
Efficacy and safety of CDK4/6 inhibitors combined with endocrine therapy versus endocrine therapy alone in hormone receptor-positive, HER2-negative, advanced breast cancer: a systematic review and meta-analysis
Highlight box
Key findings
• CDK4/6 inhibitors plus endocrine therapy effectively prolong OS, PFS, and improve ORR, CBR in patients with HR-positive, HER2-negative advanced breast cancer. The safety of CDK4/6 inhibitors was controllable.
What is known and what is new?
• Based on the previous results, some studies did not benefit significantly in OS, which requires verification of the final results.
• After the latest OS results are pooled in this analysis, the advantages of CDK4/6 inhibitors are still significant in general, but the application of palbociclib has not yet produced significant OS advantages. The combination of CDK4/6 inhibitors and endocrine therapy benefits patients with age ≥65, which is different from the previous results.
What is the implication, and what should change now?
• In subgroup analysis, palbociclib has not shown significant benefits in the results of OS. In future research, it is necessary to consider whether there are certain conditions for palbociclib's benefits to OS.
Introduction
Recent statistics indicate that breast cancer has been the most commonly diagnosed cancer with approximately 2.3 million newly diagnosed patients worldwide in 2020, which also constitutes the most common cause of death from cancer among the females (1). Hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative is the most common subtype of breast cancer (2). More than 70% of breast cancer patients have HR-positive tumors (3). The estrogen receptor (ER) signaling pathway is utilized as the main pathway for breast cancer cell survival (4). Endocrine therapies have been used in the treatment of HR-positive breast cancer and have shown improvement in prognosis, including selective ER modulators, selective ER down-regulators, and aromatase inhibitors (5,6). However, the effects are limited by the development of drug resistance, which is an evolving obstacle that researchers need to continuously overcome (4). For the HR-positive breast cancer population, an overview study demonstrated that younger patients (less than 50 years old) and older patients (between 50 and 69 years old) showed the annual recurrence rates of 4.1% and 6.1% and the annual death rates of 17.0% and 25.5%, respectively, after receiving endocrine therapy alone (7). The death from and recurrence of HR-positive breast cancer are related to the acquired resistance to endocrine therapy, which has been shown to be associated with high mutation rates and extreme subclonal diversity (8,9). Cyclin-dependent kinase 4/6 (CDK4/6) is one of the therapeutic targets for HR-positive breast cancer (10). Activation of the CDK4/6-cyclin D1 complex phosphorylates retinoblastoma gene (RB) and the complex of RB and E2F dissociates, liberating transcription factors, and allowing transformation of the cell cycle from G1 phase to S (10,11). The pooled results of several previous systematic reviews have demonstrated that adding CDK4/6 inhibitors to endocrine therapy significantly improves the progression-free survival (PFS) and objective response rate (ORR) of patients with HR-positive, HER2-negative advanced breast cancer (12,13), which also included immature or medium-term results of overall survival (OS). In addition, in the previous meta-analysis of subgroups, there were still some disputes, such as in subgroups of different ages and nature of disease, which are specific issues in the clinical application of CDK4/6 inhibitors (13). Therefore, further systematic review is needed. Up to our analysis time, the results of OS had been successively reported in studies of MONALEESA-3 (14) MONARCH-2 (15). The PALOMA-1 had updated the final OS results (16), which was especially needed for the pooled data of palbociclib. A more accurate answer is needed for clinicians, whether it is beneficial or not. The PFS results were updated in MONALEESA-3 (14), MONARCH-2 (15), MONARCH-3 (17), and PALOMA-2 (18). In addition, study of PALOMA-4 reported the primary results in 2022, which will also be included (19). Therefore, we performed this systematic review and meta-analysis in order to further assess the clinical benefits of CDK4/6 inhibitors combined with endocrine therapy on patients with HR-positive, HER2-negative advanced breast cancer. We present the following article in accordance with the PRISMA reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1306/rc).
Methods
Eligibility criteria
The included studies had to fulfill the following criteria: (I) randomized controlled trials (RCTs) of phase II and phase III including patients with HR-positive and HER2-negative advanced breast cancer, which compared the CDK4/6 inhibitors plus endocrine therapy (CDK4/6 inhibitors group) with endocrine therapy alone (control group). (II) The main results of the studies had to include hazard ratios (HRs) for PFS or OS, in the most recently updated or final reports. The other results may include or not include the results of the time from randomization to the first recorded disease progression while the patient was receiving next-line therapy or death from any cause (PFS2), time to first subsequent chemotherapy after discontinuation (TTC), ORR, clinical benefit rate (CBR) and safety assessment. (III) Articles were available in full text. Articles that did not meet the criteria above were excluded.
Search strategy and data collection
RCTs related to CDK4/6 inhibitors combined with endocrine therapy for advanced breast cancer were searched for in the electronic databases of PubMed, Cochrane Library, Embase, China National Knowledge Infrastructure (CNKI), WANFANG and China Science and Technology Journal Database (VIP) with the range of retrieval time from inception to November 2022. The search was conducted with a combination of medical subject heading (MeSH) terms and free-text terms, and used Boolean operators to connect. Two researchers independently performed screening of abstracts and full-text articles according to the eligibility criteria and extracted the data. The extracted data included general information of the study, main interventions, and outcome indicators of PFS, OS, ORR, CBR, PFS2, TTC, and safety assessment. The 2 researchers were required to consult with each other and discuss with another researcher when differences arose.
