical scars about 6 cm in length could be seen in the neck, and bilateral thyroid glands were absent. Cardiopulmonary and abdominal examinations were unremarkable, and muscle strength and muscle tone of the limbs were normal.
Laboratory and imaging examinations showed glycosylated hemoglobin A1c (HbA1c) was 6.4%, fasting blood glucose was 5.63 mmol/L, and three diabetes autoantibodies including insulin autoimmune antibody (IAA), islet cell antibody (ICA), glutamic acid decarboxylase antibody (GAD-Ab) were negative. Total cholesterol (TC) was 3.99 mmol/L, triglyceride (TG) was 1.35 mmol/L, high-density lipoprotein cholesterol (HDL-C) was 1.28 mmol/L, and low-density lipoprotein cholesterol (LDL-C) was 2.71 mmol/L. Thyroid function tests revealed thyroid-stimulating hormone (TSH) 0.006 µIU/mL (normal range, 0.27–4.20 µIU/mL), and free triiodothyronine (FT3) 6.70 pmol/L, free thyroxine (FT4) 17.21 pmol/L. Dehydroepiandrosterone sulfate (DHEA-s) was 61.47 µg/dL (normal range, 98.8–340.0 µg/dL). Liver and kidney function, electrolytes (serum potassium 3.91mmol/L), tumor markers including alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA125), carbohydrate antigen 19-9 (CA19-9), carbohydrate antigen 15-3 (CA15-3), neuron specific enolase (NSE) and cytokeratin-19 fragment (CYFRA21-1), and other endocrine hormones including growth hormone, insulin-like growth factor 1 (IGF-1), androstenedione (AD), sex hormones, aldosterone, renin, urine vanillic acid, and urinary catecholamines showed no obvious abnormality. Serum cortisol concentrations were 88.08 nmol/L at 8:00 am (6–10 am normal range, 133.00–537.00 nmol/L), 97.88 nmol/L at 4:00 pm (4–8 pm normal range, 68.20–327.00 nmol/L), and 124.20 nmol/L at 0:00 am, and serum ACTH concentrations were 6.18 pmol/L at 8:00 am (normal range, 1.60–13.90 pmol/L) and 6.81 pmol/L at 4:00 pm, 8.03 pmol/L at 0:00 am. The 2-day low dose dexamethasone suppression test (LDDST) showed cortisol was 137.80 and 197.90 nmol/L at 8:00 am before and after taking the dexamethasone, and 24-h urinary free cortisol (UFC) was 6.2 and 5.8 µg/24 h, respectively. LDDST was not suppressed. The 2-day high dose dexamethasone suppression test (HDDST) showed cortisol was 340.90 and 21.98 nmol/L at 8:00 am before and after taking the dexamethasone, and 24-h UFC was 5.8 and 12.7 µg/24 h, respectively. In HDDST, blood cortisol could be suppressed, but UFC was abnormally elevated. Pituitary MRI showed a small area of abnormal signal in the left part of the pituitary, with a lesion size of about 4 mm × 3 mm × 3 mm, suggesting pituitary microadenoma (Figure 1). The pituitary stalk was nodularly thickened and about 2 mm in diameter. The results of BIPSS combined with the desmopressin (DDAVP) stimulation test are shown in Table 1. The inferior petrosal sinus (IPS)/peripheral (P) ACTH ratio results showed the basal right IPS/P ACTH ratio was >2, and the DDAVP-stimulated ratio was >3, while the intersinus ACTH gradient was >1.4. This suggested ACTH had obvious central dominance secretion, indicating pituitary ACTH-dependent CS, and that the tumor was more likely to be on the right side of the pituitary.
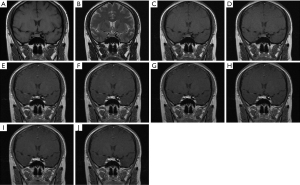
Table 1
| Time (min) | ACTH (pmol/L) | ACTH ratio | |||||
|---|---|---|---|---|---|---|---|
| Left IPS | Right IPS | P | Left IPS/P | Right IPS/P | Right IPS/left IPS | ||
| Baseline | 5.46 | 13.02 | 3.89 | 1.40 | 3.35 | 2.38 | |
| +2 | 117.10 | 9.72 | 6.46 | 18.13 | 1.50 | 0.08 | |
| +5 | 224.90 | >440.40 | 14.25 | 15.78 | >30.91 | >1.96 | |
| +10 | 289.10 | >440.40 | 21.23 | 13.62 | >20.74 | >1.52 | |
| +15 | 347.90 | >440.40 | 27.19 | 12.80 | >16.20 | >1.27 | |
ACTH, adrenocorticotrophic hormone; BIPSS, bilateral inferior petrosal sinus sampling; IPS, inferior petrosal sinus; P, peripheral.
