
Efficacy of glass membrane emulsification device in conventional transarterial chemoembolization for hepatocellular carcinoma: a preliminary study
Highlight box
Key findings
• A novel GMD produces a high percentage of water/oil emulsions with homogeneous and stable droplets for hepatocellular carcinoma patients treated with TACE.
• Clarified the differences in efficacy between c-TACE and GMD-c-TACE for patients with hepatocellular carcinoma.
What is known and what is new?
• GMD-c-TACE could suppress local recurrence and maintain hepatic reserve.
• This study adds new evidence that GMD-c-TACE allows dense lipiodol accumulation in the tumor and attainment of good local control.
What is the implication, and what should change now?
• Prospective randomized grouping study is still needed to determine whether GMD-c-TACE is useful in preventing local recurrence and is expected to become the standard treatment form of c-TACE in the future.
Introduction
Hepatocellular carcinoma (HCC) is the most common cause of cancer-related death, accounting for more than 700,000 deaths annually (1).
Transarterial chemoembolization (TACE) was developed in Japan in the early 1980s (2) and has become established as the standard treatment for HCC, not indicated for surgical resection or other forms of local treatment for patients with intermediate-stage HCC according to the Barcelona Clinic Liver Cancer classification (3-15). In particular, conventional TACE, in which ethiodized oil (Lipiodol; Guerbet, Villepinte, France) and doxorubicin/epirubicin solution are placed in separate syringes and mixed alternately through a three-way-stopcock to create emulsions, is the worldwide consensus method (16,17). Ethiodized oil (Lipiodol; Guerbet, Villepinte, France) is widely used in TACE; it is not only a carrier of anticancer drugs but also has a role in embolization (18-22). By mixing it with anticancer drugs into a water/oil emulsion, it can be possible to increase the gradual release of anticancer drugs and the embolization effect on target sites. TACE’s primary local control effect is the efficacy of resistance by embolic materials and local accumulation and stagnation of anticancer drugs and ethiodized oil. The better the accumulation of Lipiodol, the better the local control rate; thus, it is essential to improve Lipiodol accumulation in embolization therapy.
As Lipiodol is used as a drug carrier, drug properties should ideally be in a high-generation W/O (water in oil) emulsion state, but this may not be achievable in a previous three-way-stopcock because the O/W (oil in water) and W/O areas are mixed.
In addition, the mixing ratio and pumping frequency of ethiodized oil and anticancer drug solution varied among operators and institutions, and the emulsion properties are not consistent. This means that the nature of the emulsion may be one of the background factors for differences in local control of HCC.
The porous glass membrane emulsification device (GMD) (MicroMagic; Piolax Medical Devices, Yokohama, Japan), which is a connector for adjusting W/O emulsion drug solutions, can produce stable and highly pure W/O emulsions that are used when mixing drug solutions and oil-based contrast media during TACE and injecting drugs evenly (23).
Improved accumulation of lipiodol is expected to maintain good local control of HCC and reduce the concentration of anticancer drugs leaking into the systemic circulation, thereby minimizing the impact of the treatment on liver function. TACE using GMD is expected to be clinically useful. However, there are no reports of efficacy compared between c-TACE with the previous three-way stopcock and c-TACE with GMD (24).
This study investigated the outcomes and local recurrence factors in conventional-TACE (c-TACE) using GMD in c-TACE for HCC less than 5 cm in size. We present this article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1048/rc).
Methods
Subjects
This study included 71 patients with HCC (71 nodules) who had c-TACE at Saiseikai Niigata Hospital between January 2018 and November 2021. The diagnosis of HCC was made using dynamic contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) in which the contrast medium was rapidly injected through a vein, and the same area was imaged multiple times after the contrast medium reached the target area at the right time.
The exclusion criteria are listed below: (I) tumor diameter greater than 5 cm; (II) intentionally incomplete TACE performed for massive or infiltrative tumors; (III) interval between TACE and the first follow-up CT more than 4 months; (IV) CT imaging was not performed during the follow-up observation period; (V) nodules treated with local therapies such as radiofrequency ablation, microwave coagulation, laser ablation or percutaneous ethanol injection (PEI) before or after TACE as an additional treatment; (VI) microvascular invasion (MVI); (VII) another treatment was performed before or after TACE, such as surgery, chemotherapy, or radiation therapy.
