
Prevalence of gastrointestinal symptoms and their association with psychological problems in youths
Introduction
The gastrointestinal tract plays a central role in the digestion and absorption of nutrients, and its disorder may cause various gastrointestinal symptoms, primarily including abdominal pain, diarrhea, nausea, and emesis (1). Gastrointestinal symptoms are commonly observed in gastrointestinal functional disorders, such as irritable bowel syndrome, functional diarrhea, and functional constipation (2), and gastrointestinal organic diseases, such as gastritis, inflammatory bowel disease, peptic ulcer, and gastrointestinal cancer (2). Sixty-two percent of the general population have been reported to have at least one gastrointestinal symptom (3). In recent years, a growing number of studies suggest an increasing trend in the prevalence of functional gastrointestinal disorders in youths. Notably, as many as 65% of the youths are experiencing gastrointestinal symptoms, and almost one-third are seeking medical care (4). In addition, gastrointestinal symptoms are one of the most prominent somatic symptoms found in patients with mental disorders, including anxiety, depression, and autism spectrum disorder (5), which can negatively affect the youths on physical, mental, and social levels (6).
At present, over 26 million people worldwide are diagnosed with severe psychological health problems (7), mainly including depression, anxiety, hostility, and paranoid ideation. According to the World Health Organization, approximately 264 million people suffer from anxiety all over the world (8), and almost one-fifth of people experience at least one episode of depressive disorder during their lifetime (9), especially in youths. In China, phobic anxiety, depression, and anxiety are the most common psychological problems in youths (10). Psychological problems are a major precipitating factor for suicide, which is the second cause of death for youths aged 10 to 24 years old in the United States (11). Thus, early detection and correction of psychological problems are crucial among youths (12), and can effectively prevent suicide (13).
Under the action of the brain-gut axis, the digestive system and brain are linked via various bidirectional pathways, and both of them affect each other (14). Psychological problems can lead to increased stress response, inflammation, and arousal of the autonomic nervous system, which cause self-reported gastrointestinal symptoms (15). Gastrointestinal symptoms and psychological problems are often concomitant in youths (16). However, their association has not been well elucidated. Furthermore, the extent to which psychological problems affect gastrointestinal symptoms and which type of gastrointestinal symptoms is related to psychological problems are still unclear. The purpose of the present cross-sectional study was to determine the prevalence of gastrointestinal symptoms and investigate their association with psychological problems in youths. We present the following article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1316/rc).
Methods
Study design
Since attendance at college and adjustment to new social settings are considered stress-provoking environments in youths (4), college students and military recruits were selected as the study population. Before the current study was designed, several previous questionnaires-based studies had been learned (17-19), in which only the participants’ verbal informed consents were used instead of written informed consents. Two investigators (Cong Wang and Jun Liu) separately conducted face-to-face interviews with two different groups of participants to verbally introduce the objectives and significance of this questionnaire. Only if a participant gave his or her verbal informed consent to participate in the questionnaire survey, this questionnaire would be filled. All participants had been de-identified, when the data were collected and analyzed. All information obtained from these participants would be guaranteed to be kept confidential without any potential effect on them. In May 2021, the self-reported questionnaire about the health status of youths had been sent by an investigator (Cong Wang) using the online application Sojump (Changsha Ran Xing InfoTech Ltd.) to the sophomores majoring in education in a high vocational school, which is a college that trains students with certain higher education, professional skills, and technical knowledge and highlights the practical operation ability of applied technology in teaching, in the Panjin city of Liaoning province. Participants used their mobile phones to complete the online questionnaires. The online application Sojump demonstrated that each participant had a unique Internet Protocol address to avoid repeated filling. Another investigator (Jun Liu) used a paper version to the recruits in an army of the Northern Theater Command. The youths who agreed to fill out the questionnaire were included in this study. If the data in the questionnaires were invalid or unclear, the participants would be excluded. The study protocol was approved by the Medical Ethical Committee of the General Hospital of Northern Theater Command with an approval number [Y (2021) 099]. The study was performed according to the Declaration of Helsinki (as revised in 2013). The ethics committee exempted the written informed consents due to the retrospective nature of this study.
This self-reported questionnaire is composed of three parts, as follows. (I) Demographics. This part includes age, gender, height, weight, history of surgery and gastrointestinal diseases, personal habits (i.e., smoking, drinking alcohol, drinking tea, drinking coffee, and eating spicy food), lifestyles (i.e., irregular diet, eating out frequently, drinking raw water, and sharing drinking glasses), family characteristics (i.e., living area, number of family members, and annual family income), and family history of gastrointestinal diseases.