Risk of bias assessment
Cochrane risk-of-bias tool 2.0 was used to evaluate the bias risk of the included studies, and the evaluation contents were as follows: (I) random sequence generation (selection bias). (II) Allocation concealment (selection bias). (III) Blinding of participants and personnel (performance bias). (IV) Blinding of outcome assessment (detection bias). (V) Incomplete outcome data (attrition bias). (VI) Selective reporting (reporting bias). (VII) Other bias. The evaluation results were presented with the risk of bias graph and risk of bias summary by Review Manager 5.3 software (The Nordic Cochrane Center, Copenhagen, Denmark).
Statistical analysis
All of the efficacy endpoints were derived from the intention-to-treat (ITT) analyses when possible. We used Stata 14.0 software (StataCorp LLC., College Station, TX, USA) to perform the meta-analysis. The pooled outcomes of OS, PFS, PFS2, and TTC were analyzed as HR [95% confidence interval (CI)] through the inverse-variance test. The pooled dichotomous outcomes of ORR or CBR were analysed as relative risk (RR; 95% CI). The chi-squared (Chi2) test was used to detect the statistical heterogeneity, in the meta-analysis: I2<50% indicated a low statistical heterogeneity, for which fixed-effect analysis was used; I2≥50% indicated a substantial heterogeneity, for which random-effect analysis were used and the causes of heterogeneity need to explore (20). Begg test was used to detect the publication bias of the included studies. Sensitivity analysis was used to detect the stability of included studies except the only open-label phase II clinical trial. All tests were 2-sided and a P value <0.05 was considered statistically significant.
Results
According to the retrieval strategy, 2,138 records were returned, of which 58 records were obtained to view the full texts after removing the duplicates and irrelevant records. Finally, 16 records including 9 RCTs were obtained after removing the records that did not fulfill the eligible criteria (Figure 1). A total of 4,920 patients were enrolled from 9 RCTs, of which 2,971 patients received CDK4/6 inhibitors plus endocrine therapy and 1,949 patients received endocrine therapy with or without a placebo. The characteristics of the RCTs (14-19,21-30) are shown in Table 1.
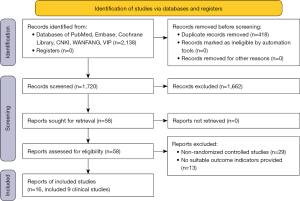
Table 1
| Study | Phase | Setting | Median age [range], years | No. of patients | Menopausal status | ECOG status, No. | HR status, No. | Therapeutic schedule | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | ≥1 | ER+ | PR+ | ||||||||
| MONALEESA-3 (14,21) | III | First line/second line | T: 63 [31–89] | T: 484 | Postmenopause | T: 310 | T: 173 | T: 481 | T: 353 | T: Ribociclib + fulvestrant | |
| C: 63 [34–86] | C: 242 | C: 158 | C: 83 | C: 241 | C: 167 | C: Placebo + fulvestrant | |||||
| MONARCH-2 (15,22) | III | Second line | T: 59 [32–91] | T: 446 | Any status | T: 264 | T: 177 | NA | T: 339 | T: Abemaciclib + fulvestrant | |
| C: 62 [32–87] | C: 223 | C: 136 | C: 87 | NA | C: 171 | C: Placebo + fulvestrant | |||||
| PALOMA-1 (16,29) | II | First line | T: 63 [54–71] | T: 84 | Postmenopause | T: 46 | T: 38 | T: 84 | NA | T: Palbocilib + letrozole | |
| C: 64 [56–70] | C: 81 | C: 45 | C: 36 | C: 81 | NA | C: Letrozole | |||||
| MONARCH-3 (17) | III | First line | T: 63 [38–87] | T: 328 | Postmenopause | T: 192 | T: 136 | NA | T: 255 | T: Abemaciclib + anastrozole/letrozole | |
| C: 63 [32–88] | C: 165 | C: 104 | C: 61 | NA | C: 127 | C: Placebo + anastrozole/letrozole | |||||
| PALOMA-2 (18,23) | III | First line | T: 62 [30–89] | T: 444 | Postmenopause | T: 257 | T: 187 | T: 444 | NA | T: Palbocilib + letrozole | |
| C: 61 [28–88] | C: 222 | C: 102 | C: 120 | C: 222 | NA | C: Placebo + letrozole | |||||
| PALOMA-4 (19) | III | First line | T:54 [31-70] | T: 169 | Postmenopause | T:84 | T:85 | T: 169 | NA | T: Palbocilib + letrozole | |
| C:54 [29-70] | C: 171 | C:81 | C:90 | C: 171 | NA | C: Placebo + letrozole | |||||
| MONALEESA-7 (24,25) | III | First line | T: 43 [25–58] | T: 335 | Premenopause or | T: 245 | T: 87 | T: 331 | T: 290 | T: Ribociclib + tamoxifen/letrozole/anastrozole | |
| C: 45 [29–58] | C: 337 | Perimenopause | C: 255 | C: 79 | C: 335 | C: 288 | C: Placebo + tamoxifen/letrozole/anastrozole | ||||
| PALOMA-3 (26,27) | III | Second line | T: 57 [30–88] | T: 347 | Any status | T: 206 | T: 141 | NA | NA | T: Palbocilib + fulvestrant | |
| C: 56 [29–80] | C: 174 | C: 116 | C: 58 | NA | NA | C: Placebo + fulvestrant | |||||
| MONALEESA-2 (28,30) | III | First line | T: 62 [23–91] | T: 334 | Postmenopause | T: 205 | T: 129 | T: 332 | T: 271 | T: Ribociclib + letrozole | |
| C: 63 [29–88] | C: 334 | C: 202 | C: 132 | C: 333 | C: 278 | C: Placebo + letrozole | |||||
RCTs, randomized controlled trials; T, CDK4/6 inhibitors group; C, control group; ECOG, Eastern Cooperative Oncology Group; HR, hormone receptor; ER, estrogen receptor; PR, progesterone receptor; NA, not available.