We analyzed local recurrence and hepatic functional reserve in the 71 patients who underwent c-TACE due to intolerance to surgery, with or without GMD.
Study methods
Treatment procedure
After puncturing the femoral artery by the Seldinger method, a 5Fr introducer was inserted in all patients. All procedures, we performed CT during angiography and CT hepatic angiography were also performed to diagnose HCC accurately. Then a 5Fr angiographic catheter and a 3Fr micro balloon catheter (Attendant, Terumo, Tokyo, Japan) were coaxially introduced into the sub-segmental or peripheral feeding arteries by the coaxial technique. Conventional TACE with a three-way stopcock or a GMD was then performed.
TACE was performed according to the standard protocol for all patients. Epirubicin hydrochloride 50 mg (Epirubicin; Nippon Kayaku, Tokyo, Japan) was dissolved in a nonionic contrast medium; the mixing ratio of aqueous Epirubicin solution to Lipiodol was 1:2 (24). Emulsions were generated by pumping 20 times with a conventional three-way stopcock or GMD before intra-arterial administration (24). The emulsion was injected through a microcatheter inserted into the tumor-feeding artery, which was subsequently embolized using gelatin particles 1-mm-diameter gelatin particles (Gelpart; Nippon Kayaku, Tokyo, Japan).
After embolization, multidirectional angiography confirmed the absence of tumor staining and sufficient accumulation of Lipiodol in the tumor. The volume of the emulsion used in both groups was determined according to the size of the nodule.
Immediately after the procedure, non-contrast CT examinations were performed using a 16-detector row scanner (Aquilion, Toshiba Medical Systems), and for lesion density measurement, a circular region of interest (ROI) lesion was set for each nodule, and the CT values of the Lipiodol that accumulated in the HCC nodule were measured. ROI dimensions were identical for each nodule. The lesion density means in Hounsfield units (HU) on CT. All conventional CT images were obtained using a multi-detector-row helical CT scanner (Aquilion PRIM; Toshiba Medical Systems, Tokyo, Japan) and a standard abdominal helical scan protocol. The tube voltage was 120 kVp; the rotational time was 0.5 seconds; detector collimation was 0.5 mm/row; and helical pitch factor was 1.388/revolution. The images were reconstructed for a 350 mm × 350 mm field of view (matrix size 512×512) with a voxel size of 0.68 mm3.
Patients were followed-up after treatment to evaluate local recurrence and accumulation of Lipiodol, and CT was subsequently performed to determine whether there was a local recurrence.
Contrast-enhanced CT was performed 1 to 3 months after TACE and every 2 to 3 months after that to determine the response to treatment in accordance with the modified RECIST (25).
Local recurrence was defined as either (I) a non-lipiodol-accumulated area in the post-treatment tumor that was contrasted in the early contrast phase and showed low ab-sorption in the delayed phase or (II) a low-absorption area in the delayed phase that was contrasted in the early contrast phase and was adjacent to the lipiodol-accumulated area.
Liver function including, albumin-bilirubin (ALBI)-score, was evaluated pre- and post-TACE procedure (26-29).
Ethical statements
This study was approved by the Institutional Review Board of Saiseikai Niigata Hospital (No. E18-18) and was conducted in accordance with the principles of the Declaration of Helsinki (as revised in 2013). Before participating in this study, written informed consent was provided by all patients.
Statistical analysis
The two groups were compared by the Chi-square test. Normally distributed continuous data were expressed as mean ± standard deviation, and compared by t-test. The differences in parameters were analyzed using a one-way repeated measures analysis of variance (ANOVA). Recurrence rates were estimated using the Kaplan-Meier method and compared using the log-rank test. The Cox proportional hazard model analyzed the predisposing factors for recurrence.
Statistical significance was defined as P<0.05. All statistical analyses were performed using EZR (Saitama Medical Centre, Jichi Medical University, Shimotsuke, Japan), a graphical user interfaces for R version 3.2.2 (The R Foundation for Statistical Computing, Vienna, Austria) (30).
