
Obstructive sleep apnea-hypopnea syndrome complicated with idiopathic intracranial hypertension: a case report
Introduction
Obstructive sleep apnea-hypopnea syndrome (OSAHS) is a common sleep disorder characterized by frequent apnea and hypopnea during sleep due to a variety of causes. The main clinical manifestations include the following: (I) irregular snoring during nighttime sleep, disordered breathing and sleep rhythm, repeated apnea and arousal, or breathlessness; (II) daytime sleepiness; and (III) memory loss, and in severe cases, psychological, intellectual, and behavioral abnormalities. Idiopathic intracranial hypertension (IIH) is a syndrome characterized by increased intracranial pressure, with the main symptoms including headache, vision loss, visual field defect, pulsatile tinnitus, unsteady gait, and nausea/vomiting. The pathogenetic mechanism of IIH remains unclear but may involve aquaporin-4, vitamin A, and the regulatory mechanism of cerebrospinal fluid (CSF) flow (1). So far, only a few studies have described IIH caused by OSAHS, although Zhan et al. (2) have published a relevant case report. The main symptoms of the patient whose CSF pressure measured by lumbar puncture was 255 mmH2O were headache and blurred vision. However, the CSF pressure of the patient with OSAHS complicated with IIH admitted to our hospital was higher and the main symptom was limb weakness. His heart ultrasound showed impaired right heart function. Herein, we report a case of OSAHS and IIH that was treated in the Department of Neurology of our center. We present the following article in accordance with the CARE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-909/rc).
Case presentation
Our patient was a 52-year-old male with a middle school educational background. He was admitted to our center on August 30, 2017, due to “progressive limb weakness for half a year, aggravated by fever and cough for 3 days”. Six months before admission, the patient began to experience weakness in all four limbs but was still able to work and live normally. Three months before admission, the feeling of weakness gradually increased, and he was experiencing obvious fatigue at work. He would fall asleep easily and had subjective memory decline. Three days before admission, he experienced a fever and cough, his sleep time increased notably, and fell asleep upon completing his daytime activities. He had difficulty in self-care and decreased appetite, but without chest tightness/palpitations or limb numbness. He had a history of glucose intolerance for 2 years but did not use any hypoglycemic agents. Also, he had no history of tobacco smoking or alcohol and no family history of genetic disease.
Physical examination at admission showed the following: body temperature (T), 37 ℃; pulse (P), 76 beats/min; respiratory rate (R), 15 breaths/min; blood pressure (BP), 112/66 mmHg; height, 170 cm; body weight, 60 kg and BMI: 20.8. He was mentally conscious but weak-minded. His lips were slightly cyanotic. The trachea was at the midline. Coarse breath sounds but no wet or dry rales were heard on lung auscultation. There was no thoracic deformity, and the thoracic mobility was fair. No murmur was heard in the auscultation areas. There was no obvious abnormality in higher cortical functions. Also, there was no cranial nerve involvement. His neck was soft, and no muscle atrophy or fasciculation was observed. The range of motion in his neck, limb muscle strength, and diaphragm muscle strength was normal, as was his muscle tone. Tendon reflex of the four limbs was positive (++). Superficial and deep sensations were normal on both sides. The Babinski sign was also positive on both sides. Meningeal irritation signs were negative, and the Finger-to-Nose (FNT) and Heel-to-Shin (HST) tests showed negative results. Blood oxygen saturation was 70% on the fingertip pulse oximeter.
The patient also underwent laboratory tests and special examinations after admission. On August 30, 2017, routine blood testing in the emergency room showed that the total white blood cell (WBC) count was 6.0×109/L and the proportion of granulocytes was 0.88. No obvious abnormality was found in the emergency coagulation assessment and emergency biochemistry. Emergency blood gas analysis showed: pH, 7.27; PCO2, 92.5 mmHg; PO2, 46 mmHg; HCO3−, 31 mmol/L; BE, 14 mmol/L; Lac, 1.2 mmol/L; and SO2, 72%. Emergency head CT showed a suspicious high-density shadow (Figure 1). Chest CT revealed pulmonary infection. On August 31, 2017, blood biochemistry showed that the high-sensitivity C-reactive protein level was 32.5 mg/L, and testing for tumor markers showed that the squamous cell carcinoma antigen (SCCA) level was 12.2 ng/mL. Also, the troponin level, coagulation profile, thyroid function, antinuclear antibody, indicators of blood transfusion, cortisol level, adrenocorticotropic hormone (ACTH) level, sex hormone levels, and growth hormone level were basically normal. Heart ultrasound revealed mild tricuspid and pulmonary valve regurgitation, mild to moderate pulmonary hypertension, left ventricular diastolic dysfunction, and obstruction of the inferior vena cava (Figure 2). No obvious abnormality was found on head magnetic resonance imaging (MRI).