OS
The pooled OS results were drawn from 6 RCTs (14-16,24,26,30), which enrolled 3,421 patients. The fixed-effect model was used due to the I2=0%, P=0.92, which indicated little heterogeneity between the groups. The pooled results showed a significant benefit in the CDK4/6 inhibitor group compared with the control group (HR 0.76; 95% CI: 0.69–0.84; P<0.001) (Figure 2). Subgroup analyses of OS were performed by stratifying the characteristics of the patients (Table 2). Among patients receiving first-line (HR 0.76, 95% CI: 0.66–0.87; P<0.001) and second-line (HR 0.77, 95% CI: 0.67–0.89; P<0.001) treatment, the OS advantage significantly supported the CDK4/6 inhibitors group. Subgroup analysis of different CDK4/6 inhibitors showed that the addition of ribociclib and abemaciclib to the endocrine therapy significantly prolonged the OS (HR 0.74; 95% CI: 0.64–0.84; P<0.001 and HR 0.76; 95% CI: 0.61–0.95; P<0.05); however, the OS benefit with palbociclib was not significant compared with the control group (HR 0.83; 95% CI: 0.68–1.02; P>0.05). The OS advantage significantly supported the CDK4/6 inhibitors group in patients with age <65 (HR 0.75; 95% CI: 0.66–0.85; P<0.01) or ≥65 (HR 0.79; 95% CI: 0.67–0.93; P<0.01). Similar advantages were observed in patients with bone-only disease (HR 0.77; 95% CI: 0.60–0.99; P<0.05) and visceral involvement (HR 0.79; 95% CI: 0.70–0.90; P<0.001).
Table 2
| Subgroups | HR (95% CI) | P value | I2, % |
|---|---|---|---|
| Age, years | |||
| <65 | 0.75 (0.66–0.85) | <0.01 | 0 |
| ≥65 | 0.79 (0.67–0.93) | <0.01 | 22.8 |
| Region | |||
| North America | 0.67 (0.54–0.84) | <0.01 | 0 |
| Asia | 0.67 (0.50–0.90) | <0.01 | 27.8 |
| Europe and Australia | 0.81 (0.69–0.94) | <0.01 | 0 |
| Latin America | 1.39 (0.44–4.41) | >0.05 | 44.5 |
| Race | |||
| Asian | 0.77 (0.53–1.10) | >0.05 | 41.1 |
| Non-Asian | 0.77 (0.68–0.86) | <0.001 | 0 |
| ECOG | |||
| 0 | 0.72(0.62–0.83) | <0.001 | 0 |
| ≥1 | 0.81(0.68–0.96) | <0.05 | 0 |
| HR status | |||
| ER+PR+ | 0.77 (0.67–0.89) | <0.001 | 0 |
| Others | 0.71 (0.56–0.90) | <0.01 | 0 |
| Menopausal | |||
| Premenopausal or perimenopausal | 0.76 (0.60–0.96) | <0.05 | 0 |
| Postmenopausal | 0.76 (0.68–0.85) | <0.001 | 0 |
| Nature of disease | |||
| Bone-only disease | 0.77 (0.60–0.99) | <0.05 | 0 |
| Visceral involvement | 0.79(0.70–0.90) | <0.001 | 0 |
| No. of sites of metastasis | |||
| <3 | 0.79 (0.62–1.01) | >0.05 | 0 |
| ≥3 | 0.67 (0.50–0.89) | <0.01 | 0 |
| Previous adjuvant or neoadjuvant chemotherapy | |||
| Yes | 0.85 (0.65–1.12) | >0.05 | 0 |
| No | 0.61 (0.42–0.88) | <0.01 | 0 |
| Previous chemotherapy in patients with metastatic disease | |||
| Yes | 0.85 (0.61–1.18) | >0.05 | 0 |
| No | 0.75 (0.66–0.84) | <0.001 | 0 |
| CDK4/6 inhibitor | |||
| Ribociclib | 0.74 (0.64–0.84) | <0.001 | 0 |
| Palbociclib | 0.83 (0.68–1.02) | >0.05 | 0 |
| Abemaciclib | 0.76 (0.61–0.95) | <0.05 | 0 |
| First line or second line | |||
| First line | 0.76 (0.66–0.87) | <0.001 | 0 |
| Second line | 0.77 (0.67–0.89) | <0.001 | 0 |
| ET resistance | |||
| Primary resistance | 0.69 (0.49–0.98) | <0.05 | 0 |
| Secondary resistance | 0.77 (0.63–0.93) | <0.01 | 0 |
OS, overall survival; ECOG, Eastern Cooperative Oncology Group; HR status, hormone receptor status; ER, estrogen receptor; PR, progesterone receptor; CI, confidence interval; HR, hazard ratio; ET, endocrine therapy.
