Translation into Portuguese, cross-cultural adaptation and validation of “The European Organization for Research and Treatment of Cancer—Quality of Life Questionnaire—Bone Metastases-22”
Introduction
Metastatic disease is the main cause of death among cancer patients. Bones are the third most common site of metastasis, after liver and lungs. Breast, prostate, lung thyroid and kidney tumors are the tumors that most frequently metastasize to bone. At the Department of Orthopedic Oncology of São Paulo Hospital, the incidence of bone metastases from breast cancers represents 45% of all cases, followed by prostate (12%) and lung cancer (9%) (1).
Skeletal-related events (SREs) due to bone metastases cause a variety of morbidities, including pain, spinal cord compression, pathologic fractures and hypercalcemia. Such events may cause significant debilitation and may have a negative impact on quality of life and functional independence (2).
Current management of bone metastases is aimed primarily at reducing morbidity due to SREs to preserve or improve both quality of life and functional independence. Preventing SREs and improving survival are the goals of both current and future research (1).
Valid and reliable instruments are required to quantify the quality of life of patients with bone metastases. The European Organization for Research and Treatment of Cancer—Quality of Life Questionnaire—Bone Metastases-22 (EORTC QLQ-BM22), a 22-item module that was designed to measure symptoms, functions, psychosocial variables and expectations of patients with bone metastases. In Brazil, prior to the present study, there was no instrument that could evaluate the quality of life of cancer patients with bone metastases (3,4).
The aim of the present study was to translate the EORTC QLQ-BM22 into Brazilian Portuguese, to adapt the instrument culturally and to validate the newly adapted instrument.
Methods
This original, longitudinal, observational study was designed with no control group and was approved by the Ethics Committee of Universidade Federal de São Paulo, Brazil (CEP-UNIFESP/EPM 1649/11). Ninety-five bone metastasis patients (31 men and 64 women, mean age 58.36±8.90 years) took part in the investigation. All participants gave their written informed consent to take part in the study. The patients were recruited prospectively between August 2012 and August 2014.
They were eligible if they were over 21 years old, with histological confirmation of primary cancer and radiologic evidence of bone metastases (X-ray, CT, or bone scintigraphy). Exclusion criteria included inability to understand the questionnaire.
The original EORTC QLQ-BM22 module comprises 22 questions that are organized into two scales: Symptom and Functional. Each of the scales has two subscales. The Symptom scale contains the painful sites (five questions) and painful characteristics (three questions) subscales, and the Functional scale contains the functional interference (eight questions) and psychosocial aspects (six questions) subscales.
After obtaining permission from the author, the English version of EORTC QLQ-BM22 was translated into Brazilian Portuguese by two independent expert translators. An additional two independent translators who were unfamiliar with the original, English EORTC QLQ-BM22 version retranslated the Brazilian Portuguese translation into English. The translated versions were discussed and compared with the original version. The Brazilian Portuguese version of EORTC QLQ-BM22 was pilot-tested in 15 Brazilian cancer patients with bone metastases. The patients understood all questions of the EORTC QLQ-BM22, reporting that all items were acceptable and understandable. No changes were made.
To test reliability, forty patients were evaluated three times, by two interviewers, using the Brazilian Portuguese version of EORTC QLQ-BM22. To assess the inter-rater reliability, the patients were evaluated twice by two distinct interviewers. Fifteen days after the first interview, the test-retest reliability was assessed by repeating the EORTC QLQ-BM22 evaluation. Intraclass correlation coefficients (ICCs) and Pearson’s correlation coefficient were used to analyze inter-rater reliability and test-retest reliability. The internal consistency of the scale (module and scale) was evaluated using Cronbach’s alpha coefficient in all EORTC QLQ-BM22 evaluations.
Face and content validity were assessed by one multidisciplinary committee (consisting of a doctor, physiotherapist, nurse and psychologist). To assess the construct validity, 40 patients were evaluated by the EORTC QLQ-BM22 and the Brazilian version of the Short Form Health Survey [36-Item Short Form Survey (SF-36)]. To analyze construct validity, Pearson’s correlation coefficient was tested between the questionnaires. A probability level less than 0.05 was considered statistically significant. The analyses were conducted using the SPSS version 16.0 software.