Diagnosis and treatment
This young woman had been suffering from menstrual disorders, weight gain, and purple skin streaks for more than half a year and had a previous history of thyroid cancer surgery. Physical examination revealed a full moon face, buffalo back, hirsutism, and purple skin striae. Auxiliary examination showed abnormal glucose tolerance, disordered circadian rhythm of cortisol, LDDST was not suppressed, and that HDDST showed a contradictory results of serum cortisol and 24-h urine free cortisol. MRI showed pituitary microadenoma, and BIPSS results suggested ACTH was centrally secreted. Based on the above medical history, physical examination, and laboratory test results, the patient was diagnosed with CD, impaired glucose tolerance, hypothyroidism, and papillary thyroid carcinoma postoperatively. During hospitalization, acarbose 100 mg tid was given orally to control blood glucose, and levothyroxine sodium 100 µg qd was given orally as replacement therapy. Subsequently, the patient underwent transsphenoidal surgery in the Department of Neurosurgery of our hospital. During the operation, 1/3 of the left pituitary gland was removed, and the suspected tumor structure on the right side of the gland was also removed. The pathological report showed tumor tissue consistent with pituitary adenoma. Immunohistochemical results showed Ki-67 (approximately 1%+), P53(−), synaptophysin (Syn) (diffuse+), cytokeratin (CK) (AE1/AE3) (approximately 1%+), ACTH (partial+), follicle stimulating hormone (FSH) (punctate+), luteinizing hormone (LH) (partial+), TSH(−), chromogranin A (CgA) (diffuse+), and prolactin (PRL) (focal+).
After the operation, the patient stopped taking acarbose, continued to take levothyroxine sodium 100 µg qd orally, and did not receive glucocorticoid replacement therapy. One month later, she returned to the hospital for a follow-up visit, and her full moon face and buffalo back were lighter than before the operation, the violaceous striae on the skin had become lighter, and her weight had decreased by 5 kg. Her fasting blood glucose was 6.09 mmol/L, her HbA1c was 5.7%, normal levels and rhythms of cortisol and ACTH were restored, and there were no obvious abnormalities in blood sodium, potassium, and sex hormones. A second follow-up 6 months after surgery showed she had no full moon face or buffalo back, the violaceous striae on her skin were less, and the color had again become lighter. Her weight had decreased by 11.8 kg compared with that before the operation, and while the thyroid axis needed to be replaced by the left thyroxine sodium hormone, the hormone levels of the adrenal axis and gonadal axis were basically normal. The patient had a natural pregnancy more than 6 months after the operation, and no obvious abnormality was found during her regular follow-up in the department of Endocrinology and Metabolism and obstetric clinic. She delivered a healthy baby boy in November 2021. A timeline of clinical symptoms, diagnosis, treatment, and prognosis in this case, is presented in Figure 2.

Case 2
A 29-year-old female was admitted to the hospital in April 2020 due to redness of the facial skin and an increase in blood pressure for 1 day. For more than 1 month she had felt her facial skin was red, and at the same time, there was acne on both sides of the cheeks and the mandible, facial hair was more obvious than before, and she felt fatigued and weak. One day earlier she has visited a doctor in another hospital, where her morning blood pressure was about 175–190/125 mmHg, fasting glucose was 5.03 mmol/L, and there was no obvious abnormality in liver and kidney function. The patient took nifedipine tablets orally as prescribed by the doctor, and in the afternoon, she had headaches, dizziness, and nausea, but no vomiting. Blood pressure was not monitored at that time. The patient’s systolic blood pressure was more than 140 mmHg on the morning of admission, and the diastolic blood pressure was unknown. For further diagnosis and treatment, she was sent for further investigation of her hypertension. Her past history included a diagnosis of polycystic ovary syndrome 5 years earlier due to a 3-month extension of the menstrual cycle, which was normalized after treatment with oral Chinese medicine. Her personal history was unremarkable, and her menstrual history included menarche at the age of 12, a menstrual period of 5–6 days, and a cycle of 28–30 days. Her last menstruation was April 3rd, 2020. The patient was married, the first child was born naturally at the age of 19, the second child was born naturally 3 months ago, and she was not breastfeeding. She reported no abnormality during the two pregnancies, and there were no obvious abnormalities in blood pressure, blood glucose, and serum potassium during the obstetric examination. Her spouse, son, and daughter were healthy. The family history was limited to her mother suffering from hypertension.
Physical examination revealed an increased pulse of 118 beats/min, blood pressure 141/100 mmHg, height 163 cm, weight 66.9 kg, BMI 25.18 kg/m2, waist circumference 92 cm, hip circumference 94 cm, and waist-to-hip ratio 0.98. A full moon face and buffalo back could be seen, and acne was evident on both sides of the cheeks and jaw. Obvious facial hair was present, and violaceous striae could be seen on the abdomen. Her abdomen was slightly distended, there was no edema in the lower extremities, and her limb muscle strength and muscle tension were normal.