PFS
The results of PFS were extracted from 9 RCTs (14,15,17-19,25,26,28,29). The fixed-effect model was applied to evaluate the pooled PFS, because of the little heterogeneity among the studies (I2=0%, P=0.83). The results demonstrated that the addition of CDK4/6 inhibitors to endocrine therapy significantly prolonged the PFS, compared with the control group (HR 0.56; 95% CI: 0.52–0.60; P<0.001) (Figure 3). Subgroup analyses of PFS were performed and were mostly consistent with the total result (Table 3). In the subgroup analysis, the heterogeneity of subgroup in Asia was substantial (HR 0.42; 95% CI: 0.27–0.66; P<0.001; I2=76.2%). Considered that the heterogeneity came from PALOMA-4 (19), since all of the patients were included from Asia, clinical heterogeneity may exist. By analyzing the pooled data of Asia except PALOMA-4, the statistical heterogeneity was reduced and the PFS benefit was consistent (HR 0.35; 95% CI: 0.26–0.47; P<0.001; I2=0%).
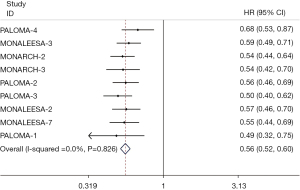
Table 3
| Subgroups | HR (95% CI) | P value | I2, % |
|---|---|---|---|
| Age, years | |||
| <65 | 0.53 (0.47–0.60) | <0.001 | 1.1 |
| ≥65 | 0.64 (0.53–0.78) | <0.001 | 0 |
| Geographical region | |||
| North America | 0.63 (0.47–0.83) | <0.01 | 0 |
| Asia | 0.42 (0.27–0.66) | <0.001 | 76.2 |
| Europe and Australia | 0.61 (0.50–0.74) | <0.001 | 0 |
| Latin America | 0.94 (0.43–2.05) | >0.05 | 0 |
| Non-Asia | 0.64 (0.55–0.75) | <0.001 | |
| Race | |||
| Asian | 0.37 (0.28–0.50) | <0.001 | 0 |
| Non-Asian | 0.64 (0.55–0.75) | <0.001 | 0 |
| ECOG | |||
| 0 | 0.58 (0.50–0.66) | <0.001 | 0 |
| ≥1 | 0.54 (0.47–0.63) | <0.001 | 0 |
| HR status | |||
| ER+PR+ | 0.59 (0.49–0.70) | <0.001 | 0 |
| Others | 0.40 (0.30–0.54) | <0.001 | 0 |
| Nature of disease | |||
| Bone-only metastasis | 0.55 (0.45–0.68) | <0.001 | 0 |
| Visceral metastasis | 0.55 (0.49–0.61) | <0.001 | 0 |
| Previous adjuvant or neoadjuvant chemotherapy | |||
| Yes | 0.55 (0.46–0.65) | <0.001 | 0 |
| No | 0.54 (0.45–0.64) | <0.001 | 38.3 |
| Measurable disease | |||
| Yes | 0.58 (0.49–0.69) | <0.001 | 14.4 |
| No | 0.43 (0.30–0.61) | <0.001 | 0 |
| CDK4/6 inhibitor | |||
| Abemaciclib | 0.54 (0.46–0.62) | <0.001 | 0 |
| Palbociclib | 0.56 (0.50–0.63) | <0.001 | 18.8 |
| Ribociclib | 0.57 (0.51–0.64) | <0.001 | 0 |
| First line or second line | |||
| First line | 0.57 (0.52–0.62) | <0.001 | 0 |
| Second line | 0.53 (0.47–0.60) | <0.001 | 0 |
PFS, progression-free survival; ECOG, Eastern Cooperative Oncology Group; HR status, hormone receptor status; ER, estrogen receptor; PR, progesterone receptor; CI, confidence interval; HR, hazard ratio.
ORR and CBR
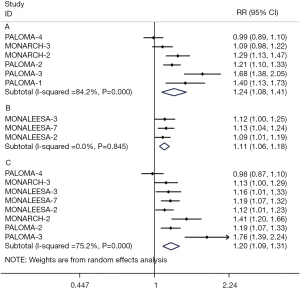
The results of ORR and CBR were analyzed in the ITT patients and patients with measurable disease, respectively. The pooled ORRs were extracted from 9 RCTs (17,19,21-23,25,27-29) and demonstrated that the addition of CDK4/6 inhibitors was beneficial to the improvement of objective response both in the ITT population (RR 1.43; 95% CI: 1.27–1.62; P<0.001) and in population with measurable disease (RR 1.43; 95% CI: 1.27–1.62; P<0.001) (Figure 4). The pooled data of CBR in the ITT population were analyzed according to the 2 different definitions of CBR respectively, of which the CBR advantages supported the CDK4/6 inhibitors group in both subgroup analyses (RR 1.24; 95% CI: 1.08–1.41; P<0.01 and RR 1.11; 95% CI: 1.06–1.18; P<0.001). The pooled results of CBR in patients with measurable disease also showed benefits in the CDK4/6 inhibitors group compared with the control group (RR 1.20; 95% CI: 1.09–1.31) (Figure 5).

