
A comparative bibliometric analysis of Omicron and Delta variants during the COVID-19 pandemic
Highlight box
Key findings
• Publications and clinical trials related to COVID-19 increased annually with different areas of research.
What is known and what is new?
• The research hotspots of Delta and Omicron variants are quite different.
• The relevant research trends of COVID-19 have shifted from vaccine development to infection control and management of complications.
What is the implication, and what should change now?
• The ongoing clinical studies will verify the safety and efficacy of promising drugs.
Introduction
Since the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) at the end of 2019, coronavirus disease 2019 (COVID-19) has spread to many countries and become a global pandemic (1). According to the World Health Organization (WHO) COVID-19 dashboard, up to November 2022, there have been 627,573,579 confirmed COVID-19 cases globally, including 6,570,363 deaths (2). As a research hotspot, there have been more than 260,000 papers about COVID-19 indexed in PubMed, with nearly 3,000 of these being clinical trials. The results of the completed studies and those still in progress are important for containing the spread of the epidemic and improving the outcomes for severe COVID-19 infections.
Variants of SARS-CoV-2 are constantly being detected, and their transmissibility and pathogenicity vary greatly. The Delta variant was first isolated in India during the second wave of the pandemic in October 2020 (3). The Omicron variant was first detected in Botswana and South Africa in November 2021 and has become a significant epidemic strain in 180 countries till now (4). Therefore, we hypothesize that a comparative bibliometric analysis of the Delta and Omicron variants in the COVID-19 pandemic should be conducted, and it may provide new research directions. Meanwhile, the relevant clinical trials that have been registered should be analyzed to identify the management prospects of the pandemic.
Methods
Research design and data collection
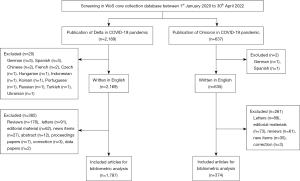
Bibliometric analysis is a rigorous method for exploring and analyzing large volumes of scientific data, and it presents the intellectual structure of a research topic based on performance analysis and science mapping (5). A bibliometric analysis aims to systematically compare various scientific publications using network maps for the Delta and Omicron research fields. The Web of Science (WoS) Core Collection database was screened to collect articles related to Delta and Omicron variants during the COVID-19 pandemic from 1 January 2020 to 30 April 2022. In addition, we conducted a comprehensive review of the relevant trials registered at ClinicalTrials.gov over the same period. To cover a greater number of publications, the search strategy was guided by previous procedural guidelines (4,5). To identify journals relevant to the Delta variant during the COVID-19 pandemic, we searched for the following keywords in both Medical Subject Headings (MeSH) and article titles: “Delta”, “B.1.617.2”, “COVID-19”, “Novel Coronavirus”, and “SARS-CoV-2”. To identify publications relevant to the Omicron variant, we used the keywords “Omicron”, “B.1.1.529”, “COVID-19”, “Novel Coronavirus”, and “SARS-CoV-2”. Searches for both Delta and Omicron studies were performed within 1 day by two authors (Yang-Xi Liu and Yue-Tian Yu) independently to avoid searching bias caused by updating the database.
Data extraction
For each publication identified, the title, author, abstract, and keywords were extracted for bibliometric analysis and downloaded in text format from the WoS Core Collection database using its citation indexing service. Studies for this analysis were restricted to original articles published in English. Studies that were not published in English and other types of essays, such as reviews, letters, and commentaries, were excluded.
We also extracted the clinical studies data of the Delta and Omicron variants registered at ClinicalTrials.gov.
Statistical analysis
Using the online functions of Bibliometric (https://bibliometric.com/), the total volume of publications and the national relationship network for studies in both the Delta and Omicron fields were established. Bibliometric analysis of authors, countries, organizations, citations, keywords, themes, and bibliographic coupling for both study areas was performed, and network maps were visualized using VOSviewer version 1.6.10 (Leiden University, Amsterdam, Netherlands). A matrix of keyword clustering for the Delta and Omicron variants during the COVID-19 pandemic was created using the Bibliographic Item Co-Occurrence Matrix Builder version 2.0 (BICOMB; Lei Cui, China Medical University, Shenyang, China), and Graphical Clustering Toolkit version 1.0 (gCLUTO; University of Minnesota, Minneapolis, MN, USA). Patterns in keywords and citations in each study field were visualized using CiteSpace Version 5.8 R3 (Chen Meichao, Drexel University, Philadelphia, PA, USA).