Results
Patient characteristics
Table 1 shows the clinical backgrounds of all patients. The mean age was 72.98±9.62 years and the male-to-female ratio was 56:15, background liver factors HBV/HCV/AIH/Alc/NASH were 14/25/4/18/10, alpha-fetoprotein (AFP) was 21.96±73.52 ng/mL, des-gamma-carboxy prothrombin (DCP) was 1,083.06±5,212.07 mAU/mL, total bilirubin was 0.88±0.42 mg/dL, albumin was 3.84±0.49 g/dL, prothrombin activity was 93.058%±12.98%, and ALBI-score was −2.52±0.47. The median observation period was 255 days (range, 71–1,086 days).
Table 1
| Variables | Number or mean ± SD |
|---|---|
| Age (years) | 72.986±9.627 |
| Gender (male/female) | 56/15 |
| Etiology (HBV/HCV/AIH/Alc/NASH) | 14/25/4/18/10 |
| Size (mm) | 32.056±5.997 |
| Location (S1/2/3/4/5/6/7/8) | 1/3/7/7/5/12/14/22 |
| Total.bilirubin (mg/dL) | 0.884±0.423 |
| Albumin (g/dL) | 3.845±0.498 |
| DCP (mAU/mL) | 1,083.06±5,212.065 |
| AFP (ng/mL) | 21.966±73.523 |
| UICC stage (I/II/III) | 4/49/18 |
| ALBI score | −2.520±0.470 |
| Child-Pugh (5/6/7/8) | 15/22/16/18 |
| Post CT value (HU) | 613.91±312.65 |
SD, standard deviation; HBV, hepatitis B virus; HCV, hepatitis C virus; AIH; autoimmune hepatitis; Alc, alcoholic hepatitis; NASH, non-alcoholic steatohepatitis; DCP, des-gamma-carboxy prothrombin; AFP, alpha-fetoprotein; UICC, Union International Contre Le Cancer; ALBI score, albumin bilirubin score; HU, Hounsfield units.
On assessing the background factors of the 27 patients in the three-way-stopcock-TACE group and the 44 patients in the GMD-c-TACE group, the mean age was 71.40±8.94 and 73.95±9.97 years, respectively. The male-to-female ratios were 22:5 and 34:10, and the causes of liver disease HBV/HCV/AIH/Alc/NASH were 6/8/2/8/3 and 8/17/2/10/7, respectively. Other background factors include total bilirubin, albumin, prothrombin activity (%), alanine aminotransferase (ALT), aspartate aminotransferase (AST), platelet count, AFP, DCP, and ALBI, which were not significantly different (Table 2). However, there were no significant differences between the two groups.
Table 2
| Variables | Non-GMD (n=27) | GMD (n=44) | P value |
|---|---|---|---|
| Age (years) | 71.407±8.984 | 73.955±9.977 | 0.2823 |
| Gender (male/female) | 22/5 | 34/10 | 0.673 |
| Etiology(viral/non-viral) | 14/13 | 25/19 | 0.683 |
| Size (mm) | 30.852±6.532 | 32.795±5.593 | 0.1869 |
| Location (S1/2/3/4/5/6/7/8) | 1/2/3/4/1/2/5/10 | 0/1/5/3/4/10/9/12 | 0.380 |
| Total bilirubin (mg/dL) | 1.002±0.494 | 0.814±0.362 | 0.0709 |
| Albumin (g/dL) | 3.733±0.524 | 3.914±0.475 | 0.1399 |
| DCP (mAU/mL) | 170.604±436.403 | 1622.23±6532.97 | 0.2632 |
| AFP (ng/mL) | 11.281±13.285 | 28.280±91.988 | 0.3537 |
| UICC (I/II/III) | 2/20/5 | 2/29/13 | 0.573 |
| ALBI score | −2.384±0.522 | −2.601±0.423 | 0.0616 |
| Child-Pugh (5/6/7/8) | 6/6/6/9 | 9/16/10/9 | 0.474 |
| Lipiodol dose (mL) | 3.344±0.611 | 3.481±0.587 | 0.349 |
| Post CT value (HU) | 527.67±303.91 | 670.16±329.31 | 0.074 |
Data are presented number or mean ± SD. TACE, transarterial chemoembolization; GMD, glass membrane emulsification device; DCP, des-gamma-carboxy prothrombin; AFP, alpha-fetoprotein; UICC, Union International Contre Le Cancer; ALBI score, albumin bilirubin score; HU, Hounsfield unit; SD, standard deviation.