PFS2 and TTC
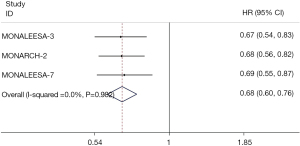
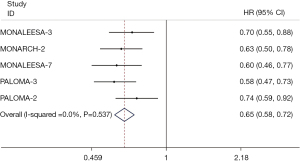
The pooled PFS2 results were extracted from 3 RCTs (14,15,24), which showed that PFS2 was statistically prolonged in the CDK4/6 inhibitors group compared with the control group (HR 0.68; 95% CI: 0.60–0.76; P<0.001) (Figure 6). Similarly, such benefit of the CDK4/6 inhibitors group was also observed in TTC results extracted from 5 RCTs (14,15,18,24,26), whereas in the control group the benefit was limited (HR 0.65; 95% CI: 0.58–0.72; P<0.001) (Figure 7).
Safety
The results of safety assessment were extracted from 9 RCTs (15,17-19,21,25,26,28,29) (Table 4). Neutropenia was the most common all-cause adverse event (AE) in the CDK4/6 inhibitors group. Any-grade and grade 3–4 neutropenia were 70.65% and 54.41% in the CDK4/6 inhibitors group and 5.96% and 1.45% in the control group, respectively. In addition to neutropenia, leukopenia, anemia, and thrombocytopenia were the most common hematologic AEs, of which the any-grade incidences were 38.37%, 27.42%, and 20.20%, respectively, in the CDK4/6 inhibitors group, compared with 4.35%, 7.98%, and 1.83% in the control group. Most of the any-grade nonhematologic AEs occurred more frequently than those in the control group, except for arthralgia, back pain, and hot flush.
Table 4
| Adverse events | CDK4/6 inhibitors group | Control group | |||
|---|---|---|---|---|---|
| Any grade, n (%) | Grade 3–4, n (%) | Any grade, n (%) | Grade 3–4, n (%) | ||
| Neutropenia | 2,092 (70.65) | 1,611 (54.41) | 115 (5.96) | 28 (1.45) | |
| Nausea | 1,165 (41.73) | 38 (1.36) | 447 (25.35) | 18 (1.02) | |
| Fatigue | 1,072 (36.20) | 70 (2.36) | 537 (27.81) | 12 (0.62) | |
| Diarrhea | 1,244 (42.01) | 121 (4.09) | 411 (21.28) | 14 (0.72) | |
| Leukopenia | 1,136 (38.37) | 585 (19.76) | 84 (4.35) | 10 (0.52) | |
| Vomiting | 693 (24.82) | 39 (1.40) | 258 (14.63) | 19 (1.08) | |
| Constipation | 577 (20.67) | 15 (0.54) | 270 (15.31) | 2 (0.11) | |
| Arthralgia | 708 (25.36) | 20 (0.72) | 459 (26.04) | 14 (0.79) | |
| Headache | 656 (23.50) | 16 (0.57) | 367 (20.82) | 11 (0.62) | |
| Back pain | 517 (18.52) | 41 (1.47) | 328 (18.60) | 15 (0.85) | |
| Anemia | 812 (27.42) | 150 (5.07) | 154 (7.98) | 33 (1.71) | |
| Decreased appetite | 538 (19.27) | 26 (0.93) | 200 (11.34) | 4 (0.23) | |
| Infections | 674 (52.13) | 56 (5.85) | 337 (37.49) | 20(3.56) | |
| Cough | 537 (20.39) | 5 (0.19) | 274 (15.48) | 0 (0) | |
| Pruritus | 191 (15.17) | 1 (0.08) | 44 (5.49) | 0 (0) | |
| Alopecia | 688 (23.24) | NA | 185 (9.58) | NA | |
| Rash | 460 (16.98) | 23 (0.85) | 129 (7.65) | 1 (0.06) | |
| Hot flush | 480 (19.47) | 2 (0.08) | 370 (23.10) | 1 (0.06) | |
| Abdominal pain | 360 (23.27) | 29 (1.87) | 97 (10.29) | 5 (0.53) | |
| Thrombocytopenia | 367 (20.20) | 47(2.59) | 22(1.83) | 4 (0.33) | |
| Stomatitis | 392 (21.57) | 12 (0.66) | 114 (9.51) | 1 (0.08) | |
| Blood creatinine level increased | 131 (17.06) | 11 (1.43) | 8 (2.08) | 0 (0) | |
| Upper respiratory tract infection | 129 (15.02) | 3 (0.35) | 49 (7.69) | 3 (0.47) | |
| Asthenia | 134 (15.55) | 16 (1.86) | 71 (11.16) | 0 (0) | |
AEs, adverse events; NA, not available.