Results
Overview of publications
Initially, 2,189 and 637 publications on Delta and Omicron variants, respectively, during the COVID-19 pandemic were identified. Of these, 1,787 and 374 original articles published in English on the Delta and Omicron variants were included in the further bibliometric analysis (Figure 1). In all, 107 and 77 countries contributed literature on the Delta and Omicron variants, respectively. The top five countries publishing articles on research into the Delta and Omicron variants were the same for each variant: the USA, followed by China, the UK, India, and Germany (Table S1). In 2021, the year with the most significant number of publications, 878 (49.1%) articles related to the Delta variant were published. However, 718 (40.2%) articles were notably published in the first 3 months of 2022. Meanwhile, reports regarding the Omicron variant were first recorded by the WoS Core Collection database in 2021, with most being published in the first 3 months of 2022 (n=341; 91.2%). There was a rapid increase in the number of publications in both areas from 1 January 2020 to 30 April 2022 (Figure S1).
Bibliometric analysis of keywords
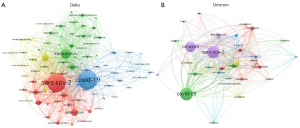
VOSviewer identified 5,999 and 1,107 keywords from articles on the Delta and Omicron variants. As shown in Figure 2, the top two keywords frequently present in articles on both Delta and Omicron were the same: “COVID-19” (713 occurrences, with a total link strength of 1,525 for the Delta variant; 137 occurrences, with a total link strength of 354 for the Omicron variant), followed by “SARS-CoV-2” (553 occurrences, with a total link strength of 1,478 for the Delta variant; 132 occurrences, with a total link strength of 395 for the Omicron variant). The other top 5 keywords in research into the Omicron variant were “variants”, “lockdown”, and “mobile cabin hospital”. In both study areas, the most vital link between keywords occurred for “lockdown” and “mobile cabin hospital” (link strength of 77 and 294 for the Delta and Omicron variants, respectively).
Analysis of research themes and topic trends
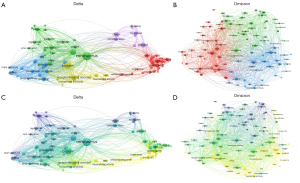
VOSviewer revealed five theme clusters in articles on the Delta variant: the origin and transmission of the Delta variant; the molecular structure of the Delta variant; binding sites and mode of activation; epidemiology and co-infection of the Delta variant; and handling of the Delta infection, including quarantine and lockdown (Figure 3A). We detected three theme clusters among articles on the Omicron variant: virus physiology and mutation; vaccine and human immune responses to Omicron; and infection control, consisting of mobile cabin hospitals (MCHs) and emergency preparedness (Figure 3B).
The trends in research themes for each variant are shown in Figure 3C,3D. The areas of intense research for both the Delta and Omicron variants changed from tracing the source of both viruses, vaccine development, and pharmacotherapy to blocking transmission and lockdown.
Figure 4 shows the matrices of keywords generated by BICOMB and biclustering by gCLUTO. There were five keyword clusters for articles related to the Delta variant. Of these clusters, Cluster 2 contained 10 items, was considered the most prominent cluster, and thus defined the keywords of virus variant related to COVID-19. There were three keyword clusters for articles pertaining to the Omicron variant. Of these, Cluster 2 was the largest, with 15 items, and focused on SARS-CoV-2 variants and immune system reaction to the virus.

Co-citation clustering and time evolution analysis
Co-citation analysis divided references in studies on the Delta and Omicron variants into 61 and 27 clusters by CiteSpace software, respectively. Cluster results related to the Delta and Omicron variants revealed a weight mean silhouette of 0.92 and 0.89, respectively (value range 0 to 1; reference value ≥0.7), and a modularity Q of 0.67 and 0.55, respectively (value range 0 to 1; reference value ≥0.3). Clusters containing >10 items (labeled by the log-likelihood ratios) were shown in Figure 5. The five largest clusters for Delta-related articles were “porcine Delta coronavirus” (#0; size: 83; silhouette: 0.99), “SARS-CoV-2 variant” (#1; size: 81; silhouette: 0.90), “main protease” (#2; size: 59; silhouette: 0.79), “CpG depletion” (#3; size: 41; silhouette: 0.86), and “COVID-19 patient” (#4; size: 39; silhouette: 0.95). In time evolution analysis, Cluster #1 was frequently cited by Clusters #2–#6 (Figure 5A,5C).