(II) Gastrointestinal symptoms. This part mainly includes the presence of gastrointestinal symptoms (i.e., nausea, emesis, abdominal pain, acid regurgitation, eructation, and heartburn). Each question on the symptom was answered by “yes” or “no”.
(III) Psychological conditions. This part was assessed using the SCL-90 (20), which is a symptom self-rating scale composed of 90 items from 9 subscales: somatization, obsessive-compulsive, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation, and psychoticism. Each item on the scale was measured on a five-point scale (1= never, 2= light, 3= moderate, 4= quite severe, and 5= severe) based on the severity of psychological conditions. A higher SCL-90 score indicates a worse psychological condition, and a total SCL-90 score beyond 160 indicates a potential psychological problem. And if the total SCL-90 score was beyond 160, the score for each subscale would be further calculated as previously recommended (21).
Statistical analyses
All statistical analyses were performed with IBM SPSS 20.0 (IBM Corp, Armonk, NY, USA) and Microsoft Office Excel 2010 software (Microsoft Corp, Redmond Washington, USA). Continuous variables were expressed as mean ± standard deviation and median (range). Categorical variables were expressed as frequency (percentage). Non-parametric Mann-Whitney U test was used for continuous variables and Chi-square and Fisher’s exact tests were used for categorical variables. Logistic regression analyses were performed to identify the independent factors associated with gastrointestinal symptoms in youths. Only variables that were statistically significant in the univariate analyses were further included in multivariate analyses. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. A two-sided P<0.05 was considered statistically significant.
Results
Participants
A total of 1,019 participants, including 708 sophomores and 311 recruits, were considered for the study inclusion. Among them, 16 sophomores’ and one recruit’s records were excluded. Finally, 1,002 participants were eligible, including 692 sophomores and 310 recruits. The median age of the sophomores was 20.0 (range, 16.0–25.0) years old; and 95.1% (n=658) were female. The median age of the recruits was 20.0 (range, 17.0–24.0) years old; and 6.5% (n=20) were female (Table S1). The prevalence of gastrointestinal symptoms was 36.7% (n=254) and 15.5% (n=48) in the sophomores and recruits, respectively (Figure 1). The prevalence of total SCL-90 score beyond 160 was 9.2% (n=64) and 2.6% (n=8) in the sophomores and recruits, respectively.
Difference between participants with and without gastrointestinal symptoms
Sophomores with gastrointestinal symptoms had a significantly higher prevalence of females, history of surgery, gastrointestinal diseases, drinking coffee, eating spicy food, irregular diet, eating out frequently, and sharing drinking glasses, halitosis, family history of gastrointestinal diseases and total SCL-90 score beyond 160 than those without (Table 1).
Table 1
| Variables | Overall | With gastrointestinal symptoms | Without gastrointestinal symptoms | P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. Pts | Values | No. Pts | Values | No. Pts | Values | ||||
| Age (years) | 692 | 20.00 (16.00–25.00), 20.04±1.38 | 254 | 20.00 (16.00–25.00), 19.98±1.25 | 438 | 20.00 (16.00–24.00), 20.05±1.40 | 0.322 | ||
| Female | 692 | 658 (95.1) | 254 | 247 (97.2) | 438 | 411 (93.8) | 0.046 | ||
| Height (m) | 692 | 1.63 (1.45–1.85), 1.64±0.06 | 254 | 1.63 (1.47–1.82), 1.63±0.05 | 438 | 1.63 (1.45–1.85), 1.64±0.06 | 0.263 | ||
| Weight (kg) | 692 | 54.00 (37.00–80.00), 55.61±9.39 | 254 | 53.00 (38.00–80.00), 54.13±8.48 | 438 | 54.00 (37.00–80.00), 55.86±9.52 | 0.182 | ||
| Body mass index (kg/m2) | 692 | 20.20 (16.65–32.05), 20.60±2.12 | 254 | 19.95 (17.59–29.74), 20.30±2.02 | 438 | 20.32 (16.65–32.05), 20.66±2.14 | 0.186 | ||
