
Association between odontogenic conditions and maxillary sinus abnormalities: a retrospective cone-beam computed-tomographic study
Highlight box
Key findings
• Adjacent odontogenic infection increased the probability of maxillary sinus abnormalities.
• The likelihood of maxillary sinus abnormality was related to the distance between the infection and the maxillary sinus, not to the type of infection.
• The maxillary root apices of older subjects tended to be farther from the maxillary sinus.
What is known, and what is new?
• It is known that adjacent odontogenic infection increases the probability of maxillary sinus abnormalities.
• The novel finding of this study is that maxillary sinus abnormality is most likely related to the distance between the infection and the maxillary sinus, not to the type of infection. The maxillary root apices of older subjects tended to be farther from the maxillary sinus.
What is the implication, and what should change now?
• Attention is recommended whenever odontogenic infections are close to the maxillary sinuses, despite infection type.
Introduction
The maxillary sinus (MS) is lined with a thin respiratory mucous membrane known as the ‘Schneiderian membrane’. When the MS mucosa is thickened, opacified, or calcified on a radiograph, it is referred to radiographically as an ‘MS abnormality’ (1,2). MS abnormalities, together with the associated symptoms and signs, constitute maxillary sinusitis. However, MS abnormalities can also be identified in asymptomatic individuals (3). Odontogenic sinusitis accounts for approximately 10% to 40% of cases of maxillary sinusitis (4). The spread of infections arising in the maxillary teeth posterior to the MS is facilitated by their close anatomical relationship. Although relatively rare, complications of maxillary sinusitis can cause orbital or intracranial infections (5).
Cone-beam computed tomography (CBCT) is useful in clarifying the relationship between dental pathology and the MS (6). CBCT allows high-contrast visualization of bone morphology, comparable to that achieved with conventional computed tomography (7), but with a radiation dose reduction of 30% to 40% and a lower cost per examination.
The relationship between odontogenic conditions and MS abnormalities on CBCT has already been described, but with conflicting results. Systematic reviews have shown that in CBCT studies, periapical lesions (PAL) in the posterior maxilla are likely to be associated with MS abnormalities. However, evidence for the relationship between periodontal bone loss (PBL) and MS abnormalities is inconclusive (8,9). Moreover, few studies have described the association between combined periodontal-endodontic lesions (CPEL) and MS abnormalities or examined the effects of the infection type or the distance between the odontogenic infection and the maxillary sinus floor (INF-MSF) on MS abnormalities. Therefore, in this study, we assessed the influence of various dental conditions on MS abnormalities. The MS septal walls are barriers of cortical bone that divide the MS floor into multiple compartments (10). The relationship between the MS septal walls and MS abnormalities is gaining attention but is controversial (11-13). Therefore, we considered MS septal walls as an influencing factor for MS abnormalities.
The main purpose of the present study was to observe the effects of age, sex, PAL, PBL, and CPEL of adjacent teeth, missing teeth, and MS septal walls on MS abnormalities and to observe the effects of the infection type, type of tooth infected, and INF-MSF on MS abnormalities. Additionally, we aimed to analyze whether the anatomical relationship between the MS and the teeth is related to age or sex. We present the following article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-950/rc).
Methods
This study was performed with a cross-sectional design and a retrospective analysis. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Medical Science Research Ethics Committee of Peking University Third Hospital (Beijing, China; No. 2020/142-03). Individual consent for this retrospective analysis was waived. Patients who received CBCT examination with a field of view of 16×8 cm2 or 8×8 cm2 in the Department of Stomatology of Peking University Third Hospital between November and December 2021 were included.
Patients who had any of the following were excluded: (I) impacted teeth in the posterior maxillary area; (II) teeth with apical lesions in the posterior maxillary area that had undergone satisfactory root canal treatment; (III) low-quality images, or the MS and posterior maxillary teeth were incompletely visible on the CBCT scan; (IV) implants or bone grafts in the posterior maxillary area; (V) trauma, cysts, tumors, or a history of any surgery related to the MS; (VI) according to medical records, periodontal curettage had been performed in the preceding 3 years.
The CBCT images of 925 consecutive patients were examined, and the images obtained from 570 patients were deemed appropriate for inclusion in the study.
The CBCT images were obtained with a KaVo 3D eXam (KaVo, Hatfield, PA, USA). All CBCT images were taken with a field of view of 16×8 cm2 or 8×8 cm2 and a basic voxel size of 0.2 mm or 0.125 mm. The operating parameters were set to 37 mA and 120 kV, and the exposure time was 26.9 s. The images were combined and observed with the KaVo eXam Vision software (KaVo) on a monitor with a 2,160×1,440 pixel resolution in a room with dimmed lighting. The collected demographic data included sex and age.