Risk of bias and sensitive analysis
All of the 9 RCTs we included described the methods of randomization and allocation concealment, which indicated low risk of bias. One study used an open-label design (29), and all other studies were double-blind. The attrition bias and reporting biases showed low risk among the RCTs. Other bias were unclear (Figures 8,9). Begg test showed that there was no significant publication bias (P=0.67), which was presented with funnel plot (Figure S1). Sensitive analysis showed that all outcomes were stable except the only open-label phase II clinical trial (PALOMA-1) (Figures S2-S5).
Discussion
CDK4/6 inhibitors affect the progression of tumor cell cycle by inhibiting the enzyme complex (31). They have been used as first-line or second-line treatment in clinical trials for patients with HR-positive, HER2-negative, advanced breast cancer (32), and researchers have reported the results of their use in early-stage breast cancer (33). Experiments have shown that the application of CDK4/6 inhibitors is related to the improvement of protective immunity (34). The pooled results from the RCTs we included showed the OS and PFS benefit was related to the addition of CDK4/6 inhibitors to the endocrine therapy. Meanwhile, the benefits were consistent in most subgroup analyses.
For different CDK4/6 inhibitor agents, the CDK4/6 inhibitors group showed a notable OS improvement among the population treated with abemaciclib and ribociclib compared with the control group, but the improvement was not significant in the palbociclib subgroup. Palbocilib, ribciclib, and abemaciclib are selective, small molecules inhibitors of CDK4/6 (35-37). Palbociclib inhibits the kinase activities of CDK4/cyclin D1, CDK4/cyclin D3, and CDK6/cyclin D2 with the half maximal inhibitory concentration (IC50) of 0.011, 0.009 and 0.015 µmol/L, respectively (38). Abamaciclib inhibits the kinase activities of CDK4/cyclinD1 with an IC50 of 2 nmol/L and CDK6/cyclinD1 with an IC50 of 10 nmol/L (39). Besides, compared with ribciclib and palbociclib, abmaciclib has greater selectivity to CDK4 (40). These differences across the CDK4/6 inhibitor agents are not enough to explain the limited OS benefit of CDK4/6 inhibitor in the subgroup of palbociclib, and more reports of clinical trials are needed to confirm this result. In the updated final OS results of PALOMA-1, the median OS in the group of palbociclib combined with endocrine therapy and the group of endocrine therapy alone was 37.5 and 34.5 months (16), respectively, which was 34.9 and 28 months in PALOMA-3 (26), although the differences are not statistically significant. Therefore, the researchers believed that a larger sample size may be needed to assess the impact of palbociclib on OS (16). The pooled data came from MONARCH-2 and MONALEESA-3, which had consistent assessment criteria of primary resistance to endocrine therapy defined as the relapse in the first 2 years while receiving neoadjuvant or adjuvant endocrine therapy, or progression within the first 6 months in the first-line endocrine therapy to advanced breast cancer (14,15,22) showed that the addition of CDK4/6 inhibitors had substantial benefits for both primary and secondary endocrine therapy resistant population. More results are needed on analysis of the application of CDK4/6 inhibitors in patients who respond differently to previous endocrine therapy. In terms of PFS, the pooled results showed that the prolonged PFS was associated with the addition of CDK4/6 inhibitors to endocrine therapy. In MONALEESA-3, the updated median PFS of the patients in CDK4/6 inhibitor group who received first-line treatment reached 33.6 months (14). Before that, the longest median PFS had been 28.18 months in CDK4/6 inhibitor arm reported from MONARCH-3 (17). The application of CDK4/6 is in the process of continuous exploration. Recently, a small sample size cohort study reported that male patients with HR+HER2 - metastatic breast cancer can also benefit from the first-line treatment of CDK4/6 inhibitors (41).
For the incidence of AEs, in this pooled analysis, most of the hematological and non-hematological AEs extracted occurred more frequently in the CDK4/6 inhibitors group, regardless of the grade 3–4 AEs and any-grade AEs. The most common AE in the CDK4/6 inhibitors group was neutropenia. According to the related safety analyses, the factor of Asian ethnicity significantly increased the risk of grade 3–4 neutropenia in the palbociclib group (42,43). A preclinical experiment showed that the suppression of bone marrow induced by palbociclib was achieved by cell cycle arrest, and it was reversible after drug discontinuation, which would not induce cell apoptosis and DNA damage, whereas the cytotoxic chemotherapy was another condition (44). Diarrhea was the most common AE in patients treated with abemaciclib, of which the percentages were 87.1% and 82.3% in MONARCH-2 and MONARCH-3, respectively (15,17). The duration of treatment was usually not affected by diarrhea, and most cases of diarrhea could be alleviated by antidiarrheal medications and dose modifications (17). In addition, the electrocardiogram (ECG) QT interval corrected for heart rate according to Friderica’s formula (QTcF) prolongation, which could be managed with dosage adjustments, was also worth noting in studies designed with ribociclib (21,25,28). However, ribociclib should be used cautiously for patients with QT interval prolongation (45). The AEs of the additional CDK4/6 inhibitors are usually considered reversible or controllable, and it is still necessary to monitor the toxicity for patients, before and during the treatment (46,47). In addition to the common AEs, there have been reports of some skin and mucosal tissue toxicity occurring during the combined application of palbociclib and radiotherapy (48).