| History of surgery | 692 | 43 (6.2) | 254 | 25 (9.8) | 438 | 18 (4.1) | 0.003 | ||
| History of gastrointestinal diseases | 692 | 99 (14.3) | 254 | 60 (23.6) | 438 | 39 (8.9) | <0.001 | ||
| History of smoking | 692 | 8 (1.2) | 254 | 5 (2.0) | 438 | 3 (0.7) | 0.151 | ||
| History of drinking alcohol | 692 | 21 (9.4) | 254 | 9 (3.5) | 438 | 12 (2.7) | 0.553 | ||
| History of drinking tea | 692 | 83 (9.4) | 254 | 37 (14.6) | 438 | 46 (10.5) | 0.113 | ||
| History of drinking coffee | 692 | 40 (5.8) | 254 | 22 (8.7) | 438 | 18 (4.1) | 0.013 | ||
| History of eating spicy food | 692 | 367 (53.0) | 254 | 163 (64.2) | 438 | 204 (46.6) | <0.001 | ||
| History of irregular diet | 692 | 293 (56.7) | 254 | 143 (56.3) | 438 | 150 (34.2) | <0.001 | ||
| History of eating out frequently | 692 | 171 (24.7) | 254 | 90 (35.4) | 438 | 81 (18.5) | <0.001 | ||
| History of drinking raw water | 692 | 349 (50.4) | 254 | 131 (51.6) | 438 | 218 (49.8) | 0.647 | ||
| History of sharing drinking glasses | 692 | 64 (9.2) | 254 | 33 (13.0) | 438 | 31 (7.1) | 0.010 | ||
| Halitosis | 692 | 66 (9.5) | 254 | 33 (13.0) | 438 | 33 (7.5) | 0.018 | ||
| Living in the countryside | 692 | 376 (54.3) | 254 | 127 (50.0) | 438 | 249 (56.8) | 0.081 | ||
| Number of family members | 692 | 4.00 (2.00–9.00), 3.84±1.12 | 254 | 4.00 (2.00–9.00), 3.76±1.14 | 438 | 4.00 (2.00–9.00), 3.85±1.12 | 0.436 | ||
| Annual family income <50,000 RMB | 692 | 455 (65.8) | 254 | 172 (67.7) | 438 | 283 (64.6) | 0.407 | ||
| Family history of gastrointestinal diseases | 692 | 23 (3.3) | 254 | 15 (5.9) | 438 | 8 (1.8) | 0.004 | ||
| SCL-90 score >160 | 692 | 64 (9.2) | 254 | 50 (19.7) | 438 | 14 (3.2) | <0.001 | ||
Data are presented as median (range), mean ± SD or frequency (percentage). SD, standard deviation; RMB, ren min bi; SCL, symptom checklist.
Recruits with gastrointestinal symptoms had a significantly higher prevalence of history of gastrointestinal diseases, drinking alcohol, drinking coffee, eating spicy food, eating out frequently, drinking raw water, and sharing drinking glasses, halitosis, family history of gastrointestinal diseases and total SCL-90 score beyond 160 than those without (Table 2).
Table 2
| Variables | Overall | With gastrointestinal symptoms | Without gastrointestinal symptoms | P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. Pts | Median (range), mean ± SD or frequency (percentage) | No. Pts | Median (range), mean ± SD or frequency (percentage) | No. Pts | Median (range), mean ± SD or frequency (percentage) | ||||
| Age (years) | 310 | 20.00 (17.00–24.00), 19.77±1.41 | 48 | 20.00 (18.00–24.00), 19.92±1.44 | 262 | 20.00 (17.00–24.00), 19.75±1.40 | 0.578 | ||
| Female | 310 | 20 (6.5) | 48 | 6 (12.5) | 262 | 14 (5.3) | 0.064 | ||
| Height (m) | 310 | 1.75 (1.60–1.96), 1.76±0.06 | 48 | 1.74 (1.60–1.91), 1.75±0.07 | 262 | 1.76 (1.60–1.96), 1.76±0.06 | 0.267 | ||
| Weight (kg) | 310 | 69.00 (51.00–100.00), 69.63±8.46 | 48 | 68.00 (55.00–100.00), 69.56±9.51 | 262 | 69.0 (51.00–95.00), 69.65±8.28 | 0.667 | ||
| Body mass index (kg/m2) | 310 | 22.30 (17.00–29.40), 22.52±2.17 | 48 | 22.25 (18.90–27.50), 22.70±2.26 | 262 | 22.40 (17.00–29.40), 22.49±2.16 | 0.815 | ||
| History of surgery | 310 | 82 (26.5) | 48 | 11 (22.9) | 262 | 71 (27.1) | 0.546 | ||
| History of gastrointestinal diseases | 310 | 14 (4.5) | 48 | 10 (20.8) | 262 | 4 (1.5) | <0.001 | ||
| History of smoking | 310 | 60 (19.4) | 48 | 10 (20.8) | 262 | 50 (19.1) | 0.778 | ||
| History of drinking alcohol | 310 | 29 (9.4) | 48 | 9 (18.8) | 262 | 20 (7.6) | 0.015 | ||
| History of drinking tea | 310 | 73 (23.5) | 48 | 14 (29.2) | 262 | 59 (22.5) | 0.318 | ||
| History of drinking coffee | 310 | 26 (8.4) | 48 | 8 (16.7) | 262 | 18 (6.9) | 0.024 | ||
| History of eating spicy food | 310 | 127 (41.0) | 48 | 28 (58.3) | 262 | 99 (37.8) | 0.008 | ||