The ipsilateral MS, maxillary alveolar bone, and posterior teeth were defined as the study unit, and 1,140 units were obtained from 570 CBCT images. In each unit, the MS abnormality, MS septal walls, infection status, and missing maxillary posterior teeth were recorded. The infection status of the teeth included PAL, PBL, and CPEL. Missing wisdom teeth were not recorded as missing teeth. Each tooth was evaluated in the coronal, sagittal, and cross-sectional view with CBCT. The anatomical relationships between the MS and maxillary teeth were also examined.
A healthy MS was defined as a Schneiderian membrane thickness of less than 2 mm, which is barely visible with radiography (Figure 1A). Abnormal membrane morphologies included ‘flat’ (horizontal thickening), ‘polypoid’ (dome-shaped thickening), ‘opacified’ (complete sinus opacification) (14), and periostitis (1).
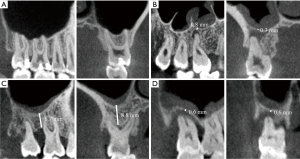
The infection status of the teeth included PAL, PBL, and CPEL. PAL was defined as radiolucency associated with the apical part of the root if the width of the radiolucency exceeded 1 mm. PBL was defined as severe periodontal bone loss involving more than half the root length. The normal height of the alveolar crest was defined as 1 mm apical to the cement-enamel junction. Teeth with both PAL and PBL were recorded as CPEL, including those in which PAL and PBL were or were not connected. The presence of a septal wall was defined as a septal wall of at least 2.5 mm in height on CBCT (15).
To analyze the correlations between the infection type, tooth position, INF-MSF, and MS abnormality, we selected units with single tooth infections: a single PAL tooth, a single PBL tooth, or a single CPEL tooth. The shortest distance between the dental infection edge and the maxillary sinus floor (MSF) was recorded as INF-MSF (Figure 1).
The vertical relationship between the MSF and teeth apex was classified as distant from the MSF (type 1); at least one root apex of the teeth in contact with the MSF (type 2); or at least one root apex of the teeth protruding into the MSF (type 3).
Two experienced endodontic specialists independently evaluated the CBCT images in a quiet environment, and the two observers showed good agreement (kappa =0.911). When results were inconsistent, the two evaluators reached consensus after discussion. An otolaryngologist instructed the observers to ensure they had a knowledge of MS abnormalities and the diagnostic skill to identify them. All CBCT images were evaluated twice at a 1-month interval by the same observer.
Statistical analysis
All data were analyzed with SPSS 26.0 software (IBM, Chicago, IL, USA). The fit of the parameters to a normal distribution was evaluated with the Shapiro-Wilk test. Medians and interquartile ranges were used to describe quantitative variables that did not conform to a normal distribution, and frequencies and percentages were used to describe categorical variables. A battery of statistical analyses was performed, including the chi-squared test, Fisher’s exact test, and Mann-Whitney U test. All variables with a P value <0.05 were incorporated into the multivariate logistic model to identify variables that exerted a significant effect. The chi-squared test and Kruskal-Wallis H test were performed to determine whether the anatomical relationship between the MS and teeth was related to age or sex. All statistical tests were two-tailed and interpreted at the 5% significance level.
A logistic regression analysis of six possible influencing factors was performed for all units, requiring at least 30 units with MS abnormality and 30 units without MS abnormality. A total of 489 units with MS abnormality and 651 units without MS abnormality met the sample size requirements for the analysis. Similarly, the logistic regression analysis of units with a single infected tooth required at least 30 units with MS abnormality and 30 units without MS abnormality. A total of 139 units with MS abnormality and 39 units without MS abnormality also met the sample size requirements for the analysis.
Results
In this study, we enrolled 570 patients who had undergone CBCT imaging, including 1,140 MSs. The 570 patients included 222 males and 348 females. The patients were aged between 13 and 91 years (mean age, 41.14±16.53 years).
MS abnormalities were found in 57.54% (328/570) of patients: 28.24% (161/570) of patients showed bilateral MS abnormalities, and 29.30% (167/570) showed unilateral MS abnormalities. MS abnormalities were detected in 42.89% (489/1,140) of MSs.