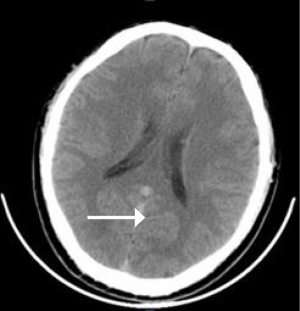
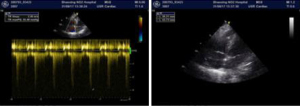
The initial diagnosis on admission was type II respiratory failure, pulmonary infection, and abnormal glucose tolerance. Non-invasive intermittent mechanical ventilation, antimicrobial therapy, and phlegm-relieving treatment were given. Subsequently, the patient’s symptoms were slightly controlled and oxygenation was improved; however, his loss of appetite worsened, and he experienced vomiting without headache. On September 3, 2017, a lumbar puncture was performed, and the results showed that the CSF pressure was higher than 400 mmH2O. Routine CSF testing showed the composition was normal. No abnormality was seen on head MRI. On September 4, 2017, whole-brain angiography showed no abnormality in the intracranial arteries and veins.
Considering the presence of IIH, we treated the patient with Mannitol 150 mL q8h for 3 consecutive days to lower the intracranial pressure. However, the symptoms were not improved, and thus, Mannitol treatment was discontinued. During monitoring of the patient, it was accidentally discovered that he had nocturnal apnea, which was accompanied by a decrease in blood oxygen saturation. Although there was no obvious snoring, the possibility of OSAHS was considered. Laryngoscopy and rhinoscopy were performed to exclude anatomical abnormalities of the upper airway. On September 8, 2017, blood gas analysis showed the following: pH, 7.3; PCO2, 59.7 mmHg; PO2, 93 mmHg; and oxygen saturation, 96.6%. On September 13, 2017, polysomnography showed that the apnea-hypopnea index (AHI) was 46.15 consisting apneas 19.12 and hypopnea 27.03 (normal value <5) (Table 1), the main symptom was obstructive sleep apnea, and the lowest blood oxygen saturation was 62% (Table 2). Severe sleep apnea with severe hypoxemia (AHI >30) was considered. After he used a fully automated ventilator and chose Autoset mode all night which pressure ranged from 8–10 cmH2O, the condition was improved.
Table 1
| Stage | Apneas | Hypopnea | AHI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Obstructive | Central | Hybrid | Total times | Obstructive | Central | Hybrid | Total times | Apneas | Hypopnea | |||
| REM | 4 | 1 | 1 | 6 | 7 | 0 | 0 | 7 | N/A | N/A | ||
| NREM | 82 | 38 | 19 | 139 | 198 | 0 | 0 | 198 | N/A | N/A | ||
| Total | 86 | 39 | 20 | 145 | 205 | 0 | 0 | 205 | 19.12 | 27.03 | ||
AHI, apnea-hypopnea index; REM, rapid eye movement; NREM, non-rapid eye movement; NA, not applicable.
Table 2
| Data | Oxygen saturation | The lowest oxygen | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <100% | <90% | <80% | <70% | <60% | <50% | <40% | 62% | ||
| Blood oxygen events (times) | 403 | 401 | 181 | 11 | 0 | 0 | 0 | N/A | |
N/A, not applicable.
On September 23, 2017, he had no obvious discomfort at the outpatient follow-up visit, and no abnormality was found on head CT (Figure 3). The CSF tests showed no abnormality, and the CSF pressure was 350 mmH2O. On October 13, 2018, the patient had no obvious discomfort at the outpatient follow-up visit. Heart ultrasound showed mild tricuspid regurgitation, and no other abnormality was found (Figure 4). Also, the CSF tests showed no abnormality, and the CSF pressure was 200 mmH2O. Subsequently, the patient was followed up via telephone. He had adhered to the NPPV and there was no complaint of discomfort (Figure 5). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.