Recurrence rate
According to m-RECIST, in the three-way-stopcock-TACE group, 12 patients (44.4%) had CR, 11 patients (40.8%) had PR, 3 patients (11.1%) had SD, and 1 patient (3.7%) had PD. In the GMD-c-TACE group, 24 patients (54.5%) had CR, 14 patients (31.8%) had PR, 5 patients (11.4%) had SD, and 1 patient (2.3%) had PD (Table 3). There was no significant difference in the response (P=0.265).
Table 3
| Variables | CR, n (%) | PR, n (%) | SD, n (%) | PD, n (%) |
|---|---|---|---|---|
| Non-GMD (n=27) | 12 (44.4) | 11 (40.8) | 3 (11.1) | 1 (3.7) |
| GMD (n=44) | 24 (54.5) | 14 (31.8) | 5 (11.4) | 1 (2.3) |
GMD, glass membrane emulsification device; CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Local recurrence was observed in 13 of the 71 nodes. The overall local recurrence rate was 3.0% at 6 months, 16.7% at 12 months, and 35.0% at 18 months, followed by a plateau (Figure 1, Log-rank test). The local recurrence rate in each group was 3.0% at 6 months, 16.7% at 12 months, and 35.0% at 18 months in the three-way stopcock-TACE (non-GMD) group, and 0.0% at 12 months and 15.41% at 18 months in the GMD-c-TACE group, and statistically significant differences were detected (Figure 2, Log-rank test; P<0.05). The cumulative 6, 12, and 24 months local recurrence rates of non-GMD group vs. GMD-c-TACE group were 12.3%, 35.9%, and 48.7% vs. 0.0%, 0.0%, 23.1%, respectively (P<0.05).
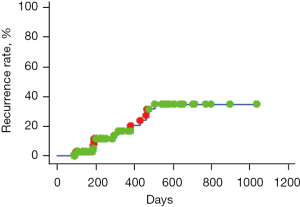
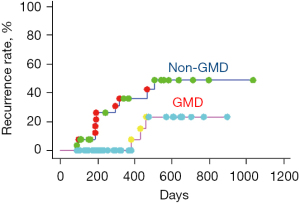
Although there were no significant differences in local recurrence rates by gender, age, background liver factors, tumor size, AFP, DCP, and post-CT values, local recurrence was significantly higher in the non-GMD group, and multivariate analysis showed significant differences in recurrence by GMD use, which was related to the recurrence factor of c-TACE without GMD. The hazard ratio was 4.655 (Table 4).
Table 4
| Variable | Categories | HR | 95% CI | P value |
|---|---|---|---|---|
| Gender | Male | 1 | 0.135–4.431 | 0.7748 |
| Female | 0.773 | |||
| Age, years | ≥70 | 2.694 | 0.740–9.808 | 0.1328 |
| <70 | 1 | |||
| Etiology | Viral | 1 | 0.458–7.347 | 0.3914 |
| Non-viral | 1.834 | |||
| Size (mm) | ≥30 | 1 | 0.081–2.112 | 0.2886 |
| <30 | 0.414 | |||
| AFP (ng/mL) | ≥10 | 1.689 | 0.343–8.325 | 0.5198 |
| <10 | 1 | |||
| DCP (mAU/mL) | ≥100 | 1 | 0.099–3.760 | 0.5938 |
| <100 | 0.610 | |||
| Post CT (HU) | Low | 1.337 | 0.299–5.977 | 0.7035 |
| High | 1 | |||
| TACE devise | Non-GMD | 4.655 | 1.192–18.171 | 0.0269 |
| GMD | 1 |
HR, hazard ratio; CI, confidence interval; AFP, alpha-fetoprotein; DCP, des-gamma-carboxy prothrombin; HU, Hounsfield unit; TACE, transarterial chemoembolization; GMD, glass membrane emulsification device.
Liver deterioration
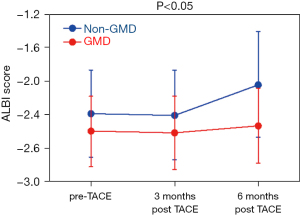
Comparison of the groups showed that c-TACE with three-way stopcock resulted in deterioration after 6 months, whereas c-TACE with GMD significantly preserved hepatic reserve during the study (Figure 3, repeated one-way ANOVA).