The associations between MS abnormalities and odontogenic factors are shown in Table 1. Individual analyses showed that the prevalence of MS abnormalities was significantly associated with age, sex, PAL, PBL, CPEL, and missing teeth (P<0.001) but not with MS septal walls (P=0.126). The multiple logistic regression results demonstrated that the frequency of MS abnormalities was significantly associated with the following factors: sex [odds ratio (OR) =1.653; 95% confidence interval (CI): 1.259, 2.170; P<0.001); PAL of adjacent teeth (OR =5.771; 95% CI: 3.636, 9.160; P<0.001), PBL of adjacent teeth (OR =2.778; 95% CI: 1.718, 4.491; P<0.001); and CPEL of adjacent teeth (OR =13.818; 95% CI: 5.812, 32.855; P<0.001). Therefore, male sex and MS adjacent to teeth with PAL, PBL, or CPEL were factors associated with a higher prevalence of MS abnormality.
Table 1
| Variables | Absence, n (%) | Presence, n (%) | Individual analyses | Adjusted analyses | ||||
|---|---|---|---|---|---|---|---|---|
| χ2 | P value | OR | 95% CI | P value | ||||
| Age, years | 104.100 | <0.001*** | ||||||
| 0–18 | 6 (75.0)a,b,c | 2 (25.0)a,b,c | 1 | 0 | ||||
| 19–25 | 295 (73.0)c | 109 (27.0)c | 1.108 | 0.218–5.632 | 0.902 | |||
| 26–40 | 217 (58.3)b | 155 (41.7)b | 1.954 | 0.384–9.942 | 0.419 | |||
| 41–60 | 101 (40.1)a | 151 (59.9)a | 2.339 | 0.452–12.104 | 0.311 | |||
| >60 | 32 (30.8)a | 72 (69.2)a | 2.924 | 0.538–15.898 | 0.214 | |||
| Sex | 17.975 | <0.001*** | <0.001*** | |||||
| Female | 432 (62.1) | 264 (37.9) | 1 | |||||
| Male | 219 (49.3) | 225 (50.7) | 1.653 | 1.259–2.170 | ||||
| PAL | 66.380 | <0.001*** | <0.001*** | |||||
| Absence | 623 (61.3) | 394 (38.7) | 1 | |||||
| Presence | 28 (22.8) | 95 (77.2) | 5.771 | 3.636–9.160 | ||||
| PBL | 68.888 | <0.001*** | <0.001*** | |||||
| Absence | 620 (61.5) | 388 (38.5) | 1 | |||||
| Presence | 31 (23.5) | 101 (76.5) | 2.778 | 1.718–4.491 | ||||
| CPEL | 99.965 | <0.001*** | <0.001*** | |||||
| Absence | 645 (61.4) | 406 (38.6) | 1 | |||||
| Presence | 6 (6.7) | 83 (93.3) | 13.818 | 5.812–32.855 | ||||
| Missing teeth | 22.353 | <0.001*** | 0.640 | |||||
| Absence | 596 (59.7) | 402 (40.3) | 1 | |||||
| Presence | 55 (38.7) | 87 (61.3) | 1.113 | 0.711–1.741 | ||||
| MS septal wall | 2.336 | 0.126 | ||||||
| Absence | 520 (56.0) | 408 (44.0) | ||||||
| Presence | 131 (61.8) | 81 (38.2) | ||||||
The corresponding data of categorical variables are frequency and composition ratio. a, b and c represent the pair comparison results of the correlation between different age groups and MS abnormality. Differing superscript letters indicate a significant difference between the different age groups, while the values with the same superscript letters indicate no statistical difference (P<0.05). ***, P<0.001. PAL, periapical lesions; PBL, periodontal bone loss; CPEL, combined periodontal-endodontic lesions; MS, maxillary sinus.
The associations between the occurrence of MS abnormalities and the infection type, the type of tooth infected, and INF-MSF in units with a single infected tooth are shown in Table 2. Individual analyses showed that the prevalence of MS abnormalities was significantly associated with sex (P=0.010), infection type (P=0.012), type of tooth infected (P=0.004), and INF-MSF (P=0.003), but not with the presence of MS septal walls (P=0.574). The results of an adjusted multiple logistic regression analysis showed that the frequency of MS abnormalities was significantly associated with the following factors: sex (OR =2.413; 95% CI: 1.021, 5.705; P=0.045), type of infected tooth (OR =3.431; 95% CI: 1.379, 8.533; P=0.008), and INF-MSF (OR =0.871; 95% CI: 0.775, 0.98; P=0.021). Therefore, in units with a single infected tooth, the probability of an MS abnormality was greater in males, units with an infected molar, or units with a shorter INF-MSF.