Discussion
Clinically, OSAHS is often manifested as irregular snoring and recurrent apnea. Due to repeated awakening and arousal responses at night, the normal sleep architecture and rhythm are destroyed, and OSAHS patients often suffer from symptoms such as fatigue, dizziness, nausea/vomiting, and inability to concentrate during the daytime. In severe cases, cognitive function decline and behavioral abnormalities may occur, which are often accompanied by cardiovascular, cerebrovascular, and metabolic diseases, and are also important causes of sudden death and road traffic deaths. Obesity and abnormal upper airway anatomy are the main risk factors. Normal cerebral circulation is characterized by considerable blood flow, high oxygen consumption, and small changes in cerebral blood flow. In a normal adult at rest, the total cerebral blood flow is about 15% of the cardiac output. In addition to its self-regulatory mechanism, cerebral blood flow can also be affected by changes in intracranial CO2 and hypoxia. In addition, the increased CO2 partial pressure in the blood and hypoxia can directly dilate the cerebral blood vessels, leading to significant cerebral edema. OSAHS patients can suffer from frequent apnea, which leads to hypoxemia, hypercapnia, altered heart rate, and many other complications (3). Hypoxemia and hypercapnia affect the hemodynamics and hemorheological features of OSAHS patients and undermine the self-regulatory system of the brain, leading to increased intracranial pressure and a higher incidence of cardiovascular and cerebrovascular diseases (4). Also, in response to chemical stimuli, the vascular regulatory system in the brains of many OSAHS patients is compromised (5). In 1989, Jennum et al. (6) carried out a study of six patients with no other conditions except for sleep apnea disease using an epidural pressure monitor. They found that intracranial pressure increased during sleep apnea; when the sleep apnea process was terminated, the intracranial pressure increased sharply. Four of these six patients also experienced elevated intracranial pressure upon awakening in the morning.
OSAHS is also an independent risk factor for stroke and can even be life-threatening in severe cases. About 75% of patients with acute ischemic stroke suffer from sleep apnea syndrome (4). In patients with either acute or chronic stroke, OSAHS can affect recovery and increase the risk of recurrence and death (7). In patients with stroke-related sleep disorders (SSD, including sleep disorders that appear for the first time after stroke or those that exist before stroke and persist or worsen after stroke), a variety of sleep disorders including insomnia and sleep-disordered breathing (SDB) may be present, among which OSAHS is the main type of stroke-related SDB. Moderate-to-severe sleep apnea occurs in up to one-third of patients in the chronic phase of ischemic stroke, affecting their recovery and long-term outcomes (8). In addition to early detection and lifestyle changes, postural intervention and continuous positive pressure ventilation may also be helpful for these patients (9). The management approaches should be tailored based on disease severity.
Our patient was admitted to the hospital due to limb weakness for 6 months, aggravated fever, and cough for 3 days. He had no signs of obesity and did not snore at night. He had no history of alcohol, tobacco, sedative and hypnotic use. Examination after admission ruled out hypothyroidism, cerebral infarction, and other diseases. OSAHS is a risk factor for the development of a variety of cardiovascular and cerebrovascular diseases and metabolic diseases. His heart ultrasound showed impaired right heart function. The pressure of the first lumbar puncture was more than 400 mmH2O which was life-threatening. After excluding other causes such as intracranial infection, tumor and venous sinus thrombosis, high intracranial pressure was considered to be related to OSAHS by Magnetic resonance Imaging, CSF examination and cerebral angiography. Up to now, the patient had no obvious risk factors of OSAHS and the symptoms of intracranial hypertension were not obvious, mainly manifested as limb weakness. The patient was admitted to the hospital due to fever and cough, which was not consistent with the typical symptoms of OSAHS. We are advised to be vigilant against patients with such atypical symptoms. STOP-Bang scale or B-APNEIC score can be used for initial screening of these patients, and neck circumference can be used as an independent correlation variable to predict the severity of obstructive sleep apnea (10). Unfortunately, neck circumference was not measured in this patient. The elevated intracranial pressure in OSAHS patients often returns to normal when they are awake during the daytime (11). However, in the case of our patient, the intracranial pressure remained high while the patient was awake. Following standardized, non-invasive mechanical ventilation, the patient’s condition was controlled and the intracranial hypertension was improved. Unfortunately, after 1 year of follow-up, the patient refused to return to our center for lumbar puncture and CSF pressure measurement due to improvement in his condition. Therefore, there is no follow-up data on the CSF pressure. In addition, the patient had no obvious snoring during the diagnosis and treatment processes, which is common in OSAHS patients, and led to the delayed diagnosis of OSAHS.