Adverse events
The maximum change in grade evaluated c-TACE-related adverse events within 3 months of treatment according to the National Cancer Institute Common Terminology Criteria version 4.0. In the three-way-stopcock group (non-GMD), events such as increased AST in 12 patients (44.4%), increased ALT in 13 patients (48.18%), and hyperbilirubinemia in 1 patient (3.7%) were observed. In the GMD group, events such as increased AST in 4 patients (9.1%), and increased ALT in 7 patients (15.9%). The incidence of thrombocytopenia was 1 case (2.2%). All patients healed spontaneously without additional treatment for elevated liver enzymes.
Discussion
According to the Barcelona Clinic Liver Cancer (BCLC) algorithm, TACE is the standard-of-care treatment for patients with intermediate-stage HCC (6,12,13,31).
However, by definition, TACE is a palliative and repetitive treatment in achieving a complete response rate and risk of disease recurrence (10,16,17).
C-TACE with ethiodized oil has been performed since the 1980s for inoperable hepatocellular carcinoma in the world (2,18,32,33).
In c-TACE, the standard technique is the forming ethiodized oil-cytotoxic drug emulsion using a three-way stopcock. It involves pumping Lipiodol and doxorubicin/epirubicin solution emulsification using a three-way-stopcock. In other words, to obtain a high W/O emulsion forming rate, it has been reported as a technical recommendation to mix a small amount of aqueous doxorubicin/epirubicin solution rather than lipiodol (16). It is widely known that technical parameters affect the physicochemical properties of emulsions, and previous studies focusing on the emulsion form (W/O or O/W) have proved that W/O is superior to HCC in c-TACE. W/O emulsions have a higher viscosity than O/W emulsions, are denser than the O/W emulsion, and have a higher embolic effect (16,19). Higher viscosity means better tumor retention (34), higher drug loading capacity, and longer release time (35,36).
In c-TACE, the emulsion properties are essential to control the drug release rate, and W/O emulsions have slower drug release rates than O/W emulsions (37-39).
However, there is a limitation in the emulsion forming by pumping with a three-way-stopcock. Even when the mixing ratio of aqueous anticancer drug solution and lipiodol is 1:2, the forming ratio of W/O emulsion is reported to be approximately 70% (40). A pumping emulsification device using a porous glass membrane has recently been developed to solve this issue, which enables the formation of W/O emulsions close to 100% (23).
Theoretically, the pure W/O emulsion could achieve a slow drug release.
Furthermore, recent advances in systemic therapy for HCC have changed the indications for TACE, and c-TACE is now required to achieve more selective and better local control (41). Therefore, how efficiently Lipiodol can be retained in tumor tissues is an important issue (38,42,43). Lipiodol is an ethyl ester of iodized fatty acids of poppy-seed oil. It accumulates in HCC by improving permeability and retention in solid tumors. In combination with the embolization material, it blocks blood flow to the nutrient supply vessels of the HCC and the surrounding sinuses through intratumoral sinuses, resulting in ischemia and necrosis (44,45). The conventional pumping method using a three-way stop-cock is widely used for the emulsification of water-soluble anticancer drugs and ethyl ester of iodinated poppy-seed oil fatty acid (Lipiodol), but this method can only achieve a W/O production rate of about 70% and the droplet size, and viscosity are unstable (40,46).
From the perspective of TACE, this means that conventional TACE is more susceptible to the operator’s technical factors than DEB-TACE, that the particle size is homogenized, and the drug load per particle is constant. Several issues are to be considered for the standardization of conventional TACE, including drug selection, emulsion properties, and treatment techniques. Homogenizing these as much as possible is essential in future discussions of TACE, and GMD was developed to enhance the effect of TACE by standardizing the emulsion preparing process that is performed in conventional TACE. That consists of a disk-shaped glass membrane made from volcanic ash with numerous 50 µm pores (23).
A porous GMD has been developed to enhance the therapeutic effect of conventional TACE. Porous GMD consists of a disk-shaped glass membrane made from volcanic ash with numerous 100 µm pores (47).
The use of GMD is expected to increase the therapeutic efficacy of TACE, as compared with the conventional three-way stopcock pumping, as it enables the production of a higher proportion of a W/O emulsion with more homogenous and stable droplets. It has been shown to be more effective in retaining anticancer drugs in tumors in the VX2 Rabbit liver cancer model (48).