Table 2
| Variable | Absence, n (%) | Presence, n (%) | Individual analyses | Adjusted analyses | ||||
|---|---|---|---|---|---|---|---|---|
| χ2/z | P value | OR | 95% CI | P value | ||||
| Age, years | 4.472 | 0.215 | ||||||
| 19–25 | 10 (37.0) | 17 (63.0) | ||||||
| 26–40 | 10 (20.0) | 40 (80.0) | ||||||
| 41–60 | 14 (20.0) | 56 (80.0) | ||||||
| >60 | 5 (16.1) | 26 (83.9) | ||||||
| Sex | 6.704 | 0.010* | 0.045* | |||||
| Female | 29 (29.0) | 71 (71.0) | 1 | |||||
| Male | 10 (12.8) | 68 (87.2) | 2.413 | 1.021–5.705 | ||||
| Infection type | 8.870 | 0.012* | ||||||
| PAL | 22 (24.4)a | 68 (75.6)a | 1 | 0.244 | ||||
| PBL | 16 (29.1)a | 39 (70.9)a | 0.868 | 0.332–2.275 | 0.774 | |||
| CPEL | 1 (3.0)b | 32 (97.0)b | 5.458 | 0.669–44.504 | 0.113 | |||
| Infected tooth type | 8.333 | 0.004** | 0.008** | |||||
| Premolar | 14 (40.0) | 21 (60.0) | 1 | |||||
| Molar | 25 (17.5) | 118 (82.5) | 3.431 | 1.379–8.533 | ||||
| IF-MSF† | 5‡ (1.4§, 8.65¶) | 2.4‡ (0.7§, 5.25¶) | −2.94 | 0.003** | 0.871 | 0.775–0.980 | 0.021* | |
| MS septal wall | 0.315 | 0.574 | ||||||
| Absence | 35 (22.6) | 120 (77.4) | ||||||
| Presence | 4 (17.4) | 19 (82.6) | ||||||
†, continuous variable; ‡, median; §, lower quartile; ¶, upper quartile; *, P<0.05; **, P<0.01. The corresponding data of categorical variables are frequency and composition ratio. a and b represent the pair comparison results of the correlation between different infection type groups and MS abnormality. Differing superscript letters indicate a significant difference between the different infection type groups, while the values with the same superscript letters indicate no statistical difference (P<0.05). PAL, periapical lesions; PBL, periodontal bone loss; CPEL, combined periodontal-endodontic lesions; IF-MSF, the distance between the dental infection and the maxillary sinus floor (mm); MS, maxillary sinus.
In terms of the closest relationship between the tooth root tip and the MSF, we found that in 17.28% (197/1,140) of units, the root apices were distant from the MSF; in 17.63% (201/1,140) of units, at least one root apex contacted the MSF, but did not enter it; and in 57.89% (660/1,140) of units, at least one root apex entered the MS, and the cortical bone of the MSF was incomplete. In 7.19% (82/1,140) of units, the relationship between the MS and teeth could not be determined because one or some teeth were missing in the unit.
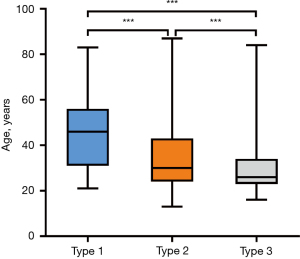
The anatomical relationship between the MS and the maxillary teeth correlated significantly with age (P<0.001; Table 3). The median age of subjects with the type 1 relationship was 46 years (range, 31–56 years); with a type 2 relationship was 30 years (range, 24–43 years); and with a type 3 relationship was 26 years (range, 23–34 years). A pairwise Bonferroni post hoc comparison of the corrected significance levels showed statistically significant differences in the age distribution among all three groups (Figure 2). That is, the maxillary apices of older subjects tended to be farther from the MSF. The anatomical relationship between the MS and the maxillary teeth did not correlate significantly with sex (P=0.184; Table 3).
Table 3
| Variables | Anatomic relationship between the MS and maxillary teeth | H/χ2 | P value | ||
|---|---|---|---|---|---|
| Type 1 | Type 2 | Type 3 | |||
| Age, years† | 46‡ (31§, 56¶) | 30‡ (24§, 43¶) | 26‡ (23§, 34¶) | 138.614 | <0.001*** |
| Gender, n (%) | 3.384 | 0.184 | |||
| Female | 131 (19.8) | 132 (19.9) | 399 (60.3) | ||
| Male | 66 (16.7) | 69 (17.4) | 261 (65.9) | ||
†, continuous variable; ‡, median; §, lower quartile; ¶, upper quartile; ***, P<0.001. The corresponding data of categorical variables are frequency and composition ratio. MS, maxillary sinus.