Conclusions
In conclusion, for patients with unexplained respiratory failure, detailed history-taking is required to rule out the possibility of secondary OSAHS. For these patients, testing for sleep apnea is recommended even if there are no typical snoring symptoms. Special attention should be paid to IIH, which can be life-threatening. Overnight pulse oximetry can be used as a screening tool for OSAHS patients (12). Currently, there is still a lack of awareness and knowledge regarding sleep apnea, and patients with OSAHS (including those associated with stroke) are often not diagnosed or treated promptly. A multidisciplinary approach that includes specialists from the departments of neurology, cardiology, endocrinology, otolaryngology, respiratory medicine, and other disciplines is required to jointly explore the pathological mechanism of this disease. Patient education should be carried out through social media and community engagement. These efforts may help to achieve the early and correct diagnosis of OSAHS and allow for tailored treatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-909/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-909/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- You C, Zhou F, Huang L. Research progress of idiopathic cranial hypertension. Chinese Journal of Neuromedicine 2018;17:856-62.
- Zhan S, Huang C, Li N, et al. Idiopathic intracranial hypertension and obstructive sleep apnea: a case study. Chinese Journal of Neuromedicine 2011;44:520-3.
- Vorlová T, Dlouhá O, Kemlink D, et al. Decreased perception of high frequency sound in severe obstructive sleep apnea. Physiol Res 2016;65:959-67. [Crossref] [PubMed]
- Culebras A. Sleep and stroke. Semin Neurol 2009;29:438-45. [Crossref] [PubMed]
- Tsivgoulis G, Zhang Y, Alexandrov AW, et al. Safety and tolerability of early noninvasive ventilatory correction using bilevel positive airway pressure in acute ischemic stroke. Stroke 2011;42:1030-4. [Crossref] [PubMed]
- Jennum P, Børgesen SE. Intracranial pressure and obstructive sleep apnea. Chest 1989;95:279-83. [Crossref] [PubMed]
- Martínez-García MA, Galiano-Blancart R, Román-Sánchez P, et al. Continuous positive airway pressure treatment in sleep apnea prevents new vascular events after ischemic stroke. Chest 2005;128:2123-9. [Crossref] [PubMed]
- Baillieul S, Dekkers M, Brill AK, et al. Sleep apnoea and ischaemic stroke: current knowledge and future directions. Lancet Neurol 2022;21:78-88. [Crossref] [PubMed]
- Beijing Academy of Neurology Sleep Disorders Professional Committee, Committee of Neuropsychiatry and Clinical Psychology, Beijing Academy of Neurology, Sleep Science Branch of Chinese Gerontology and Geriatrics Society. Chinese expert consensus on the assessment and management of stroke-related sleep disorders. Chinese Journal of Internal Medicine 2019;58:17-26. [PubMed]
- Morinigo R, Quraishi SA, Ewing S, et al. The B-APNEIC score: distilling the STOP-Bang questionnaire to identify patients at high risk for severe obstructive sleep apnoea. Anaesthesia 2022;77:286-92. [Crossref] [PubMed]
- Onder H, Ergun O, Kaygisiz M, et al. Total improvement after surgery for obstructive sleep apnea syndrome in a patient with concurrent malignant idiopathic intracranial hypertension. J Neurosurg 2018;131:582-6. [Crossref] [PubMed]
- Kok LT, Gnoni V, Muza R, et al. Prevalence and utility of overnight pulse oximetry as a screening tool for obstructive sleep apnoea in newly diagnosed idiopathic intracranial hypertension. Eye (Lond) 2022;1-6. Epub ahead of print. [Crossref] [PubMed]
(English Language Editor: A. Kassem)