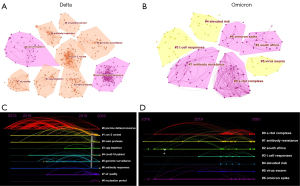
The five largest clusters for Omicron-related articles were “spike protein receptor-binding domain (S-RBD) complexes” (#0; size: 31; silhouette: 0.91), “antibody resistance” (#1; size: 31; silhouette: 0.80), “South Africa” (#2; size: 22; silhouette: 0.84), “T cell responses” (#3; size: 21; silhouette: 0.96), and “elevated risk” (#4; size: 19; silhouette: 0.99). Time evolution analysis for Omicron-related research indicated that clusters including over 10 items were highly cited after 2019 (Figure 5B,5D).
Bibliometric analysis of co-authorship
In all, there were 12,896 authors of Delta-related publications, with 32 publishing >5 articles. Shaobo Xiao from Huazhong Agricultural University (Wuhan, China) co-authored 13 reports with 71 citations (total link strength of 25). Among the 32 authors who published >5 articles, Gavin Dabrera from the UK Health Security Agency (London, UK) had the most citations (n=908) of the five articles he co-authored, with a total link strength of 8. In all, 2,759 authors contributed to Omicron-related research publications, with six authors being listed on a maximum of four articles. Kwok-Yung Yuen from the University of Hong Kong (Hong Kong, China) was the author with the most citations (n=80) of the four articles he co-authored (Figure S2).
The literature search revealed 3,619 and 1,089 organizations published Delta- and Omicron-related research papers, respectively. Of these organizations, 228 and 31 produced >5 Delta- and Omicron-related research papers, respectively. The Chinese Academy of Sciences published the most Delta- and Omicron-related articles. Details of the top 5 organizations publishing Delta- and Omicron-related research papers are presented in Table S2. Cooperation network maps for the organizations in each field are shown in Figure S3.
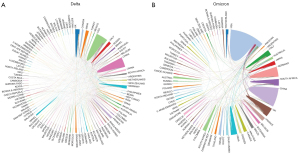
The US contributed the most Delta- and Omicron-related articles had a more significant interaction with other countries, and had the greatest total link strength. For Delta- and Omicron-related research, the US collaborated with 52 (total link strength 433) and 25 (total link strength 121) other countries, respectively. Notably, the US collaborated with China the most in research on the Delta variant (total link strength 53) and with the UK and South Africa the most in research on the Omicron variant (total link strength with both countries =12; Figure 6).
Analysis of trials registered with ClinicalTrials.gov
From 1 January 2020 to 30 April 2022, 12 trials related to research on the Delta variant and nine related to the Omicron variant were registered with ClinicalTrials.gov. Of the 12 clinical trials connected to the Delta variant, 11 (91.7%) were observational studies, and 4 (33.3%) included children as participants. Only 1 (8.3%) trial was reported completed, and none of the trials achieved precise results during the study period. Most clinical trials were conducted by the pharmaceutical industry (n=9; 75%). The characteristics of the Omicron-related trials were similar to those of the Delta-related trials. All nine Omicron-related trials were interventional studies, and 4 (44.4%) involved children. None of the Omicron-related trials were reported as completed, with clinical results, and most were pharmaceutical industry-funded (n=8; 88.8%). No Omicron-related trials were registered from the Asia-Pacific region. All registered trials of the Delta and Omicron variants aimed to evaluate the immunogenicity and safety of different vaccines in various populations. Further details of all registered trials are provided in Table 1.