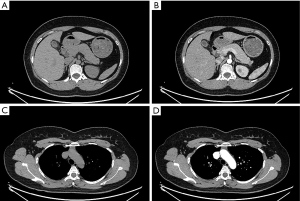
Laboratory and imaging examinations showed TC was 6.53 mmol/L, TG was 1.67 mmol/L, HDL-C was 1.25 mmol/L, and LDL-C was 5.47 mmol/L. OGTT and insulin release tests showed blood glucose at 0, 0.5, 1, 2, and 3 h was 7.06, 14.50, 20.22, 18.56, and 5.93 mmol/L, respectively, and the insulin level was 137.30, 348.90, 457.30, 585.70, and 249.40 pmol/L respectively. HbA1c was 6.3%, and three diabetes autoantibodies (IAA, ICA, GAD-Ab) were negative. Urine routine showed urine glucose 1+, and electrolyte results revealed potassium was 2.62 mmol/L (normal range, 3.50–5.50 mmol/L) and sodium was 147.10 mmol/L (normal range, 137.00–147.00 mmol/L). Blood gas analysis showed pH7.54, HCO3− 30.5mmol/L, standard bicarbonate (SBC) 32.1 mmol/L. FSH was 5.10 IU/L, LH 5.84 IU/L, estradiol (E2) 137.90 pmol/L, PRL 209.50 µIU/L, progesterone (PROG) 12.77 nmol/L, testosterone 6.48 nmol/L (normal range, 0.29–1.67 nmol/L). and AD >10.00 ng/mL (normal range, 0.30–3.30 ng/mL). DHEA-s was 799.50 µg/dL (normal range, 98.80–340.00 µg/dL) and 17α-hydroxyprogesterone (17α-OHP) was 22.54 ng/mL (normal range, follicular phase 0.05–1.02 ng/mL). Thyroid function, growth hormone, IGF-1, parathyroid hormone (PTH), aldosterone, renin, urinary vanillylmandelic acid, urinary catecholamines, calcitonin, tumor markers including AFP, CEA, CA125, CA19-9, CA15-3, NSE, and CYFRA21-1, and gastrin-releasing peptide precursor showed no obvious abnormality. Cortisol at 8:00 am, 4:00 pm, and 0:00 am were all >1,750.00 nmol/L, and ACTH was 74.91 pmol/L at 8:00 am, 73.06 pmol/L at 4:00pm, and 76.54 pmol/L at 0:00 am, respectively (Table 2). The 2-day LDDST showed cortisol at 8:00 am before and after taking the dexamethasone was both more than 1,750.00 nmol/L, and LDDST was not suppressed. The 2-day HDDST showed cortisol was 1,308.00 nmol/L and 1,351.00 nmol/L at 8:00 am before and after taking the dexamethasone, respectively, while the 2-day HDDST was also not suppressed. An electrocardiogram showed sinus tachycardia and ST-T changes, and ambulatory blood pressure showed a 24-h average blood pressure of 196/117 mmHg, with an inversion of the circadian rhythm. No obvious abnormalities were found in bilateral renal arteries and renal, cardiac, and thyroid ultrasound. Bone mineral density indicated a decrease in bone mass. Gastroscopy revealed chronic superficial gastritis with erosions, and colonoscopy showed multiple polyps in the colon (removed), subsequently diagnosed as serrated polyps. Chest CT plain scan showed minor inflammation in the posterior basal segment of the lower lobe of the right lung, while adrenal plain and enhanced CT scan showed diffuse hyperplasia of bilateral adrenal glands (Figure 3A,3B). Chest, abdomen, and pelvis plain and enhanced CT scan showed a round isodensity lesion in the anterior superior mediastinum, with a maximum diameter of 16 mm × 24 mm (Figure 3C,3D). Imaging revealed a soft tissue mass in the anterior superior mediastinum, considered as a possible thymoma, bilateral adrenal diffuse hyperplasia, and multiple kidney stones. Pituitary MRI showed no definite abnormality.
Table 2
| Feature | Preoperative | POD 1 | POD 10 | 3.5 months after surgery | 1 year after surgery | Normal range |
|---|---|---|---|---|---|---|
| FBG (mmol/L) | 7.06 | 5.70 | 5.00 | 4.61 | 4.60 | 3.89–6.11 |
| 2hPG (mmol/L) | 18.56 | 11.00 | 8.00 | 7.19 | 6.42 | 3.89–7.80 |
| HbA1c (%) | 6.3 | / | / | 5.7 | 5.5 | 4.0–6.0 |
| Serum potassium (mmol/L) | 2.62 | 3.57 | 4.60 | 4.31 | 3.82 | 3.50–5.50 |
| Testosterone (nmol/L) | 6.48 | / | 0.685 | 0.613 | 0.965 | 0.29–1.67 |
| AD | >10.00 ng/mL | / | 2.09 ng/mL | 6.21 nmol/L | 5.37 nmol/L | 0.30–3.30 ng/mL |
| Follicular phase 1.22–8.73 nmol/L | ||||||
| Luteal phase 1.05–8.20 nmol/L | ||||||
| DHEA-s (μg/dL) | 799.50 | / | 98.48 | 166.20 | 230.20 | 98.80–340.00 |
| 17α-OHP (ng/mL) | 22.54 | / | 0.71 | 1.69 | 1.63 | Follicular phase 0.05–1.02 |
| Luteal phase 0.30–2.34 | ||||||
| Cortisol at 8:00 am (nmol/L) | >1,750.00 | 156.80 | 375.80 | 161.30 | 234.90 | 133.00–537.00 |
| Cortisol at 4:00 pm (nmol/L) | >1,750.00 | / | 248.10 | 68.74 | 315.50 | 68.20–327.00 |
| Cortisol at 0:00 am (nmol/L) | >1,750.00 | / | 49.27 | 16.13 | 17.84 | – |
| ACTH at 8:00 am (pmol/L) | 74.91 | 4.48 | 6.79 | 4.24 | 4.82 | 1.60–13.90 |
| ACTH at 4:00 pm (pmol/L) | 73.06 | / | 4.30 | 4.25 | 5.74 | 1.60–13.90 |
| ACTH at 0:00 am (pmol/L) | 76.54 | / | 1.91 | 2.24 | 1.63 | 1.60–13.90 |
| LDDST, cortisol at 8:00 am (nmol/L) | >1,750.00 | / | / | 8.60 | 8.88 | – |
POD, postoperative day; FBG, fasting blood glucose; 2hPG, 2-hour postprandial glucose; HbA1c, glycosylated hemoglobin A1c; AD, androstenedione; DHEA-s, dehydroepiandrosterone sulfate; 17α-OHP, 17α-hydroxyprogesterone; ACTH, adrenocorticotrophic hormone; /, this check was not performed; LDDST, low dose dexamethasone suppression test.