| History of irregular diet | 310 | 26 (8.4) | 48 | 7 (14.6) | 262 | 19 (7.3) | 0.092 | ||
| History of eating out frequently | 310 | 52 (16.8) | 48 | 18 (37.5) | 262 | 34 (13.0) | <0.001 | ||
| History of drinking raw water | 310 | 59 (19.0) | 48 | 15 (31.3) | 262 | 44 (16.8) | 0.019 | ||
| History of sharing drinking glasses | 310 | 9 (2.9) | 48 | 5 (10.4) | 262 | 4 (1.5) | 0.006 | ||
| Halitosis | 310 | 15 (4.8) | 48 | 10 (20.8) | 262 | 5 (1.9) | <0.001 | ||
| Living in the countryside | 310 | 200 (64.5) | 48 | 29 (60.4) | 262 | 171 (65.3) | 0.518 | ||
| Number of family members | 310 | 4.00 (1.00–8.00), 3.73±0.97 | 48 | 4.00 (2.00–8.00), 3.85±1.20 | 262 | 4.00 (1.00–7.00), 3.71±0.92 | 0.604 | ||
| Annual family income <50,000 RMB | 310 | 147 (47.4) | 48 | 26 (54.2) | 262 | 121 (46.2) | 0.309 | ||
| Family history of gastrointestinal diseases | 310 | 13 (4.2) | 48 | 6 (12.5) | 262 | 7 (2.7) | 0.002 | ||
| SCL-90 score >160 | 310 | 8 (2.6) | 48 | 5 (10.4) | 262 | 3 (1.1) | 0.003 | ||
SD, standard deviation; RMB, ren min bi; SCL, symptom checklist.
Risk factors for gastrointestinal symptoms
In the sophomore population, univariate logistic regression analysis showed that body mass index, history of surgery, gastrointestinal diseases, drinking coffee, eating spicy food, irregular diet, eating out frequently, and sharing drinking glasses, halitosis, family history of gastrointestinal diseases and total SCL-90 score beyond 160 (OR =7.423; 95% CI: 4.010–13.740; P<0.001) were significant risk factors of gastrointestinal symptoms. Multivariate logistic regression analysis showed that history of surgery, gastrointestinal diseases, irregular diet, eating out frequently, and sharing drinking glasses and total SCL-90 score beyond 160 (OR =5.467; 95% CI: 2.855–10.470; P<0.001) were independently associated with gastrointestinal symptoms (Table 3).
Table 3
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Age (years) | 2.318 | 0.995–5.403 | 0.051 | ||||
| Gender (female vs. male) | 0.980 | 0.876–1.096 | 0.719 | ||||
| Height (m) | 0.190 | 0.014–2.541 | 0.209 | ||||
| Weight (kg) | 0.984 | 0.967–1.001 | 0.059 | ||||
| Body mass index (kg/m2) | 0.925 | 0.857–0.998 | 0.046 | 0.933 | 0.858–1.015 | 0.105 | |
| History of surgery (yes vs. no) | 2.547 | 1.361–4.768 | 0.003 | 2.491 | 1.244–4.991 | 0.010 | |
| History of gastrointestinal diseases (yes vs. no) | 3.164 | 2.042–4.904 | <0.001 | 2.156 | 1.324–3.511 | 0.002 | |
| History of smoking (yes vs. no) | 2.912 | 0.690–12.287 | 0.146 | ||||
| History of drinking alcohol (yes vs. no) | 1.304 | 0.542–3.139 | 0.554 | ||||
| History of drinking tea (yes vs. no) | 1.453 | 0.914–2.310 | 0.114 | ||||
| History of drinking coffee (yes vs. no) | 2.213 | 1.163–4.210 | 0.016 | 1.416 | 0.681–2.944 | 0.352 | |
| History of eating spicy food (yes vs. no) | 2.055 | 1.495–2.823 | <0.001 | 1.369 | 0.957–1.958 | 0.085 | |
| History of irregular diet (yes vs. no) | 2.474 | 1.802–3.396 | <0.001 | 1.750 | 1.232–2.486 | 0.002 | |
| History of eating out frequently (yes vs. no) | 2.419 | 1.700–3.441 | <0.001 | 1.828 | 1.233–2.712 | 0.003 | |
| History of drinking raw water (yes vs. no) | 0.930 | 0.683–1.268 | 0.648 | ||||
| History of sharing drinking glasses | 1.960 | 1.169–3.287 | 0.011 | 1.971 | 1.120–3.468 | 0.019 | |
| Halitosis (yes vs. no) | 1.833 | 1.101–3.051 | 0.020 | 1.258 | 0.705–2.244 | 0.437 | |
| Living in the countryside (yes vs. no) | 0.759 | 0.557–1.035 | 0.082 | ||||
| Number of family members | 1.063 | 0.927–1.219 | 0.384 | ||||
| Annual family income <50,000 RMB (yes vs. no) | 1.149 | 0.828–1.595 | 0.407 | ||||
| Family history of gastrointestinal diseases (yes vs. no) | 3.373 | 1.410–8.072 | 0.006 | 1.631 | 0.602–4.418 | 0.336 | |
| SCL-90 score >160 (yes vs. no) | 7.423 | 4.010–13.74 | <0.001 | 5.467 | 2.855–10.470 | <0.001 | |
OR, odds ratio; CI, confidence interval; RMB, ren min bi; SCL, symptom checklist.