Results
Patient demographics
A total of 95 patients were enrolled, with 64 (67.4%) female and 31 (32.6%) male, in whom the breast (n=50; 53%), prostate (n=17, 18%) and lung (n=11, 12%) were the most common primary cancer sites and spine (n=42, 27%), pelvis (n=28, 18%), and femur (n=28, 18%) were the most common bone metastasis site. The median age was 58.36±8.90 years (Table 1).
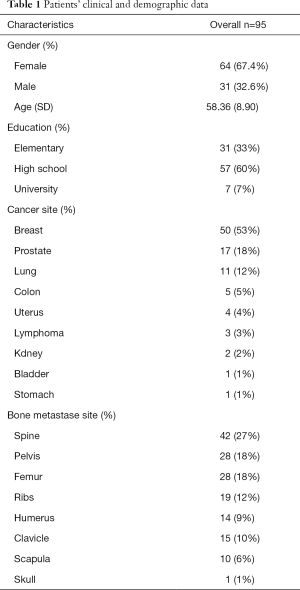
Full table
Translation
After the translation process by two independent translators, two Portuguese versions of the EORTC QLQ-BM22 were prepared and were then reviewed by a multidisciplinary committee that drafted a single version in Portuguese. This Portuguese version was retranslated into English by two independent translators, who created two Portuguese versions of the questionnaire. These two versions were analyzed and compared to the original version to discern possible differences or discrepancies in the translation process. At the end of this stage, the final version of the EORTC QLQ-BM22 was drafted in Portuguese.
Transcultural adaptation
One patient (6.6%) did not understand the term “constant” in question number 6 (You had constant pain?), and one patient (6.6%) did not understand the term “intermittent” in question 7 (You had intermittent pain?). Because the proportion of patients affected was less than 10%, it was not necessary to modify these terms. Thus, the Portuguese measure was considered culturally equivalent to the English measure, and there was no need for any changes to the questionnaire. The average time taken to answer the questionnaire was 8 minutes, with a range of 5 to 14 minutes. The degree of internal consistency of the questionnaire was measured in each of the three interviews. It was determined that the Cronbach’s alpha value was high in all three interviews, and these alpha values show that the questionnaire is fairly consistent (Table 2).
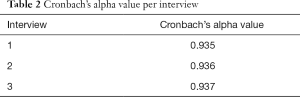
Full table
The accuracy of the EORTC QLQ-BM22 Brazilian Portuguese version was evaluated by Cronbach’s alpha analysis. The alpha value for the entire scale exceeded 0.8 (Table 2). Only one items measures presented Cronbach’s alpha value less than 0.8 (painful sites =0.696). The others items measures exceeding 0.8 (painful characteristics =0.845; functional interference =0.938; psychosocial aspects =0.806).
We can conclude from these data that both the Pearson correlation and the intraclass correlation values are statistically significant and very high, showing that the first interview has excellent reliability and validity with the two additional interviews (Table 3).
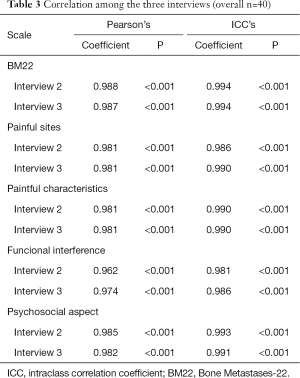
Full table
Validity
We analyzed the results of the correlation between symptom QLQ-BM22 with the SF-36. This indicates the existence of a significant correlation between the symptom domain of the QLQ-BM22 and the areas of functioning, bodily pain, general health, vitality, and social aspects as well as the domains of limitation by emotional aspects and mental health from the SF-36 (P<0.05). Only the domain of limited by physical appearance showed no correlation with the symptom domain of the QLQ-BM22 (Table 4).
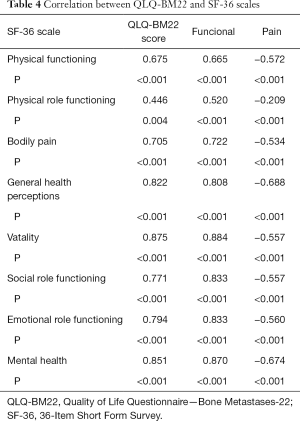
Full table
Analysis of construct validity revealed moderate and strong correlations between the SF-36 coefficients and the EORTC QLQ-BM22 score (total and subscale).