As Lipiodol is used as a drug carrier, the drug properties are ideal for a state of W/O with a high generation rate, but until now, the problem has been the mixture of O/W, as W/O areas are also present in a three-way stopcock system. Microscopic examination was performed by emulsion-by-device (emulsion formed by the device). Almost 100% pure W/O is shown. Homogenous sizes and shape of droplets solution in dispersion phase are seen. The GMD formed almost 100% water-in-oil (47).
Based on these essential evaluations, GMD, which allows any operator to prepare stable emulsions, has the potential to offer the following advantages:
- Improved intravascular distribution with stable emulsion (even, smaller vessels, peripheral drug distribution);
- Improved intra-tumor stagnation rate due to viscosity;
- Sustained release effect of the drug;
- Reduction of side effects [decrease in blood concentration of anticancer drugs (suppression of rapid increase)] is expected.
Recently, Imai et al. reported that TACE with GMD could be considered an effective and safely treatment option for the management of solitary HCC as compared to stereotactic body radiotherapy (SBRT) (24,49).
Although better TACE outcomes are expected with GMD, its efficacy in clinical practice is still unclear.
This study observed local recurrence in 13 of the total 71 nodes, and the overall local recurrence rate was 3.0% at 6 months, 16.7% at 12 months, and 35.0% at 18 months, after which it plateaued. The GMD-c-TACE group was 7.7% at 14 months and 23.1% at 20 months, showing that local recurrence could be suppressed significantly. In addition, when multivariate analysis was performed on the factors that suppressed local recurrence, GMD-c-TACE suppressed local recurrence and maintained hepatic reserve. Multivariate analysis also showed that non-GMD-c-TACE was the only factor associated with local recurrence and showed a hazard ratio of 4.655.
Furthermore, the hepatic functional reserve was considerably preserved in the c-TACE group that used GMD. This may be because the W/O emulsion of GMD had a significantly higher rate of permeation of epirubicin and a significantly longer drug elution rate than the three-way stopcock, resulting in fewer systemic effects (50).
The present study also showed that there were no Grade 4 or higher adverse events, no adverse events of clinical concern, and the hepatic reserve was maintained with GMD-c-TACE.
There are several limitations to this study. First, the number of patients was limited. Second, we believe it is necessary to investigate a large number of patients in various stages of the disease. Third, the retrospective design of this study may introduce bias in the selection of patients for HCC treatment. Finally, the data are from a single-center, which necessarily eliminates selection bias. More extensive prospective clinical trials are needed to confirm these findings with high accuracy.
Conclusions
In conclusion, although further studies with a large number of patients at various disease stages are needed, GMD-c-TACE therapy can be considered to be a useful treatment strategy because of its ability to maintain the hepatic functional reserve. It also can homogenize the emulsion preparing process in c-TACE and move closer to standardization of the procedure.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1048/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1048/dss
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1048/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1048/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Institutional Review Board of Saiseikai Niigata Hospital (No. E18-18) and was conducted in accordance with the principles of the Declaration of Helsinki (as revised in 2013). All patients provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Yamada R, Sato M, Kawabata M, et al. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology 1983;148:397-401. [Crossref] [PubMed]
- Takayasu K, Arii S, Ikai I, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology 2006;131:461-9. [Crossref] [PubMed]
- Takayasu K, Arii S, Kudo M, et al. Superselective transarterial chemoembolization for hepatocellular carcinoma. Validation of treatment algorithm proposed by Japanese guidelines. J Hepatol 2012;56:886-92. [Crossref] [PubMed]