This young woman experienced an abrupt onset of her symptoms, which combined with the physical examination findings and auxiliary examination results prompted a diagnosis of ACTH-dependent CS, EAS with high possibility (thymoma?), diffuse bilateral adrenal hyperplasia, secondary hypertension, hypokalemia, steroid-induced diabetes, osteopenia, and dyslipidemia. She was further diagnosed with pneumonia, chronic superficial gastritis with erosion, multiple colon polyps, and multiple bilateral small kidney stones. During hospitalization, the patient received urapidil and sodium nitroprusside by intravenous infusion to control blood pressure, followed by nifedipine controlled-release tablets 30 mg bid and benazepril 10 mg bid orally to lower blood pressure. She was prescribed oral and intravenous potassium supplementation and had spironolactone 20 mg bid to correct hypokalemia. Metformin sustained-release tablets 1 g qd, acarbose 100 mg tid, and canagliflozin 100 mg qd were taken orally to control blood glucose. Other oral medications included levofloxacin 0.5 g qd for anti-infection treatment, atorvastatin 20 mg qn to lower blood lipids, and calcitriol 0.25 µg qd to supplement vitamin D and improve osteopenia. The patient then underwent thoracoscopic anterior mediastinal tumor resection, and the pathological results showed a neuroendocrine tumor prone to carcinoid (G1). Immunohistochemical results revealed CK(+), CD56(+), CD57(+), CgA(+), Syn(+), Ki-67(1%+), epithelial membrane antigen (EMA)(−), vimentin(−), CK19(+), CK7(−), CK20(−), CK5/6(−), P63(−), CD5(−), CD34(−), S-100(−), HMB45(−), melan-A(−), and ACTH(weak +).
At the first follow-up 1 day after surgery, the cortisol was 156.80 nmol/L and ACTH was 4.48 pmol/L at 8:00 am, while 10 days after the operation, the cortisol had returned to a normal rhythm, and testosterone, AD, DHEA-s, and 17α-OHP had all decreased to the normal range (Table 2). The patient’s blood pressure, blood glucose, and serum potassium stabilized, and the antihypertensive, hypoglycemic, and potassium supplementation drugs were gradually reduced and eventually stopped. The patient was finally diagnosed with EAS, thymic carcinoid (G1), and was followed up regularly, during which time her symptoms and signs of CS gradually improved. Blood glucose, blood pressure, and serum potassium were monitored to be normal, and one year after surgery, an adrenal CT scan showed the morphology of the bilateral adrenal glands had returned to normal (Figure 4A,4B), and there was no recurrence sign on chest CT (Figure 4C,4D). A timeline of clinical symptoms, diagnosis, treatment, and prognosis in this case is presented in Figure 5.

Discussion
CS is rare, and according to the level of ACTH, can be divided into ACTH-dependent CS and ACTH-independent CS. The former includes CD, EAS, and the rare ectopic corticotropin-releasing hormone (CRH) syndrome. In clinical work, after making a clear diagnosis of ACTH-dependent CS, distinguishing CD and EAS and finding the lesions are often difficult. In general, CD patients may have a longer course of disease than EAS patients, and the onset is relatively insidious. The typical symptoms and signs of CS, such as full moon face, buffalo back, and violaceous striae, are obvious at the time of consultation. However, EAS patients often have a short course of the disease and rapid onset. The typical manifestations of CS may not be prominent, and patients often seek medical treatment for obvious hypertension, hypokalemia, hyperglycemia, and other reasons. Cortisol levels in these patients are often moderately or severely elevated, and androgen levels may also be elevated to varying degrees. A meta-analysis based on 5,367 patients found the mean time to diagnosis of CS was 34 months, and the mean time to diagnosis of CD and EAS was 38 and 14 months, respectively (8). Some authors have further divided EAS into aggressive EAS and indolent EAS according to clinical manifestations, hormone levels, and prognosis. The clinical manifestations of the former may not be typical, with symptoms and signs mainly due to protein catabolism, while ACTH and cortisol are extraordinarily elevated. These patients have a rapid onset, short course of disease, and poor prognosis. Conversely, the clinical manifestations of indolent EAS overlap considerably with those of CD, with ACTH and cortisol levels showing only a mild increase. These patients have a relatively long course of disease and an acceptable prognosis (9). In case 2, the blood pressure, blood glucose, serum potassium, and other indicators were not significantly abnormal during delivery in another hospital 3 months earlier, but 2 months later the patient began to experience atypical symptoms such as facial redness, acne, fatigue, and weakness. During hospitalization, the patient’s blood pressure was significantly increased, along with refractory hypokalemia and alkalemia. The cortisol level exceeded the upper limit of detection, androgen was also significantly increased, and bone mass decreased. The above test results suggested an abrupt onset and rapid progress.