Discussion
The incidence of SREs has been increasing, possibly due to the improvements in treatment of the primary site and treatment of disseminated cancer, thereby increasing the survival of these patients (5).
Bone metastases cause various co-morbidities, including pain, pathologic fractures, hypercalcemia and spinal cord compression. These events have a negative impact on the quality of life and functional independence of cancer patients (6).
Currently, there are concerns not only about the results of the treatment or surgical intervention but also about the functional and emotional impact on the quality of life. The challenge for researchers is to quantify the subjective character data and determine what issues should be addressed. Generally, measurement tools are created originally in English, so it is necessary to complete the translation process and then evaluate the measure’s properties in a specific cultural context (7,8).
Each country has its own customs, beliefs, behaviors and habits that directly reflect its culture and differences. To translate a tool, we should aim to present the tool in easy-to-understand language and simple, clear words that are appropriate to the culture, without losing the essence of the original version.
The choice of a tool for evaluation depends on a few factors. Initially, the tool must have credibility and comprehensiveness, accuracy and reproducibility. The tool should also be easy to apply and have acceptability among the interviewees and interviewers (9). Evaluation questionnaires must be reproducible over time and should thus produce the same or similar results in two or more administrations to the same patient if the patient’s medical condition did not change (10). None of patients in this study had any changes in their treatment or intervention during the research, thus justifying the great inter-rater agreement.
There are functional assessment for patients with musculoskeletal tumors: the Musculoskeletal Tumor Society Rating Scale (MSTS) measures the functional impairment of patients with musculoskeletal tumors submitted to member preservation surgery, and Toronto Extremity Salvage Score (TESS) evaluates physical disability, according to the report based on this same group of patients (11,12). TESS was translated into Brazilian Portuguese and validated in patients with osteosarcoma, and the MSTS was validated in patients with diagnoses of musculoskeletal tumors, osteosarcoma, chondrosarcoma, and Ewing’s sarcoma. Although the two tools assess patients with musculoskeletal tumors, they are very specific, and it is thus not possible to use them to evaluate bone metastasis patients (13,14).
Among the cases of musculoskeletal cancer, bone metastasis is the most frequent complication, and it can lead to the presence of pain and loss of function, thereby worsening the quality of life of these patients. There was a need for a specific tool that aids in the clinical and therapeutic monitoring of these patients by evaluating the quality of life. This study sought to evaluate a tool that met this need and addressed issues related solely to the characteristic symptoms of patients with bone metastasis in a simple and objective way. The QLQ-BM22 is an instrument available in the literature that has been translated and validated for use in Japan, Iran and Poland (4,15-18).
In the validation phase of the QLQ- BM22, it was observed that 68.2% of the patients had primary site as breast cancer, followed by prostate (11.5%) and lung (7.8%) cancer (4). By analyzing the characteristics of the sample according to the primary site of cancer, we found that breast (49.47%), prostate (21.05%) and lung (11.57%) were the most common primary sites of metastases, as reported in previous studies (1,19).
For a long time, assessments of a given intervention were performed using clinical criteria. Currently, there is a consensus on the need for standardized evaluation systems. There is also a need to compare results of different treatment methods in patients with the same problem and to analyze the effectiveness of a treatment modality with greater reliability.
Because of the high incidence of patients with bone metastases and the progress of treatment, the QLQ-BM22 can help multidisciplinary professionals evaluate patients by following the evolution of symptoms and functioning. However, the tool can also be used to determine the effect of different treatments on a patient, so the best approach can be chosen to improve the patient’s quality of life and well-being (1,20).
Conclusions
The QLQ-BM22 was translated into Brazilian Portuguese, and it was culturally adapted and proved to be reproducible. The translated, culturally adapted tool was determined to have face, content and construct validity.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethics Committee of Universidade Federal de São Paulo, Brazil (No. CEP-UNIFESP/EPM 1649/11) and written informed consent was obtained from all patients.
References
- Garcia Filho RJ. Princípios básicos em Ortopedia Oncológica - Diagnóstico dos tumores ósseos. In: Garcia Filho RJ, editor. Clínica Ortopédica da SBOT - Tumores Ósseos e Sarcomas dos Tecidos Moles. 1 ed. Rio de Janeiro: Editora Guanabara Koogan S.A., 2009:12-20.