In this analysis, we included updated OS and PFS results to further evaluate the clinical efficacy of CDK inhibitors combined with endocrine therapy in HR-positive HER2-negative advanced breast cancer. There are some limitations in our study. First, in the 9 RCTs included, the interventions were not completely consistent, such as the application of different CDK4/6 inhibitor agents and the different setting of first-line or second-line treatment. Although the subgroup analyses were used to manage, the clinical heterogeneity could not be eliminated. Second, the quality of the studies we included was generally high, but 1 study was conducted in an open-label phase-II setting, which could have resulted in the risk of performance bias. Third, small part of OS and PFS results in the included RCTs were interim results and more reports are needed to further confirm the effect of CDK4/6 inhibitors. Therefore, the outcomes of this study should be applied with caution.
Conclusions
The addition of CDK4/6 inhibitors effectively prolonged PFS and OS, regardless of whether they were used as first-line or second-line therapy, compared with endocrine therapy alone, in HR-positive, HER2-negative advanced breast cancer. It was worth mentioning that the subgroup analysis of this meta-analysis showed that the combination of CDK4/6 inhibitors and endocrine therapy benefited the patients ≥65 years old and patients with bone-only disease in OS, which was different from the previous studies. Similarly, CDK4/6 inhibitors could also improve the results of ORR, CBR, PFS2, and TTC. The application of CDK4/6 increased the incidence of AEs, most of which were controllable and tolerable. Familiarity with the AEs caused by different CDK4/6 inhibitors may be instrumental to the practice of clinicians.
Acknowledgments
Funding: This work was supported by the Medical and Engineering Integration Project of Shanghai Jiaotong University (No. YG2021ZD15); Project of Shanghai Municipal Commission of Science and Technology (No. 21Y11923000).
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1306/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1306/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 2014;106:dju055. [Crossref] [PubMed]
- Rakha EA, El-Sayed ME, Lee AH, et al. Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J Clin Oncol 2008;26:3153-8. [Crossref] [PubMed]
- Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med 2011;62:233-47. [Crossref] [PubMed]
- AlFakeeh A, Brezden-Masley C. Overcoming endocrine resistance in hormone receptor-positive breast cancer. Curr Oncol 2018;25:S18-27. [Crossref] [PubMed]
- Riggins RB, Schrecengost RS, Guerrero MS, et al. Pathways to tamoxifen resistance. Cancer Lett 2007;256:1-24. [Crossref] [PubMed]
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365:1687-717. [Crossref] [PubMed]
- Lei JT, Anurag M, Haricharan S, et al. Endocrine therapy resistance: new insights. Breast 2019;48:S26-S30. [Crossref] [PubMed]
- Haricharan S, Punturi N, Singh P, et al. Loss of MutL Disrupts CHK2-Dependent Cell-Cycle Control through CDK4/6 to Promote Intrinsic Endocrine Therapy Resistance in Primary Breast Cancer. Cancer Discov 2017;7:1168-83. [Crossref] [PubMed]
- Bilgin B, Sendur MAN, Şener Dede D, et al. A current and comprehensive review of cyclin-dependent kinase inhibitors for the treatment of metastatic breast cancer. Curr Med Res Opin 2017;33:1559-69. [Crossref] [PubMed]
- Gampenrieder SP, Rinnerthaler G, Greil R. CDK4/6 inhibition in luminal breast cancer. Memo 2016;9:76-81. [Crossref] [PubMed]
- Li J, Fu F, Yu L, et al. Cyclin-dependent kinase 4 and 6 inhibitors in hormone receptor-positive, human epidermal growth factor receptor-2 negative advanced breast cancer: a meta-analysis of randomized clinical trials. Breast Cancer Res Treat 2020;180:21-32. [Crossref] [PubMed]
- Wang L, Gao S, Li D, et al. CDK4/6 inhibitors plus endocrine therapy improve overall survival in advanced HR+/HER2- breast cancer: A meta-analysis of randomized controlled trials. Breast J 2020;26:1439-43. [Crossref] [PubMed]
- Slamon DJ, Neven P, Chia S, et al. Overall Survival with Ribociclib plus Fulvestrant in Advanced Breast Cancer. N Engl J Med 2020;382:514-24. [Crossref] [PubMed]
- Sledge GW Jr, Toi M, Neven P, et al. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy-MONARCH 2: A Randomized Clinical Trial. JAMA Oncol 2020;6:116-24. [Crossref] [PubMed]
- Finn RS, Boer K, Bondarenko I, et al. Overall survival results from the randomized phase 2 study of palbociclib in combination with letrozole versus letrozole alone for first-line treatment of ER+/HER2- advanced breast cancer (PALOMA-1, TRIO-18). Breast Cancer Res Treat 2020;183:419-28. [Crossref] [PubMed]
- Johnston S, Martin M, Di Leo A, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 2019;5:5. [Crossref] [PubMed]
- Rugo HS, Finn RS, Diéras V, et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res Treat 2019;174:719-29. [Crossref] [PubMed]