- Ikeda M, Arai Y, Park SJ, et al. Prospective study of transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: an Asian cooperative study between Japan and Korea. J Vasc Interv Radiol 2013;24:490-500. [Crossref] [PubMed]
- Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-38. [Crossref] [PubMed]
- Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology 2004;127:S179-88. [Crossref] [PubMed]
- Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734-9. [Crossref] [PubMed]
- Ikeda K, Kumada H, Saitoh S, et al. Effect of repeated transcatheter arterial embolization on the survival time in patients with hepatocellular carcinoma. An analysis by the Cox proportional hazard model. Cancer 1991;68:2150-4. [Crossref] [PubMed]
- Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35:1164-71. [Crossref] [PubMed]
- Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology 2002;224:47-54. [Crossref] [PubMed]
- Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int 2015;35:2155-66. [Crossref] [PubMed]
- Bargellini I, Florio F, Golfieri R, et al. Trends in utilization of transarterial treatments for hepatocellular carcinoma: results of a survey by the Italian Society of Interventional Radiology. Cardiovasc Intervent Radiol 2014;37:438-44. [Crossref] [PubMed]
- Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 2010;33:41-52. [Crossref] [PubMed]
- Golfieri R, Giampalma E, Renzulli M, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer 2014;111:255-64. [Crossref] [PubMed]
- de Baere T, Arai Y, Lencioni R, et al. Treatment of Liver Tumors with Lipiodol TACE: Technical Recommendations from Experts Opinion. Cardiovasc Intervent Radiol 2016;39:334-43. [Crossref] [PubMed]
- Lencioni R, de Baere T, Soulen MC, et al. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology 2016;64:106-16. [Crossref] [PubMed]
- Nakamura H, Hashimoto T, Oi H, et al. Transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology 1989;170:783-6. [Crossref] [PubMed]
- Demachi H, Matsui O, Abo H, et al. Simulation model based on non-newtonian fluid mechanics applied to the evaluation of the embolic effect of emulsions of iodized oil and anticancer drug. Cardiovasc Intervent Radiol 2000;23:285-90. [Crossref] [PubMed]
- Takayasu K, Arii S, Matsuo N, et al. Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. AJR Am J Roentgenol 2000;175:699-704. [Crossref] [PubMed]
- Lencioni R. Chemoembolization for hepatocellular carcinoma. Semin Oncol 2012;39:503-9. [Crossref] [PubMed]
- Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology 2012;262:43-58. [Crossref] [PubMed]
- Tanaka T, Masada T, Nishiofuku H, et al. Development of pumping emulsification device with glass membrane to form ideal lipiodol emulsion in transarterial chemoembolization. Eur Radiol 2018;28:2203-7. [Crossref] [PubMed]
- Imai N, Yokoyama S, Yamamoto K, et al. Safety and Efficacy of Glass Membrane Pumping Emulsification Device in Transarterial Chemoembolization for Hepatocellular Carcinoma: First Clinical Outcomes. Anticancer Res 2021;41:5817-20. [Crossref] [PubMed]
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. [Crossref] [PubMed]
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 2015;33:550-8. [Crossref] [PubMed]
- Kudo M. Newly Developed Modified ALBI Grade Shows Better Prognostic and Predictive Value for Hepatocellular Carcinoma. Liver Cancer 2021;11:1-8. [Crossref] [PubMed]
- Hiraoka A, Kumada T, Kudo M, et al. Albumin-Bilirubin (ALBI) Grade as Part of the Evidence-Based Clinical Practice Guideline for HCC of the Japan Society of Hepatology: A Comparison with the Liver Damage and Child-Pugh Classifications. Liver Cancer 2017;6:204-15. [Crossref] [PubMed]
- Hiraoka A, Michitaka K, Kumada T, et al. Validation and Potential of Albumin-Bilirubin Grade and Prognostication in a Nationwide Survey of 46,681 Hepatocellular Carcinoma Patients in Japan: The Need for a More Detailed Evaluation of Hepatic Function. Liver Cancer 2017;6:325-36. [Crossref] [PubMed]
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907-17. [Crossref] [PubMed]
- Ohishi H, Uchida H, Yoshimura H, et al. Hepatocellular carcinoma detected by iodized oil. Use of anticancer agents. Radiology 1985;154:25-9. [Crossref] [PubMed]