- Patrick DL, Ferketich SL, Frame PS, et al. National institutes of health state-of-the-science conference statement: symptom management in cancer: pain, depression, and fatigue, July 15-17, 2002. J Natl Cancer Inst 2003;95:1110-7. [Crossref] [PubMed]
- Chow E, Hird A, Velikova G, et al. The European organisation for research and treatment of cancer quality of life questionnaire for patients with bone metastases: the EORTC QLQ-BM22. Eur J Cancer 2009;45:1146-52. [Crossref] [PubMed]
- Chow E, Nguyen J, Zhang L, et al. International field testing of the reliability and validity of the EORTC QLQ-BM22 module to assess health-related quality of life in patients with bone metastases. Cancer 2012;118:1457-65. [Crossref] [PubMed]
- Yu HH, Tsai YY, Hoffe SE. Overview of diagnosis and management of metastatic disease to bone. Cancer Control 2012;19:84-91. [PubMed]
- Galasko CS. Skeletal metastases. Clin Orthop Relat Res 1986.18-30. [PubMed]
- Guillemin F. Cross-cultural adaptation and validation of health status measures. Scand J Rheumatol 1995;24:61-3. [Crossref] [PubMed]
- Guyatt GH, Naylor CD, Juniper E, et al. Users' guides to the medical literature. XII. How to use articles about health-related quality of life. Evidence-Based Medicine Working Group. JAMA 1997;277:1232-7. [Crossref] [PubMed]
- Brandão L, Ferraz MB, Zerbini AC. Avaliação da qualidade de vida na artrite reumatoide. Ver Bra Reumatol 1997;37:275-81.
- Jenkinson C. Evaluating the efficacy of medical treatment: possibilities and limitations. Soc Sci Med 1995;41:1395-401. [Crossref] [PubMed]
- Enneking WF, Dunham W, Gebhardt MC, et al. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res 1993.241-6. [PubMed]
- Davis AM, Wright JG, Williams JI, et al. Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual Life Res 1996;5:508-16. [Crossref] [PubMed]
- Saraiva D, de Camargo B, Davis AM. Cultural adaptation, translation and validation of a functional outcome questionnaire (TESS) to Portuguese with application to patients with lower extremity osteosarcoma. Pediatr Blood Cancer 2008;50:1039-42. [Crossref] [PubMed]
- Rebolledo DC. Tradução e validação do instrument Musculoskeletal Tumor Society Rating Scale (MSTS) para avaliação da função em paciente com sarcomas ósseos dos membros inferiores. São Paulo. Universidade de São Paulo 2011:94.
- Satoh T, Kobayashi K, Hori T, et al. The European organisation for research and treatment of cancer (EORTC) quality of life questionnaire for Japanese patients with bone metastases--The Japanese version of the EORTC QLQ-BM22. Gan To Kagaku Ryoho 2010;37:1507-12. [PubMed]
- Yekaninejad MS, Ahmadzadeh A, Mosavi SH, et al. The reliability and validity of the Iranian version of the European organization for research and treatment of cancer quality of life questionnaire for patients with bone metastases: the EORTC QLQ-BM22. Expert Rev Pharmacoecon Outcomes Res 2014;14:147-56. [Crossref] [PubMed]
- Püsküllüoğlu M, Tomaszewski KA, Bottomley A, et al. Validation of the Polish version of the EORTC QLQ-BM22 module for the assessment of health-related quality of life in patients with bone metastases. Qual Life Res 2014;23:527-32. [Crossref] [PubMed]
- Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer 1987;55:61-6. [Crossref] [PubMed]
- Bedard G, Zeng L, Poon M, et al. Comparison of the EORTC QLQ-BM22 and the BOMET-QOL quality of life questionnaires in patients with bone metastases. Asia Pac J Clin Oncol 2014;10:118-23. [Crossref] [PubMed]
- Zeng L, Chow E, Bedard G, et al. Quality of life after palliative radiation therapy for patients with painful bone metastases: results of an international study validating the EORTC QLQ-BM22. Int J Radiat Oncol Biol Phys 2012;84:e337-42. [Crossref] [PubMed]