Table 1
| Characteristics | Delta (n=12) | Omicron (n=9) | |||
|---|---|---|---|---|---|
| Number | Percentage (%) | Number | Percentage (%) | ||
| Study type | |||||
| Interventional | 11 | 91.7 | 9 | 100.0 | |
| Observational | 1 | 8.3 | 0 | 0.0 | |
| Participant age | |||||
| Children (<18 years) | 4 | 33.3 | 4 | 44.4 | |
| Adults (18–64 years) | 12 | 100.0 | 9 | 100.0 | |
| Older adults (≥65 years) | 11 | 91.7 | 9 | 100.0 | |
| Trial status | |||||
| Not recruiting | 6 | 50.0 | 4 | 44.4 | |
| Recruiting | 5 | 41.7 | 3 | 33.3 | |
| Active | 0 | 0.0 | 1 | 11.1 | |
| Completed | 1 | 8.3 | 0 | 0.0 | |
| Results | |||||
| With results | 0 | 0.0 | 0 | 0.0 | |
| Without result | 12 | 100.0 | 9 | 100.0 | |
| Funding source | |||||
| NIH | 1 | 8.3 | 1 | 11.1 | |
| Industry | 9 | 75.0 | 8 | 88.8 | |
| Other | 2 | 16.7 | 0 | 0.0 | |
| Locations | |||||
| Africa | 1 | 8.3 | 1 | 11.1 | |
| Asia | 1 | 8.3 | 0 | 0.0 | |
| Europe | 2 | 16.7 | 2 | 22.2 | |
| Middle East | 3 | 25.0 | 1 | 11.1 | |
| United States | 2 | 16.7 | 3 | 33.3 | |
| Pacifica | 1 | 8.3 | 0 | 0.0 | |
NIH, National Institutes of Health.
Discussion
As a prevailing global pandemic, the epidemiological strains of COVID-19 have changed constantly since SARS-CoV-2 was first detected. Of the COVID-19 variants, Delta and Omicron are the most highly represented and have become the dominant strain in most countries because of their increased transmissibility and ability to cause a severe form of the disease (1,2).
Based on the results of the research themes analysis, it was revealed that the study hotspots of the two variants were distinguishing. Researchers devoted more attention to the vaccine and infection control in Omicron compared with Delta, which were more interested in the origin from the beginning on account of the topic trends. Studies showed great interest in exploring the mutations that emerged, structures of spike complexes, and neutralizing antibodies in both Delta and Omicron variants. Kumar et al. stated that significant changes in the RBD region of the Omicron variant might contribute to high binding specificity with human angiotensin-converting enzyme 2 (hACE2), which may result in a higher transmission rate and considerable impact on pathogenesis when compared to Delta variant (6). Planas et al. proved Omicron escapes most therapeutic monoclonal antibodies and vaccine-elicited antibodies (7). Mlcochova et al. observed that Delta spike protein was able to mediate highly efficient syncytium formation that was less sensitive to inhibition by neutralizing antibodies and had higher replication and spike-mediated entry (8). Such researchers hint at a higher level of difficulty in vaccine development.
Although our bibliometric analysis of articles and clinical trials revealed that vaccine development is still in progress, however, as nearly 12.84 billion vaccine doses have been administered (2), the demand for vaccines has started to decline. Moreover, the high levels of mutations in Delta and Omicron variants lead to severe concerns in vaccine failure, immune escape, and increasing transmissibility (9), which is also responsible for decreasing vaccine need. Contrary to previous bibliometric analyses in the past 2 years (10,11), the research trends are now more focused on optimizing the prevention of the pandemic spread and recruitment of international cooperation. Therefore, lockdowns and the establishment of MCHs may be effective during periods of rapid SARS-CoV-2 transmission as shown in topic trends of Omicron. Since the predominant SARS-CoV-2 variant has changed from Alpha to Delta and Omicron, treatment strategies have been revised accordingly. Many new medications, such as nirmatrelvir and ritonavir, have been developed, and more risk factors have been identified, which may prevent the progression of COVID-19 infection from mild to critical conditions (12-14). Previous studies have estimated that because of changes in the virulence genome of the Omicron variant, more confirmed cases are associated with a lower risk of progression to severe clinical outcomes, including symptomatic hospital admission, intensive care unit (ICU) admission, and mechanical ventilation (MV), compared with infection with the Delta variant (15-17). The most significant real-world sample is 222,688 outpatient-diagnosed cases from the Kaiser Permanente of Southern California healthcare system (18). In that study, the cumulative 30-day risk of hospital admission, symptomatic hospital admission, ICU admission, the onset of MV, and death among cases with Omicron variant infection were 4.5, 3.9, 0.2, 0.1, and 0.1 per 1,000 cases, respectively (18); these values are considerably lower than corresponding values for infection with the Delta variant. We also observed shorter durations of hospital stay following inpatient admission among cases with Omicron compared with Delta variant infection. As many as 66.2% of patients were reported as requiring a hospital stay ≤5 days in the Kaiser Permanente of Southern California study (18). As for the protection afforded by vaccines, this seems to have been further weakened with the Omicron variant, and the establishment of a pan-coronavirus vaccine pipeline is encouraged (19,20).