- Ohishi H, Yoshimura H, Uchida H, et al. Transcatheter arterial embolization using iodized oil (lipiodol) mixed with an anticancer drug for the treatment of hepatocellular carcinoma. Cancer Chemother Pharmacol 1989;23:S33-6. [Crossref] [PubMed]
- Becker S, Lepareur N, Cadeillan V, et al. Optimization of hepatocarcinoma uptake with radiolabeled lipiodol: development of new lipiodol formulations with increased viscosity. Cancer Biother Radiopharm 2012;27:149-55. [Crossref] [PubMed]
- Kan Z, Wright K, Wallace S. Ethiodized oil emulsions in hepatic microcirculation: in vivo microscopy in animal models. Acad Radiol 1997;4:275-82. [Crossref] [PubMed]
- Idée JM, Guiu B. Use of Lipiodol as a drug-delivery system for transcatheter arterial chemoembolization of hepatocellular carcinoma: a review. Crit Rev Oncol Hematol 2013;88:530-49. [Crossref] [PubMed]
- Choi JW, Cho HJ, Park JH, et al. Comparison of drug release and pharmacokinetics after transarterial chemoembolization using diverse lipiodol emulsions and drug-eluting beads. PLoS One 2014;9:e115898. [Crossref] [PubMed]
- Deschamps F, Farouil G, Gonzalez W, et al. Stabilization Improves Theranostic Properties of Lipiodol(®)-Based Emulsion During Liver Trans-arterial Chemo-embolization in a VX2 Rabbit Model. Cardiovasc Intervent Radiol 2017;40:907-13. [Crossref] [PubMed]
- de Baere T, Zhang X, Aubert B, et al. Quantification of tumor uptake of iodized oils and emulsions of iodized oils: experimental study. Radiology 1996;201:731-5. [Crossref] [PubMed]
- Masada T, Tanaka T, Nishiofuku H, et al. Techniques to Form a Suitable Lipiodol-Epirubicin Emulsion by Using 3-Way Stopcock Methods in Transarterial Chemoembolization for Liver Tumor. J Vasc Interv Radiol 2017;28:1461-6. [Crossref] [PubMed]
- Kudo M, Han KH, Ye SL, et al. A Changing Paradigm for the Treatment of Intermediate-Stage Hepatocellular Carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements. Liver Cancer 2020;9:245-60. [Crossref] [PubMed]
- Deschamps F, Moine L, Isoardo T, et al. Parameters for Stable Water-in-Oil Lipiodol Emulsion for Liver Trans-Arterial Chemo-Eembolization. Cardiovasc Intervent Radiol 2017;40:1927-32. [Crossref] [PubMed]
- Dioguardi Burgio M, Sartoris R, Libotean C, et al. Lipiodol retention pattern after TACE for HCC is a predictor for local progression in lesions with complete response. Cancer Imaging 2019;19:75. [Crossref] [PubMed]
- Miyayama S, Matsui O, Yamashiro M, et al. Ultraselective transcatheter arterial chemoembolization with a 2-f tip microcatheter for small hepatocellular carcinomas: relationship between local tumor recurrence and visualization of the portal vein with iodized oil. J Vasc Interv Radiol 2007;18:365-76. [Crossref] [PubMed]
- Miyayama S, Matsui O, Yamashiro M, et al. Iodized oil accumulation in the hypovascular tumor portion of early-stage hepatocellular carcinoma after ultraselective transcatheter arterial chemoembolization. Hepatol Int 2007;1:451-9. [Crossref] [PubMed]
- Boulin M, Schmitt A, Delhom E, et al. Improved stability of lipiodol-drug emulsion for transarterial chemoembolisation of hepatocellular carcinoma results in improved pharmacokinetic profile: Proof of concept using idarubicin. Eur Radiol 2016;26:601-9. [Crossref] [PubMed]
- Tanaka T, Iwamoto H, Fujihara M, et al. Efficacy of a Glass Membrane Emulsification Device to Form Mixture of Cisplatin Powder with Lipiodol on Transarterial Therapy for Hepatocellular Carcinoma. Cardiovasc Intervent Radiol 2021;44:766-73. [Crossref] [PubMed]
- Masada T, Tanaka T, Nishiofuku H, et al. Use of a Glass Membrane Pumping Emulsification Device Improves Systemic and Tumor Pharmacokinetics in Rabbit VX2 Liver Tumor in Transarterial Chemoembolization. J Vasc Interv Radiol 2020;31:347-51. [Crossref] [PubMed]
- Imai N, Ishigami M, Oie Y, et al. Effectiveness of Porous Glass Membrane Pumping Emulsification Device in Transarterial Chemoembolization for Solitary Hepatocellular Carcinoma. Anticancer Res 2022;42:3947-51. [Crossref] [PubMed]
- Tanaka T, Nishiofuku H, Masada T, et al. Drug Release Property of Lipiodol Emulsion Formed by Glass Membrane Emulsification Device for Transarterial Chemoembolization. Cardiovasc Intervent Radiol 2020;43:135-9. [Crossref] [PubMed]