- Xu B, Hu X, Li W, et al. Palbociclib plus letrozole versus placebo plus letrozole in Asian postmenopausal women with oestrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: Primary results from PALOMA-4. Eur J Cancer 2022;175:236-45. [Crossref] [PubMed]
- Deeks JJ, Higgins JPT, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available online: www.training.cochrane.org/handbook
- Slamon DJ, Neven P, Chia S, et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J Clin Oncol 2018;36:2465-72. [Crossref] [PubMed]
- Sledge GW Jr, Toi M, Neven P, et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J Clin Oncol 2017;35:2875-84. [Crossref] [PubMed]
- Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med 2016;375:1925-36. [Crossref] [PubMed]
- Im SA, Lu YS, Bardia A, et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N Engl J Med 2019;381:307-16. [Crossref] [PubMed]
- Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol 2018;19:904-15. [Crossref] [PubMed]
- Turner NC, Slamon DJ, Ro J, et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N Engl J Med 2018;379:1926-36. [Crossref] [PubMed]
- Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 2016;17:425-39. [Crossref] [PubMed]
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol 2018;29:1541-7. [Crossref] [PubMed]
- Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015;16:25-35. [Crossref] [PubMed]
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. N Engl J Med 2022;386:942-50. [Crossref] [PubMed]
- Harbeck N, Bartlett M, Spurden D, et al. CDK4/6 inhibitors in HR+/HER2- advanced/metastatic breast cancer: a systematic literature review of real-world evidence studies. Future Oncol 2021;17:2107-22. [Crossref] [PubMed]
- Braal CL, Jongbloed EM, Wilting SM, et al. Inhibiting CDK4/6 in Breast Cancer with Palbociclib, Ribociclib, and Abemaciclib: Similarities and Differences. Drugs 2021;81:317-31. [Crossref] [PubMed]
- Cunningham NC, Turner NC. Understanding divergent trial results of adjuvant CDK4/6 inhibitors for early stage breast cancer. Cancer Cell 2021;39:307-9. [Crossref] [PubMed]
- Heckler M, Ali LR, Clancy-Thompson E, et al. Inhibition of CDK4/6 Promotes CD8 T-cell Memory Formation. Cancer Discov 2021;11:2564-81. [Crossref] [PubMed]
- De Luca A, Maiello MR, D'Alessio A, et al. Pharmacokinetic drug evaluation of palbociclib for the treatment of breast cancer. Expert Opin Drug Metab Toxicol 2018;14:891-900. [Crossref] [PubMed]
- Syed YY. Ribociclib: First Global Approval. Drugs 2017;77:799-807. [Crossref] [PubMed]
- Robert M, Frenel JS, Bourbouloux E, et al. Pharmacokinetic drug evaluation of abemaciclib for advanced breast cancer. Expert Opin Drug Metab Toxicol 2019;15:85-91. [Crossref] [PubMed]
- Fry DW, Harvey PJ, Keller PR, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther 2004;3:1427-38. [Crossref] [PubMed]
- Gelbert LM, Cai S, Lin X, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs 2014;32:825-37. [Crossref] [PubMed]
- Hamilton E, Infante JR. Targeting CDK4/6 in patients with cancer. Cancer Treat Rev 2016;45:129-38. [Crossref] [PubMed]
- Yıldırım HÇ, Mutlu E, Chalabiyev E, et al. Clinical outcomes of cyclin-dependent kinase 4-6 (CDK 4-6) inhibitors in patients with male breast cancer: A multicenter study. Breast 2022;66:85-8. [Crossref] [PubMed]
- Verma S, Bartlett CH, Schnell P, et al. Palbociclib in Combination With Fulvestrant in Women With Hormone Receptor-Positive/HER2-Negative Advanced Metastatic Breast Cancer: Detailed Safety Analysis From a Multicenter, Randomized, Placebo-Controlled, Phase III Study (PALOMA-3). Oncologist 2016;21:1165-75. [Crossref] [PubMed]
- Diéras V, Harbeck N, Joy AA, et al. Palbociclib with Letrozole in Postmenopausal Women with ER+/HER2- Advanced Breast Cancer: Hematologic Safety Analysis of the Randomized PALOMA-2 Trial. Oncologist 2019;24:1514-25. [Crossref] [PubMed]
- Hu W, Sung T, Jessen BA, et al. Mechanistic Investigation of Bone Marrow Suppression Associated with Palbociclib and its Differentiation from Cytotoxic Chemotherapies. Clin Cancer Res 2016;22:2000-8. [Crossref] [PubMed]
- Bøttcher TM, Cold S, Jensen AB. Treatment of advanced HR+/HER2- breast cancer with new targeted agents in combination with endocrine therapy: a review of efficacy and tolerability based on available randomized trials on everolimus, ribociclib, palbociclib and abemaciclib. Acta Oncol 2019;58:147-53. [Crossref] [PubMed]
- Boyle F, Beith J, Burslem K, et al. Hormone receptor positive, HER2 negative metastatic breast cancer: Impact of CDK4/6 inhibitors on the current treatment paradigm. Asia Pac J Clin Oncol 2018;14:3-11. [Crossref] [PubMed]
- Hui R, de Boer R, Lim E, et al. CDK4/6 inhibitor plus endocrine therapy for hormone receptor-positive, HER2-negative metastatic breast cancer: The new standard of care. Asia Pac J Clin Oncol 2021;17:3-14. [Crossref] [PubMed]
- van Aken ESM, Beeker A, Houtenbos I, et al. Unexpected toxicity of CDK4/6 inhibitor palbociclib and radiotherapy. Cancer Rep (Hoboken) 2022;5:e1470. [Crossref] [PubMed]