At present, the clinical differentiation of CD and EAS remains challenging and needs to be comprehensively evaluated in combination with clinical manifestations, endocrine dynamic function tests, BIPSS, and various imaging examinations, although each method has its own precautions and deficiencies (10). While a suppression of more than 50% in the HDDST usually indicates a greater likelihood of CD, previous studies suggest the sensitivity and specificity of HDDST are different, with a 2020 study reporting a sensitivity of only 84.5% and specificity of 47.6%. This shows HDDST could be inhibited in 84.5% of CD patients but could also be inhibited in up to 52.4% of EAS patients (11). However, the study also pointed out the sensitivity and specificity of the CRH stimulation test in differentiating CD and EAS was greater than 80% (12), and the combined HDDST and CRH stimulation test had a sensitivity of 95% and a specificity of 93% for the detection of CD (13). Despite this, CRH is often difficult to obtain in our country, and CRH stimulation tests or the above-mentioned combined tests cannot be performed. Therefore, the results of endocrine function tests alone often bring uncertainty to clinical diagnosis.
On the other hand, pituitary MRI is often required to be perfected for localization diagnosis, although an occasional pituitary tumor with a diameter of 3–6 mm can be found in pituitary MRI in about 10% of healthy individuals (14). Therefore, CD cannot be diagnosed by pituitary MRI finding lesions less than or equal to 6 mm in diameter alone. In addition, it has been reported that about half of CD patients have no visible lesions on pituitary MRI (15,16). With the development of MRI technology, investigators reported that in a series study using 3-T MRI and including 115 CD patients, the sensitivity and specificity of detecting pituitary tumors were 80% and 100%, respectively. Further, 3D spoiled gradient-echo (SGE) was the best detection sequence and could detect pituitary tumors with a diameter of only 2 mm (17). In addition, Law et al. reported 7-T MRI could accurately locate a pituitary microadenoma in a CD patient which was negative on both 1.5-T and 3-T MRI (18). However, a recent study found the positive rate of pituitary MRI lesions in EAS patients was 29.2%, and even 9.5% of EAS patients had pituitary lesions with a diameter of more than 6 mm (11). In summary, the pituitary MRI results of patients with ACTH-dependent CS should be interpreted with caution, and a comprehensive judgment should be made in combination with endocrine function tests and other examination results.
At present, BIPSS is regarded as the gold standard for differentiating CD from EAS (19,20), with a sensitivity and specificity for diagnosing CD of more than 95% in experienced medical centers (21,22). When pituitary MRI shows the diameter of a pituitary lesion is less than 6 mm or the results of functional tests are inconsistent, BIPSS is required to confirm the diagnosis. Therefore, in the guideline for the treatment of CS issued in 2015, it was suggested that, if feasible, all patients with ACTH-dependent CS and patients with pituitary tumors <6 mm be immediately referred to a pituitary tumor center of excellence to complete BIPSS to identify CD and EAS (23). The results show that if the basal IPS/P ACTH ratio is >2, and/or the CRH or DDAVP-stimulated ratio is >3, CD is indicated, and if otherwise, EAS is indicated (20,21,24,25). However, some authors have put forward different opinions on BIPSS over the past 2 years, including the view that a combination of some non-invasive examinations including CRH and DDAVP stimulation test, pituitary MRI, and whole body thin-layer CT scan exempt about 50% of patients with ACTH-dependent CS from BIPSS (26). Despite this, a recently published consensus on diagnosis and management of CD (27) points out that combined testing is currently limited to an experienced multidisciplinary team, the quality of evidence is very low, and it is a discretionary recommendation. At the same time, the consensus recommends (27) all patients with ACTH-dependent CS with pituitary lesions less than 6 mm in diameter should undergo BIPSS, although it is not required if the pituitary MRI shows the adenoma is greater than 10 mm or larger and endocrine function test results support the diagnosis of CD. For pituitary adenoma with a diameter of 6–9 mm, experts have different opinions, but most recommend perfecting BIPSS for a definitive diagnosis. In case 1, although HDDST suggested serum cortisol could be suppressed, pituitary MRI showed the pituitary microadenoma diameter was only 4 mm. This raised the questions of whether the patient could be diagnosed with CD and whether the pituitary microadenoma was the responsible lesion. Therefore, combined with the recommendations of the guidelines, we requested the neurosurgery department perform BIPSS, and the results suggested ACTH had an obvious central predominant secretion, which supported the diagnosis of CD. Although BIPSS is the gold standard for discriminating CD from EAS, its significance in the tumor lateralization of the pituitary gland is limited (27). Taking interpetrosal (side-to-side) ratios ≥1.4 as the cutoff point, the accurate prediction rate of left and right localization of pituitary microadenoma was about 69% (28). Although the accurate prediction rate of BIPSS for the lateralization of pituitary microadenoma is not very high, it still has certain significance for clinical guidance on surgical localization. In case 1, pituitary MRI showed a microadenoma with a diameter of about 4 mm on the left side of the pituitary. However, the BIPSS results showed that after stimulation by DDAVP, the ACTH ratio of the right IPS/left IPS was greater than 1.4, suggesting the lesion may be on the right side of the pituitary. During surgery, the lesion on the right side and 1/3 of the pituitary gland on the left were removed after making a detailed exploration. The postoperative pathology and improvement of symptoms, signs, and hormone levels in the follow-up indicated localization in this case was accurate.