In the recruit population, univariate logistic regression analysis showed that history of gastrointestinal diseases, drinking alcohol, drinking coffee, eating spicy food, eating out frequently, drinking raw water, and sharing drinking glasses, halitosis, family history of gastrointestinal diseases and total SCL-90 score beyond 160 (OR =10.039; 95% CI: 2.314–43.543; P=0.002) were significant risk factors of gastrointestinal symptoms. Multivariate logistic regression analysis showed that history of gastrointestinal diseases and eating out frequently, halitosis and total SCL-90 score beyond 160 (OR =6.734; 95% CI: 1.226–36.999; P=0.028) were independently associated with gastrointestinal symptoms (Table 4).
Table 4
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| Age (years) | 1.088 | 0.877–1.350 | 0.445 | ||||
| Gender (female vs. male) | 2.531 | 0.921–6.953 | 0.072 | ||||
| Height (m) | 0.082 | 0.001–10.530 | 0.312 | ||||
| Weight (kg) | 0.999 | 0.963–1.036 | 0.941 | ||||
| Body mass index (kg/m2) | 1.044 | 0.907–1.202 | 0.551 | ||||
| History of surgery (yes vs. no) | 0.800 | 0.387–1.653 | 0.546 | ||||
| History of gastrointestinal diseases (yes vs. no) | 16.947 | 5.069–58.836 | <0.001 | 15.945 | 4.204–60.475 | <0.001 | |
| History of smoking (yes vs. no) | 1.116 | 0.521–2.390 | 0.778 | ||||
| History of drinking alcohol (yes vs. no) | 2.792 | 1.186–6.574 | 0.019 | 0.799 | 0.231–2.764 | 0.723 | |
| History of drinking tea (yes vs. no) | 1.417 | 0.713–2.815 | 0.320 | ||||
| History of drinking coffee (yes vs. no) | 2.711 | 1.105–6.651 | 0.029 | 2.267 | 0.749–6.862 | 0.147 | |
| History of eating spicy food (yes vs. no) | 2.305 | 1.233–4.310 | 0.009 | 1.441 | 0.664–3.126 | 0.356 | |
| History of irregular diet (yes vs. no) | 2.184 | 0.864–5.521 | 0.099 | ||||
| History of eating out frequently (yes vs. no) | 4.024 | 2.025–7.994 | <0.001 | 2.897 | 1.207–6.954 | 0.017 | |
| History of drinking raw water (yes vs. no) | 2.252 | 1.128–4.494 | 0.021 | 1.478 | 0.615–3.550 | 0.382 | |
| History of sharing drinking glasses | 7.500 | 1.937–29.042 | 0.004 | 3.016 | 0.471–19.339 | 0.244 | |
| Halitosis (yes vs. no) | 13.526 | 4.386–41.717 | <0.001 | 18.962 | 5.376–66.881 | <0.001 | |
| Living in the countryside (yes vs. no) | 0.812 | 0.432–1.528 | 0.519 | ||||
| Number of family members | 1.161 | 0.853–1.581 | 0.342 | ||||
| Annual family income <50,000 RMB (yes vs. no) | 1.377 | 0.743–2.544 | 0.310 | ||||
| Family history of gastrointestinal diseases (yes vs. no) | 5.204 | 1.667–16.243 | 0.005 | 3.403 | 0.651–17.791 | 0.147 | |
| SCL-90 score >160 (yes vs. no) | 10.039 | 2.314–43.543 | 0.002 | 6.734 | 1.226–36.999 | 0.028 | |
OR, odds ratio; CI, confidence interval; RMB, ren min bi; SCL, symptom checklist.
Various types of psychological problems between participants with and without gastrointestinal symptoms
Sophomores with gastrointestinal symptoms had a significantly higher prevalence of obsessive-compulsive, interpersonal sensitivity, depression, anxiety, and hostility than those without. Recruits with gastrointestinal symptoms had a significantly higher prevalence of somatization, obsessive-compulsive, and depression than those without (Table 5).