Discussion
There is no consensus about how thick the mucosa should be to indicate mucosal thickening, and threshold values range from 1 to 6 mm (1,2,9,14,16-26). A threshold value of 2 mm was selected in the largest number of past studies (14,17-19,21-23,25). A recent study demonstrated an association between mucosal thickening larger than 2 mm and maxillary sinusitis (27), so we chose 2 mm as the threshold value for mucosal thickening (24). Penarrocha-Oltra’s study classified MS abnormalities and studied the relationship between different types of MS abnormalities and different types of odontogenic infections (9). In this study, we found that two or more abnormalities frequently occurred simultaneously in one MS, and some MS abnormalities could not be accurately classified after consulting otolaryngologists, so we did not pursue this analysis.
In the present study, MS abnormalities were detected in 57.54% of patients and in 42.89% of MSs, consistent with previous studies where MS abnormalities were detected in 48.4–60.62% of patients and 39.2–53.6% of sinuses (17,21,22,24,28).
Because of the close anatomical relationship between dentoalveolar units and the MS, conditions arising from dentoalveolar units can cause MS abnormalities or even maxillary sinusitis (1,21,23). We found that PAL, PBL, and CPEL were significantly associated with MS abnormalities.
In previous studies, the risk of MS abnormality was significantly higher in the presence of PAL (9,17-18,21,28-32), consistent with our results. The resolution of MS abnormalities after root canal treatment of a PAL tooth has been reported (33), which confirmed the correlation between PAL and MS abnormalities.
Previous research has produced controversial results on the relationship between PBL and MS abnormalities. Some studies found an association between periodontal health and maxillary sinusitis (17,24,30,31,34,35), whereas others found no such relationship (18,21,25). These differences are due to different definitions of ‘periodontitis’ from one study to another, with some including all and others only severe periodontitis. The probability of MS abnormalities increases as PBL worsens, and severe PBL is associated with MS abnormalities (9,24,29,36), consistent with our study. Mucosa thickening was reduced within 4 months with thorough debridement after tooth extraction in units with severe PBL (34), further confirming the correlation between severe PBL and MS abnormalities.
The risk of MS abnormality was significantly higher in the presence of CPEL, consistent with a previous study (31). In the first regression model containing all units, the OR value for CPEL was higher than that for PAL or PBL, whereas in the second regression model, which contained units with a single infected tooth, the type of infection was not associated with the occurrence of MS abnormalities. The difference in results is attributable to the different factors taken into account in the two models. In the first model, the cumulative effect of multiple infected teeth and INF-MSF could not be taken into account, so the second regression model showed a more objective analysis of the relationship between the infection type and the occurrence of MS abnormality. This finding also confirmed that CPEL should receive as much attention as PAL and PBL in terms of its relationship with MS abnormalities.
Mucosal thickening is reportedly more common in males (21,22,25,28-30), consistent with our finding, although no significant difference was observed between males and females in Sakir’s study (18). The specific characteristics of each study population may be responsible for this discrepancy.
Previous research has presented controversial results on the relationship between age and MS abnormalities. In most studies, univariate analyses identified such an association (14,21,22,28), whereas multivariate regression analyses did not (21,30), consistent with our study. In Huang’s study, an association was detected with multivariate regression analysis (29). Various inflammatory diseases accumulate with increasing age, and it is possible that the results of these univariate analyses were confounded by the presence of inflammatory diseases, which have also been shown to increase the likelihood of MS abnormalities. Therefore, no correlation was detected after adjustment for these variables.
The removal of unhealthy teeth did not completely resolve the thickening of the sinus membrane, and the resultant membrane thickness may have changed in structure and thickened secondary to metaplasia rather than being infected (19). Previous studies have shown a correlation between tooth loss and MS abnormalities when using univariate analyses (17,28,34), consistent with the present study. However, we performed a further multivariate logistic regression analysis and found that a missing tooth was not associated with MS abnormality. This finding may be due to infection in other maxillary posterior teeth that also initiated MS abnormality and should be considered in any study.
No significant association was detected between the presence of MS septal walls and MS abnormalities in our study, as reported in Bornstein’s study (37). However, one study found that the presence of MS septal walls resulted in MS thickening (11), whereas several studies attributed the thinness of the MS membrane to MS septal walls (12,13). The discrepancies in these findings may be due to the age and ethnicity of the study populations or the imaging modality used. The effect of MS septal walls on the thickness of the MS membrane needs to be demonstrated in more carefully designed studies.
As the effects of multiple infected teeth are probably cumulative, we selected units with a single infected tooth to study the influence of the infection type, the type of infected tooth, and INF-MSF on the occurrence of MS abnormalities, which has rarely been mentioned in previous studies (9,32).