While EAS is one cause of ACTH-dependent CS, it brings great difficulties to the localization diagnosis because its lesions can be distributed in various body organs. A review published in the Lancet in 2015 suggested that when considering a diagnosis of EAS, a neck-to-pelvis thin-slice CT scan or MRI should be used to identify ectopic lesions, and advanced imaging examination such as octreotide scan or positron emission tomography (PET)-CT should be used if necessary (16). According to a recent study, 68Ga DOTATATE PET-CT had relatively high sensitivity for the detection of primary and metastatic ectopic ACTH secreting tumors, and more easily identified occult tumors compared with a routine examination (29). In a published review that included the largest number of EAS cases, the localization of lesions in 383 EAS patients was analyzed. The most common tumors were thoracic tumors, such as pulmonary or mediastinal carcinoids, small cell lung carcinoma, thymic tumors, and medullary thyroid carcinoma. Abdominal tumors were also not uncommon, such as islet cell tumors, pheochromocytomas, and gastrointestinal carcinoids (30). In case 2, after completing the LDDST and HDDST, pituitary MRI and other examinations to confirm the diagnosis of EAS, the chest, abdomen and pelvis CT, thyroid ultrasound, and gastrointestinal endoscopy were improved according to the recommendations in the literature. A suspicious lesion was finally found in the anterior superior mediastinum, and postoperative pathology demonstrated a thymic carcinoid, and ACTH was weakly positive. The improvement of blood pressure, blood glucose, and serum potassium in the follow-up and the recovery of various elevated hormones indicated the qualitative and localization diagnosis of this case were correct.
Early recognition and clinical management
As CS is a rare and serious disease with a high mortality rate, early identification of suspected patients and timely diagnosis can help improve their prognosis. For young patients with hypertension or osteoporosis, women with acne, hirsutism and irregular menstruation, and patients with full moon face, buffalo back, facial plethora, purple striae, thin skin, muscle weakness and other signs, it is recommended to complete the screening of CS to make a clear diagnosis (31,32). In a consensus on the diagnosis and treatment of hypertension in patients with CS published this year, it is suggested to screen CS in the following hypertensive populations: patients under 40 years old with grade 2 hypertension, patients with childhood onset hypertension, refractory hypertension, patients with previously chronic stable hypertension presenting with acute exacerbation of blood pressure, hypertension with adrenal lesions, and hypertension with clinical features specific for hypercortisolism (33). Some authors investigated 18 key symptoms of CS in CS patients and non-CS patients, and 5 of them were more common in CS patients than those excluding CS: osteoporosis incompatible with age, adrenal incidentaloma, metabolic syndrome, myopathy, and multiple symptoms such as hypertension, diabetes, and sleep disorders. Obesity was more common in patients excluding CS, while recent significant weight gain was more common in patients with CS (34). The female patient of case 1 complained of irregular menstruation, short-term weight gain, and purple striae, while the young female patient of case 2 presented with facial plethora, acne, and new-onset refractory hypertension. Therefore, we performed CS related examinations for the patients and finally confirmed the diagnosis. At present, surgical resection of the lesion is the first-line treatment for CS. During the perioperative period, hypercortisolemia related complications such as diabetes, hypertension, hypokalemia, infection, hyperlipidemia, osteoporosis and other diseases should be well managed (23). Especially for patients with severe hypercortisolism, the comorbidity of hypokalemia, hypertension, diabetes with ketoacidosis, pulmonary embolism, opportunistic infection and acute psychosis may endanger life. It is more meaningful to try to improve the general condition of the patient before the operation (35). After surgery, the hypercortisolemia is reversed, leading to improvements in diabetes and hypertension. Therefore, it is important to closely monitor blood glucose and blood pressure after surgery and adjust medication as needed (36). Postoperative patients should receive long-term follow-up and lifelong recurrence monitoring (23). In case 1 with impaired glucose tolerance and hypothyroidism, hypoglycemic therapy was administered preoperatively and thyroid hormone replacement therapy was continued. Compared with case 1, the situation in case 2 was more complex and serious. The patient had resistant hypertension and refractory hypokalemia, accompanied by diabetes, pneumonia, hyperlipidemia and osteopenia. We gave corresponding treatment for the above conditions, especially for the treatment of hypertension, combined with benazepril, nifedipine and spironolactone. According to the consensus issued this year, angiotensin conversion-enzyme inhibitors (ACE-Is) or angiotensin receptor blockers (ARBs) is recommended as the first choice of antihypertensive drugs for CS patients with hypertension. If the blood pressure is still greater than 130/80 mmHg, calcium channel blockers can be added. If the blood pressure is still not up to standard, mineralocorticoid receptor antagonists can be added (33). After active and effective preoperative management, the general conditions of the two patients were significantly improved and received surgical treatment. After operation, the blood glucose of case 1, both blood glucose and blood pressure of case 2 gradually decreased to normal, and the dose of hypoglycemic and antihypertensive drugs gradually decreased and were discontinued. The patients were followed up regularly in our department after the operation, and no signs of recurrence were found.