Table 5
| Variables | Overall | With gastrointestinal symptoms | Without gastrointestinal symptoms | P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. Pts | Frequency (percentage) | No. Pts | Frequency (percentage) | No. Pts | Frequency (percentage) | ||||
| Sophomores | |||||||||
| Somatization | 692 | 4 (0.6) | 254 | 3 (1.2) | 438 | 1 (0.2) | 0.143 | ||
| Obsessive-compulsive | 692 | 11 (1.6) | 254 | 11 (4.3) | 438 | 0 (0.0) | <0.001 | ||
| Interpersonal sensitivity | 692 | 6 (0.9) | 254 | 6 (2.4) | 438 | 0 (0.0) | 0.002 | ||
| Depression | 692 | 14 (2.0) | 254 | 14 (5.5) | 438 | 0 (0.0) | <0.001 | ||
| Anxiety | 692 | 8 (1.2) | 254 | 8 (3.1) | 438 | 0 (0.0) | <0.001 | ||
| Hostility | 692 | 6 (0.9) | 254 | 6 (2.4) | 438 | 0 (0.0) | 0.002 | ||
| Phobic anxiety | 692 | 2 (0.3) | 254 | 2 (0.8) | 438 | 0 (0.0) | 0.134 | ||
| Paranoid ideation | 692 | 2 (0.3) | 254 | 2 (0.8) | 438 | 0 (0.0) | 0.134 | ||
| Psychoticism | 692 | 1 (0.1) | 254 | 1 (0.4) | 438 | 0 (0.0) | 0.367 | ||
| Recruits | |||||||||
| Somatization | 310 | 2 (0.6) | 48 | 2 (4.2) | 262 | 0 (0.0) | 0.024 | ||
| Obsessive-compulsive | 310 | 2 (0.6) | 48 | 2 (4.2) | 262 | 0 (0.0) | 0.024 | ||
| Interpersonal sensitivity | 310 | 2 (0.6) | 48 | 1 (2.1) | 262 | 1 (0.4) | 0.286 | ||
| Depression | 310 | 2 (0.6) | 48 | 2 (4.2) | 262 | 0 (0.0) | 0.024 | ||
| Anxiety | 310 | 1 (0.3) | 48 | 1 (2.1) | 262 | 0 (0.0) | 0.155 | ||
| Hostility | 310 | 0 (0.0) | 48 | 0 (0.0) | 262 | 0 (0.0) | NA | ||
| Phobic anxiety | 310 | 0 (0.0) | 48 | 0 (0.0) | 262 | 0 (0.0) | NA | ||
| Paranoid ideation | 310 | 0 (0.0) | 48 | 0 (0.0) | 262 | 0 (0.0) | NA | ||
| Psychoticism | 310 | 0 (0.0) | 48 | 0 (0.0) | 262 | 0 (0.0) | NA | ||
NA, not available.
Prevalence of gastrointestinal symptoms according to the total SCL-90 score
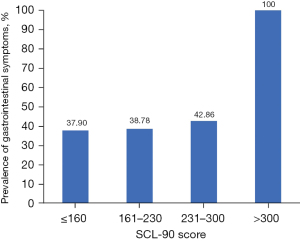
In the sophomore population, the prevalence of gastrointestinal symptoms was increased with the total SCL-90 score. The prevalence was 37.9% (n=198), 38.8% (n=19), 42.9% (n=6), and 100.0% (n=1) in the groups with total SCL-90 scores less than or equal to 160, 161–230, 231–300, and beyond 300, respectively (Figure 2).
Various types of gastrointestinal symptoms among participants with a total SCL-90 score beyond and less than or equal to 160
In the sophomore population, the prevalence of nausea, emesis, abdominal pain, acid regurgitation, eructation, heartburn, anorexia, abdominal bloating, diarrhea, constipation, and hematochezia was significantly higher in the group with a total SCL-90 score beyond 160 than in the group with a total SCL-90 score less than or equal to 160.
In the recruit population, the prevalence of abdominal pain, anorexia, abdominal bloating, constipation, and hematochezia was significantly higher in the group with a total SCL-90 score beyond 160 than in the group with a total SCL-90 score less than or equal to 160 (Table 6).