The principle by which odontogenic infection causes MS abnormalities involves the spread of infection, so the distance between the infection and the MS, as well as the host’s resistance, are important factors. In most studies, a close spatial relationship between the apex (17,18,31,32) or lesion edge (1) of a tooth with PAL and the MSF resulted in more frequent MS abnormalities, whereas no such association was found in Lu’s study (22). Whereas previous studies considered the relationship between PAL and MSF and the occurrence of MS abnormality, this study is the first to expand the research to common odontogenic infections. We concluded that the shorter the INF-MSF distance, the more significant the impact on MS abnormalities. Therefore, caution is recommended whenever areas close to the sinuses are being treated.
The sinus floor acts as a barrier that rarely allows the direct penetration of dental infections into the sinus interior. In 84% of second molars, at least one root is in contact with or has entered the sinus floor. This is also true of 77% of first molars (28). In the present study, we found that a relatively high percentage (57.89%) of MSFs were incomplete, with only a layer of mucous membrane separating the MS from the root tip, which favors the dissemination of odontogenic infections into the sinus (38). Histological examination in a previous study has confirmed bone perforations of the MS cortex in areas with root contact (39). So, we consider that the sinus cortex in this study was not intact in all cases, although not confirmed by histology. The maxillary apices of older subjects tended to be farther from the MSF, consistent with previous studies (38,40,41). The greater distance between the root tip and the MS with age appears to be a protective physiological response, increasing the distance between the MS and the increasing number of odontogenic infections that may occur, thus reducing the risk of MS abnormalities. Studies have shown that the MS volume typically reaches its maximum in the 30s for men and 20s for women (42) and then decreases as age increases (43-45). In this study, we found that the distance between the MS floor and the root tip increased with age, which may reflect the reduction in the MS volume in the direction of the alveolar process.
This was a retrospective study based only on CBCT images and patient medical records. Similar to other CBCT-based studies (1,18,29), factors such as smoking, systemic diseases, otolaryngology history, etc., that may affect MS abnormalities were not analyzed as confounding factors, and this was a limitation of this study. Another limitation was that it was a single-center study, and the results should be verified by further investigations. In the future, a collaborative study with otolaryngologists is required to determine the MS abnormalities that require therapy and to assess the therapeutic and prognostic consequences of mucosal changes.
Conclusions
PAL, PBL, or CPEL of the adjacent teeth increased the probability of MS abnormalities. The likelihood of MS abnormality was related to the distance between the infection and MS and not to the type of infection.
Acknowledgments
The authors thank Dr. Jin Zeng (Department of Otolaryngology, Peking University Third Hospital), who served as an otolaryngology consultant.
Funding: This work was supported by the Clinical Cohort Construction Program of Peking University Third Hospital (No. BYSYDL2019011).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-950/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-950/dss
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-950/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-950/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nunes CA, Guedes OA, Alencar AH, et al. Evaluation of Periapical Lesions and Their Association with Maxillary Sinus Abnormalities on Cone-beam Computed Tomographic Images. J Endod 2016;42:42-6. [Crossref] [PubMed]
- Souza-Nunes LA, Verner FS, Rosado LPL, et al. Periapical and Endodontic Status Scale for Endodontically Treated Teeth and Their Association with Maxillary Sinus Abnormalities: A Cone-beam Computed Tomographic Study. J Endod 2019;45:1479-88. [Crossref] [PubMed]
- Fokkens WJ, Lund VJ, Hopkins C, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020;58:1-464. [Crossref] [PubMed]
- Ferguson M. Rhinosinusitis in oral medicine and dentistry. Aust Dent J 2014;59:289-95. [Crossref] [PubMed]