In this paper, two cases of ACTH-dependent CS were introduced. In the process of diagnosis and treatment, endocrine function tests, pituitary MRI, whole-body CT, and BIPSS were used for differential diagnosis, to accurately locate the focus and lay a foundation for the success of subsequent treatment. At the same time, there are some deficiencies in the diagnosis of case 1. The patient had papillary thyroid cancer, pituitary adenoma, and CD, and attention should be paid to the investigation of multiple endocrine neoplasia. In these cases, it is recommended patients undergo a relevant genetic examination if agreed to.
Conclusions
In conclusion, the differentiation of CD and EAS should be comprehensively evaluated in combination with the medical history, clinical manifestations, dynamic tests such as HDDST, pituitary MRI, and other examinations. If the dynamic test results are discordant or pituitary MRI shows the lesion is less than 6 mm, BIPSS should be performed to confirm the diagnosis. If necessary, BIPSS can also be performed when the pituitary adenoma with a diameter of 6–9 mm. EAS tends to have a shorter course of the disease, more prominent clinical manifestations, and higher levels of cortisol, ACTH, and other hormones. Due to the lesions of EAS being complex and diverse, attention should be paid to imaging examinations of the neck, chest, abdomen, and pelvic organs in localization diagnosis. If conventional CT or MRI examinations are negative, advanced imaging examinations such as PET-CT and octreotide imaging can be used to locate lesions.
Acknowledgments
Funding: This study was supported by the Natural Science Foundation of Shenzhen University General Hospital (No. SUGH2018QD008).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1177/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1177/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hakami OA, Ahmed S, Karavitaki N. Epidemiology and mortality of Cushing's syndrome. Best Pract Res Clin Endocrinol Metab 2021;35:101521. [Crossref] [PubMed]
- Wengander S, Trimpou P, Papakokkinou E, et al. The incidence of endogenous Cushing's syndrome in the modern era. Clin Endocrinol (Oxf) 2019;91:263-70. [Crossref] [PubMed]
- Park JS, Yun SJ, Lee JK, et al. Descriptive Epidemiology and Survival Analysis of Prolactinomas and Cushing's Disease in Korea. Endocrinol Metab (Seoul) 2021;36:688-96. [Crossref] [PubMed]
- Papakokkinou E, Olsson DS, Chantzichristos D, et al. Excess Morbidity Persists in Patients With Cushing's Disease During Long-term Remission: A Swedish Nationwide Study. J Clin Endocrinol Metab 2020;105:dgaa291. [Crossref] [PubMed]
- Clayton RN, Jones PW, Reulen RC, et al. Mortality in patients with Cushing's disease more than 10 years after remission: a multicentre, multinational, retrospective cohort study. Lancet Diabetes Endocrinol 2016;4:569-76. [Crossref] [PubMed]
- Ferriere A, Tabarin A. Biochemical testing to differentiate Cushing's disease from ectopic ACTH syndrome. Pituitary 2022;25:705-8. [Crossref] [PubMed]
- Hayes AR, Grossman AB. Distinguishing Cushing's disease from the ectopic ACTH syndrome: Needles in a haystack or hiding in plain sight? J Neuroendocrinol 2022;34:e13137. [Crossref] [PubMed]
- Rubinstein G, Osswald A, Hoster E, et al. Time to Diagnosis in Cushing's Syndrome: A Meta-Analysis Based on 5367 Patients. J Clin Endocrinol Metab 2020;105:dgz136. [Crossref] [PubMed]
- Araujo Castro M, Marazuela Azpiroz M. Two types of ectopic Cushing syndrome or a continuum? Pituitary 2018;21:535-44. Review. [Crossref] [PubMed]
- Hayes AR, Grossman AB. The Ectopic Adrenocorticotropic Hormone Syndrome: Rarely Easy, Always Challenging. Endocrinol Metab Clin North Am 2018;47:409-25. [Crossref] [PubMed]
- Chen S, Chen K, Wang S, et al. The Optimal Cut-off of BIPSS in Differential Diagnosis of ACTH-dependent Cushing's Syndrome: Is Stimulation Necessary? J Clin Endocrinol Metab 2020;105:dgz194. [Crossref] [PubMed]
- Ceccato F, Tizianel I, Vedolin CK, et al. Human Corticotropin-Releasing Hormone Tests: 10 Years of Real-Life Experience in Pituitary and Adrenal Disease. J Clin Endocrinol Metab 2020;105:dgaa564. [Crossref] [PubMed]
- Isidori AM, Kaltsas GA, Mohammed S, et al. Discriminatory value of the low-dose dexamethasone suppression test in establishing the diagnosis and differential diagnosis of Cushing's syndrome. J Clin Endocrinol Metab 2003;88:5299-306. [Crossref] [PubMed]