Table 6
| Variables | Overall | SCL-90 score >160 | SCL-90 score ≤160 | P value | |||||
|---|---|---|---|---|---|---|---|---|---|
| No. Pts | Frequency (percentage) | No. Pts | Frequency (percentage) | No. Pts | Frequency (percentage) | ||||
| Sophomores | |||||||||
| Nausea | 692 | 56 (8.1) | 64 | 19 (29.7) | 628 | 37 (5.9) | <0.001 | ||
| Emesis | 692 | 10 (1.4) | 64 | 4 (6.2) | 628 | 6 (1.0) | 0.009 | ||
| Abdominal pain | 692 | 55 (7.9) | 64 | 20 (31.2) | 628 | 35 (5.6) | <0.001 | ||
| Acid regurgitation | 692 | 34 (4.9) | 64 | 13 (20.3) | 628 | 21 (3.3) | <0.001 | ||
| Eructation | 692 | 121 (17.5) | 64 | 26 (40.6) | 628 | 95 (15.1) | <0.001 | ||
| Heartburn | 692 | 27 (3.9) | 64 | 12 (18.8) | 628 | 15 (2.4) | <0.001 | ||
| Anorexia | 692 | 77 (11.1) | 64 | 25 (39.1) | 628 | 52 (8.3) | <0.001 | ||
| Abdominal bloating | 692 | 74 (10.7) | 64 | 25 (39.1) | 628 | 49 (7.8) | <0.001 | ||
| Diarrhea | 692 | 41 (5.9) | 64 | 14 (21.9) | 628 | 27 (4.3) | <0.001 | ||
| Constipation | 692 | 80 (11.6) | 64 | 15 (23.4) | 628 | 65 (10.4) | 0.002 | ||
| Hematemesis | 692 | 1 (0.1) | 64 | 1 (1.6) | 628 | 0 (0.0) | 0.092 | ||
| Hematochezia | 692 | 13 (1.9) | 64 | 4 (6.2) | 628 | 9 (1.4) | 0.025 | ||
| Recruits | |||||||||
| Nausea | 310 | 17 (5.5) | 8 | 2 (25.0) | 302 | 15 (5.0) | 0.065 | ||
| Emesis | 310 | 6 (1.9) | 8 | 1 (12.5) | 302 | 5 (1.7) | 0.146 | ||
| Abdominal pain | 310 | 12 (3.9) | 8 | 2 (25.0) | 302 | 10 (3.3) | 0.034 | ||
| Acid regurgitation | 310 | 4 (1.3) | 8 | 0 (0.0) | 302 | 4 (1.3) | 1.000 | ||
| Eructation | 310 | 1 (0.3) | 8 | 0 (0.0) | 302 | 1 (0.3) | 1.000 | ||
| Heartburn | 310 | 2 (0.6) | 8 | 0 (0.0) | 302 | 2 (0.7) | 1.000 | ||
| Anorexia | 310 | 4 (1.3) | 8 | 2 (25.0) | 302 | 2 (0.7) | 0.003 | ||
| Abdominal bloating | 310 | 9 (2.9) | 8 | 3 (37.5) | 302 | 6 (2.0) | 0.001 | ||
| Diarrhea | 310 | 6 (1.9) | 8 | 0 (0.0) | 302 | 6 (2.0) | 1.000 | ||
| Constipation | 310 | 10 (3.2) | 8 | 2 (25.0) | 302 | 8 (2.6) | 0.024 | ||
| Hematemesis | 310 | 0 (0.0) | 8 | 0 (0.0) | 302 | 0 (0.0) | NA | ||
| Hematochezia | 310 | 8 (2.6) | 8 | 2 (25.0) | 302 | 6 (2.0) | 0.015 | ||
SCL, symptom checklist; NA, not available.
Discussion
A major finding of our study was that the prevalence of gastrointestinal symptoms in youths was high, and the prevalence of gastrointestinal symptoms was lower in the recruits than those in the sophomores (15.5% vs. 36.7%). Several potential explanations are made for this phenomenon. Firstly, gastrointestinal symptoms are gender-specific. Females have a higher prevalence of gastrointestinal symptoms (22), probably because estrogens directly affect the intestinal microbes and immune cells (23), and the menstrual cycle affects gastrointestinal transit duration (24), then leading to gastrointestinal symptoms. In our study, the sophomore population had a predominance of females. Secondly, the daily management of the sophomores is less strict than that of recruits, so they may have more unhealthy lifestyles that lead to the occurrence of gastrointestinal symptoms. Thirdly, the recruits should meet strict criteria for conscription of the troops, and those with poor physical fitness will be eliminated (25).
The prevalence of sophomores who experienced gastrointestinal symptoms was lower in our study than in previous studies. It was 36.7% in our sophomore population, but 51.2% in 127 university students of Canada (26), 64.2% in 668 university students of Switzerland (27), and 65% in 715 university students of Korea (28). Such a difference may be related to the heterogeneity in regions, ethnicities, living conditions, and sample size among studies. Similarly, the prevalence of gastrointestinal symptoms was lower in our recruit population than in fighting forces previously reported. It was 15.5% in our recruit population, but 25% in veterans from the United States who participated in the Persian Gulf War (29). Gastrointestinal symptoms in recruits may be related to an increase in segmental gastrointestinal permeability during combat training (30). Military soldiers aged 17 to 25 years old need to go through numerous high-intensity training, and have a higher prevalence of Helicobacter pylori infection than civilians of the same age (31), which may cause gastrointestinal symptoms (32).