- Carr TF. Complications of sinusitis. Am J Rhinol Allergy 2016;30:241-5. [Crossref] [PubMed]
- Kanagasingam S, Lim CX, Yong CP, et al. Diagnostic accuracy of periapical radiography and cone beam computed tomography in detecting apical periodontitis using histopathological findings as a reference standard. Int Endod J 2017;50:417-26. [Crossref] [PubMed]
- Al Abduwani J. Cone beam CT paranasal sinuses versus standard multidetector and low dose multidetector CT studies. Am J Otolaryngol 2016;37:59-64. [Crossref] [PubMed]
- Eggmann F, Connert T, Bühler J, et al. Do periapical and periodontal pathologies affect Schneiderian membrane appearance? Systematic review of studies using cone-beam computed tomography. Clin Oral Investig 2017;21:1611-30. [Crossref] [PubMed]
- Peñarrocha-Oltra S, Soto-Peñaloza D, Bagán-Debón L, et al. Association between maxillary sinus pathology and odontogenic lesions in patients evaluated by cone beam computed tomography. A systematic review and meta-analysis. Med Oral Patol Oral Cir Bucal 2020;25:e34-48. [Crossref] [PubMed]
- Pommer B, Ulm C, Lorenzoni M, et al. Prevalence, location and morphology of maxillary sinus septa: systematic review and meta-analysis. J Clin Periodontol 2012;39:769-73. [Crossref] [PubMed]
- Rancitelli D, Borgonovo AE, Cicciù M, et al. Maxillary Sinus Septa and Anatomic Correlation With the Schneiderian Membrane. J Craniofac Surg 2015;26:1394-8. [Crossref] [PubMed]
- Çakur B, Sümbüllü MA, Durna D. Relationship among Schneiderian membrane, Underwood's septa, and the maxillary sinus inferior border. Clin Implant Dent Relat Res 2013;15:83-7. [Crossref] [PubMed]
- Sánchez-Pérez A, Boracchia AC, López-Jornet P, et al. Characterization of the Maxillary Sinus Using Cone Beam Computed Tomography. A Retrospective Radiographic Study. Implant Dent 2016;25:762-9. [Crossref] [PubMed]
- Garcia-Font M, Abella F, Patel S, et al. Cone-beam Computed Tomographic Analysis to Detect the Association between Primary and Secondary Endodontic Infections and Mucosal Thickness of Maxillary Sinus. J Endod 2020;46:1235-40. [Crossref] [PubMed]
- Maestre-Ferrín L, Galán-Gil S, Rubio-Serrano M, et al. Maxillary sinus septa: a systematic review. Med Oral Patol Oral Cir Bucal 2010;15:e383-6. [Crossref] [PubMed]
- Rege IC, Sousa TO, Leles CR, et al. Occurrence of maxillary sinus abnormalities detected by cone beam CT in asymptomatic patients. BMC Oral Health 2012;12:30. [Crossref] [PubMed]
- Kuligowski P, Jaroń A, Preuss O, et al. Association between Odontogenic and Maxillary Sinus Conditions: A Retrospective Cone-Beam Computed Tomographic Study. J Clin Med 2021;10:2849. [Crossref] [PubMed]
- Sakir M, Ercalik Yalcinkaya S. Associations between Periapical Health of Maxillary Molars and Mucosal Thickening of Maxillary Sinuses in Cone-beam Computed Tomographic Images: A Retrospective Study. J Endod 2020;46:397-403. [Crossref] [PubMed]
- Block MS, Dastoury K. Prevalence of sinus membrane thickening and association with unhealthy teeth: a retrospective review of 831 consecutive patients with 1,662 cone-beam scans. J Oral Maxillofac Surg 2014;72:2454-60. [Crossref] [PubMed]
- Sheikhi M, Pozve NJ, Khorrami L. Using cone beam computed tomography to detect the relationship between the periodontal bone loss and mucosal thickening of the maxillary sinus. Dent Res J (Isfahan) 2014;11:495-501. [PubMed]
- Shanbhag S, Karnik P, Shirke P, et al. Association between periapical lesions and maxillary sinus mucosal thickening: a retrospective cone-beam computed tomographic study. J Endod 2013;39:853-7. [Crossref] [PubMed]
- Lu Y, Liu Z, Zhang L, et al. Associations between maxillary sinus mucosal thickening and apical periodontitis using cone-beam computed tomography scanning: a retrospective study. J Endod 2012;38:1069-74. [Crossref] [PubMed]
- Maillet M, Bowles WR, McClanahan SL, et al. Cone-beam computed tomography evaluation of maxillary sinusitis. J Endod 2011;37:753-7. [Crossref] [PubMed]
- Phothikhun S, Suphanantachat S, Chuenchompoonut V, et al. Cone-beam computed tomographic evidence of the association between periodontal bone loss and mucosal thickening of the maxillary sinus. J Periodontol 2012;83:557-64. [Crossref] [PubMed]
- Janner SF, Caversaccio MD, Dubach P, et al. Characteristics and dimensions of the Schneiderian membrane: a radiographic analysis using cone beam computed tomography in patients referred for dental implant surgery in the posterior maxilla. Clin Oral Implants Res 2011;22:1446-53. [Crossref] [PubMed]