- Hall WA, Luciano MG, Doppman JL, et al. Pituitary magnetic resonance imaging in normal human volunteers: occult adenomas in the general population. Ann Intern Med 1994;120:817-20. [Crossref] [PubMed]
- Grober Y, Grober H, Wintermark M, et al. Comparison of MRI techniques for detecting microadenomas in Cushing's disease. J Neurosurg 2018;128:1051-7. [Crossref] [PubMed]
- Lacroix A, Feelders RA, Stratakis CA, et al. Cushing's syndrome. Lancet 2015;386:913-27. [Crossref] [PubMed]
- Fukuhara N, Inoshita N, Yamaguchi-Okada M, et al. Outcomes of three-Tesla magnetic resonance imaging for the identification of pituitary adenoma in patients with Cushing's disease. Endocr J 2019;66:259-64. [Crossref] [PubMed]
- Law M, Wang R, Liu CJ, et al. Value of pituitary gland MRI at 7 T in Cushing's disease and relationship to inferior petrosal sinus sampling: case report. J Neurosurg 2018; Epub ahead of print. [Crossref]
- Loriaux DL. Diagnosis and Differential Diagnosis of Cushing's Syndrome. N Engl J Med 2017;376:1451-9. [Crossref] [PubMed]
- Perlman JE, Johnston PC, Hui F, et al. Pitfalls in Performing and Interpreting Inferior Petrosal Sinus Sampling: Personal Experience and Literature Review. J Clin Endocrinol Metab 2021;106:e1953-67. [Crossref] [PubMed]
- Oldfield EH, Doppman JL, Nieman LK, et al. Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis of Cushing's syndrome. N Engl J Med 1991;325:897-905. [Crossref] [PubMed]
- Deipolyi AR, Alexander B, Rho J, et al. Bilateral inferior petrosal sinus sampling using desmopressin or corticotropic-releasing hormone: a single-center experience. J Neurointerv Surg 2015;7:690-3. [Crossref] [PubMed]
- Nieman LK, Biller BM, Findling JW, et al. Treatment of Cushing's Syndrome: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2015;100:2807-31. [Crossref] [PubMed]
- Vassiliadi DA, Mourelatos P, Kratimenos T, et al. Inferior petrosal sinus sampling in Cushing's syndrome: usefulness and pitfalls. Endocrine 2021;73:530-9. [Crossref] [PubMed]
- Feng M, Liu Z, Liu X, et al. Tumour lateralization in Cushing's disease by inferior petrosal sinus sampling with desmopressin. Clin Endocrinol (Oxf) 2018;88:251-7. [Crossref] [PubMed]
- Frete C, Corcuff JB, Kuhn E, et al. Non-invasive Diagnostic Strategy in ACTH-dependent Cushing's Syndrome. J Clin Endocrinol Metab 2020;105:dgaa409. [Crossref] [PubMed]
- Fleseriu M, Auchus R, Bancos I, et al. Consensus on diagnosis and management of Cushing's disease: a guideline update. Lancet Diabetes Endocrinol 2021;9:847-75. [Crossref] [PubMed]
- Wind JJ, Lonser RR, Nieman LK, et al. The lateralization accuracy of inferior petrosal sinus sampling in 501 patients with Cushing's disease. J Clin Endocrinol Metab 2013;98:2285-93. [Crossref] [PubMed]
- Wannachalee T, Turcu AF, Bancos I, et al. The Clinical Impact of [68 Ga]-DOTATATE PET/CT for the Diagnosis and Management of Ectopic Adrenocorticotropic Hormone - Secreting Tumours. Clin Endocrinol (Oxf) 2019;91:288-94. [Crossref] [PubMed]
- Isidori AM, Lenzi A. Ectopic ACTH syndrome. Arq Bras Endocrinol Metabol 2007;51:1217-25. [Crossref] [PubMed]
- Tabarin A, Assié G, Barat P, et al. Consensus statement by the French Society of Endocrinology (SFE) and French Society of Pediatric Endocrinology & Diabetology (SFEDP) on diagnosis of Cushing's syndrome. Ann Endocrinol (Paris) 2022;83:119-41. [Crossref] [PubMed]
- Scoffings K, Morris D, Pullen A, et al. Recognising and diagnosing Cushing's syndrome in primary care: challenging but not impossible. Br J Gen Pract 2022;72:399-401. [Crossref] [PubMed]
- Fallo F, Di Dalmazi G, Beuschlein F, et al. Diagnosis and management of hypertension in patients with Cushing's syndrome: a position statement and consensus of the Working Group on Endocrine Hypertension of the European Society of Hypertension. J Hypertens 2022;40:2085-101. [Crossref] [PubMed]
- Braun LT, Vogel F, Zopp S, et al. Whom Should We Screen for Cushing Syndrome? The Endocrine Society Practice Guideline Recommendations 2008 Revisited. J Clin Endocrinol Metab 2022;107:e3723-30. [Crossref] [PubMed]
- Castinetti F. Medical management of Cushing's disease: When and how? J Neuroendocrinol 2022;34:e13120. [Crossref] [PubMed]
- Honegger J, Nasi-Kordhishti I. Surgery and perioperative management of patients with Cushing's disease. J Neuroendocrinol 2022;34:e13177. [Crossref] [PubMed]
(English Language Editor: B. Draper)