Conclusions
The COVID-19 pandemic has emerged in a great number of articles and clinical trials. Our comparative bibliometric analysis included research materials worldwide and obtained adequate knowledge about the Delta and Omicron variants to present substantial evidence for future studies. In this comparative bibliometric analysis, we provide the current state, and hotpots of COVID-19 variants. Furthermore, we identified that the future trends have changed from the origin of variants and vaccine development to mutation and prevention of the rapid transmission of the virus. It is also noticed that more clinical trials have been registered, and the results are promising, which call focus on the efficacy of antiviral drugs against the Delta and Omicron variants.
Acknowledgments
We also thank all the staff who participated in the data collection.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1111/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med 2020;383:2451-60. [Crossref] [PubMed]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 2022. Available online: https://covid19.who.int/
- Mistry P, Barmania F, Mellet J, et al. SARS-CoV-2 Variants, Vaccines, and Host Immunity. Front Immunol 2022;12:809244. [Crossref] [PubMed]
- Rubin EJ, Baden LR, Abdool Karim SS, et al. Audio Interview: The Omicron Variant of SARS-CoV-2. N Engl J Med 2021;385:e96. [Crossref] [PubMed]
- Pai RR, Alathur S. Bibliometric Analysis and Methodological Review of Mobile Health Services and Applications in India. Int J Med Inform 2021;145:104330. [Crossref] [PubMed]
- Kumar S, Thambiraja TS, Karuppanan K, et al. Omicron and Delta variant of SARS-CoV-2: A comparative computational study of spike protein. J Med Virol 2022;94:1641-9. [Crossref] [PubMed]
- Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022;602:671-5. [Crossref] [PubMed]
- Mlcochova P, Kemp SA, Dhar MS, et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature 2021;599:114-9. [Crossref] [PubMed]
- Guo H, Gao Y, Li T, et al. Structures of Omicron spike complexes and implications for neutralizing antibody development. Cell Rep 2022;39:110770. [Crossref] [PubMed]
- Yu Y, Li Y, Zhang Z, et al. A bibliometric analysis using VOSviewer of publications on COVID-19. Ann Transl Med 2020;8:816. [Crossref] [PubMed]
- Hashemi H, Rajabi R, Brashear-Alejandro TG. COVID-19 research in management: An updated bibliometric analysis. J Bus Res 2022;149:795-810. [Crossref] [PubMed]
- Zhong H, Wang Y, Zhang ZL, et al. Efficacy and safety of current therapeutic options for COVID-19 - lessons to be learnt from SARS and MERS epidemic: A systematic review and meta-analysis. Pharmacol Res 2020;157:104872. [Crossref] [PubMed]
- Yu Y, Zhu C, Yang L, et al. Identification of risk factors for mortality associated with COVID-19. PeerJ 2020;8:e9885. [Crossref] [PubMed]
- Liu L, Xie J, Wu W, et al. A simple nomogram for predicting failure of non-invasive respiratory strategies in adults with COVID-19: a retrospective multicentre study. Lancet Digit Health 2021;3:e166-74. [Crossref] [PubMed]
- Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet 2022;399:1303-12. [Crossref] [PubMed]
- Madhi SA, Kwatra G, Myers JE, et al. Population Immunity and Covid-19 Severity with Omicron Variant in South Africa. N Engl J Med 2022;386:1314-26. [Crossref] [PubMed]
- Bager P, Wohlfahrt J, Bhatt S, et al. Risk of hospitalisation associated with infection with SARS-CoV-2 omicron variant versus delta variant in Denmark: an observational cohort study. Lancet Infect Dis 2022;22:967-76. [Crossref] [PubMed]
- Lewnard JA, Hong VX, Patel MM, et al. Clinical outcomes associated with SARS-CoV-2 Omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in Southern California. Nat Med 2022;28:1933-43. [Crossref] [PubMed]
- Dolgin E. Pan-coronavirus vaccine pipeline takes form. Nat Rev Drug Discov 2022;21:324-6. [Crossref] [PubMed]
- Yu Y, Xu C, Zhu C, et al. Emergency preparedness for COVID-19: experience from one district general hospital in Wuhan. J Emerg Crit Care Med 2020;4:30. [Crossref]
(English Language Editors: N. Korszniak and J. Jones)