- Manji A, Faucher J, Resnik RR, et al. Prevalence of maxillary sinus pathology in patients considered for sinus augmentation procedures for dental implants. Implant Dent 2013;22:428-35. [Crossref] [PubMed]
- Capelli M, Gatti P. Radiological Study of Maxillary Sinus using CBCT: Relationship between Mucosal Thickening and Common Anatomic Variants in Chronic Rhinosinusitis. J Clin Diagn Res 2016;10:MC07-10. [Crossref] [PubMed]
- Aksoy U, Orhan K. Association between odontogenic conditions and maxillary sinus mucosal thickening: a retrospective CBCT study. Clin Oral Investig 2019;23:123-31. [Crossref] [PubMed]
- Huang YT, Hu SW, Huang JY, et al. Assessment of relationship between maxillary sinus membrane thickening and the adjacent teeth health by cone-beam computed tomography. J Dent Sci 2021;16:275-9. [Crossref] [PubMed]
- Nascimento EH, Pontual ML, Pontual AA, et al. Association between Odontogenic Conditions and Maxillary Sinus Disease: A Study Using Cone-beam Computed Tomography. J Endod 2016;42:1509-15. [Crossref] [PubMed]
- Curi FR, Pelegrine RA, Nascimento MDCC, et al. Odontogenic infection as a predisposing factor for pathologic disorder development in maxillary sinus. Oral Dis 2020;26:1727-35. [Crossref] [PubMed]
- Estrela CRA, Bueno MR, Estrela MRA, et al. Frequency and Risk Factors of Maxillary Sinusitis of Endodontic Origin Evaluated by a Dynamic Navigation and a New Filter of Cone-Beam Computed Tomography. J Endod 2022;48:1263-72. [Crossref] [PubMed]
- Van Den Munckhof T, Patel S, Koller G, et al. Schneiderian membrane thickness variation following endodontic procedures: a retrospective cone beam computed tomography study. BMC Oral Health 2020;20:133. [Crossref] [PubMed]
- Cao Z, Yuan J. Changes in Maxillary Sinus Mucosal Thickening following the Extraction of Teeth with Advanced Periodontal Disease: A Retrospective Study Using Cone-Beam Computed Tomography. Biomed Res Int 2021;2021:6688634. [Crossref] [PubMed]
- Bisla S, Gupta A, Singh H, et al. Evaluation of relationship between odontogenic infections and maxillary sinus changes: A Cone Beam Computed Tomography-based study. J Oral Biol Craniofac Res 2022;12:645-50. [Crossref] [PubMed]
- Ren S, Zhao H, Liu J, et al. Significance of maxillary sinus mucosal thickening in patients with periodontal disease. Int Dent J 2015;65:303-10. [Crossref] [PubMed]
- Bornstein MM, Seiffert C, Maestre-Ferrín L, et al. An Analysis of Frequency, Morphology, and Locations of Maxillary Sinus Septa Using Cone Beam Computed Tomography. Int J Oral Maxillofac Implants 2016;31:280-7. [Crossref] [PubMed]
- Tian XM, Qian L, Xin XZ, et al. An Analysis of the Proximity of Maxillary Posterior Teeth to the Maxillary Sinus Using Cone-beam Computed Tomography. J Endod 2016;42:371-7. [Crossref] [PubMed]
- Wehrbein H, Diedrich P. The initial morphological state in the basally pneumatized maxillary sinus--a radiological-histological study in man. Fortschr Kieferorthop 1992;53:254-62. [Crossref] [PubMed]
- Gu Y, Sun C, Wu D, et al. Evaluation of the relationship between maxillary posterior teeth and the maxillary sinus floor using cone-beam computed tomography. BMC Oral Health 2018;18:164. [Crossref] [PubMed]
- Abdulghani EA, Al-Sosowa AA, Alhammadi MS, et al. Three-dimensional assessment of the favorability of maxillary posterior teeth intrusion in different skeletal classes limited by the vertical relationship with the maxillary sinus floor. Head Face Med 2022;18:13. [Crossref] [PubMed]
- Whyte A, Boeddinghaus R. The maxillary sinus: physiology, development and imaging anatomy. Dentomaxillofac Radiol 2019;48:20190205. [Crossref] [PubMed]
- Aktuna Belgin C, Colak M, Adiguzel O, et al. Three-dimensional evaluation of maxillary sinus volume in different age and sex groups using CBCT. Eur Arch Otorhinolaryngol 2019;276:1493-9. [Crossref] [PubMed]
- Kalabalık F, Tarım Ertaş E. Investigation of maxillary sinus volume relationships with nasal septal deviation, concha bullosa, and impacted or missing teeth using cone-beam computed tomography. Oral Radiol 2019;35:287-95. [Crossref] [PubMed]
- Wu X, Cai Q, Huang D, et al. Cone-beam computed tomography-based analysis of maxillary sinus pneumatization extended into the alveolar process in different age groups. BMC Oral Health 2022;22:393. [Crossref] [PubMed]