Another finding of our study was that the total SCL-90 score beyond 160 was independently associated with gastrointestinal symptoms in both sophomore and recruit populations. Such an association between gastrointestinal symptoms and psychological problems should be mainly attributed to the brain-gut axis (33), which functions via three major pathways. Firstly, psychological problems activate the Hypothalamic-Pituitary-Adrenal (HPA) axis, increasing the levels of glucocorticoid and gastrin, thereby leading to endocrine and gastrointestinal dysfunction (34). On the other hand, abdominal pain leads to anxiety- and depression-like behaviors via the HPA axis (35). Secondly, when people have psychological problems, the balance between the limbic system and the hypothalamus is broken, which affects the vagal tone and decreases the circular muscle contractility, thereby delaying gastric emptying (36). On the other hand, when people have gastrointestinal symptoms, the vagus nerve transmits signals from the gastrointestinal tract to the central nervous system to regulate cognition and affect emotion (37). Thirdly, the central nervous system indirectly regulates the composition and function of intestinal microbes by releasing cytokines and antimicrobial peptides (38). On the other hand, the imbalance of intestinal flora will cause the dysfunction of bacteria in the brain-gut axis, decreasing 5-hydroxytryptamine content in the hippocampus and brain-derived neurotrophic factor mRNA expression, which will cause psychological problems (39).
Our study also found that depression and anxiety are the most common psychological problems in youths according to the psychological scale. Some previous studies explored the correlation between gastrointestinal symptoms and psychological problems. Both studies by Ballou et al. (40) and Lu et al. (41) demonstrated a close association of depression with diarrhea. Both studies by Cunningham et al. (42) and Aggarwal Dutta et al. (43) suggested a correlation between anxiety and functional abdominal pain. Additionally, depression and anxiety might be associated with severe nausea and vomiting (44) and functional constipation (45) (Table S2). Some psychological interventions, such as music therapy, are promising to improve psychological health and are helpful for preventing and treating psychological problems in youths (46), thereby improving gastrointestinal symptoms.
Our study had some limitations. Firstly, the study was performed based on a questionnaire survey without any objective evaluation. Participants’ response to the questionnaire survey was potentially unreliable. Secondly, existing and validated questionnaires, such as the GI-PROMIS scales (5), could not fully cover what we want to know or address the specific objectives of our study, so the present questionnaires had to be specially designed in our study. We should acknowledge that its credibility and validity are not sufficient. Thirdly, diet, such as vitamin D (47), may influence both mood and gastrointestinal symptoms. But dietary questions had not been specified in our questionnaire yet. Fourthly, the population characteristics, especially living environments and gender, were very different between the two independent cohorts of youths. However, the findings from the two cohorts were very similar, providing more solid evidence for supporting the association of gastrointestinal symptoms with psychological problems in youths. Fifthly, some questions regarding personal habits and lifestyles are often subjective and a bit ambiguous. Timeframe or frequency of irregular diet, eating out, or sharing drinking glasses had not been specified in our questionnaire yet.
Conclusions
Based on the current findings, gastrointestinal symptoms may be common in youths and are strongly associated with psychological problems. In the future, prospective cohort studies are necessary to observe whether psychological problems are potential causes of gastrointestinal symptoms and explore whether the improvement of psychological problems can alleviate gastrointestinal symptoms.
Acknowledgments
We thank all the participants who have participated in this study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1316/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1316/dss
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1316/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1316/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Medical Ethical Committee of the General Hospital of Northern Theater Command with an approval number [Y (2021) 099]. The study was performed according to the Declaration of Helsinki (as revised in 2013). All participants gave verbal informed consent to participate in the study, and the ethics committee of the hospital exempted the written informed consents due to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Clevers E, Törnblom H, Simrén M, et al. Relations between food intake, psychological distress, and gastrointestinal symptoms: A diary study. United European Gastroenterol J 2019;7:965-73. [Crossref] [PubMed]
- Black CJ, Drossman DA, Talley NJ, et al. Functional gastrointestinal disorders: advances in understanding and management. Lancet 2020;396:1664-74. [Crossref] [PubMed]
- Clevers E, Whitehead WE, Palsson OS, et al. Factor Analysis Defines Distinct Upper and Lower Gastrointestinal Symptom Groups Compatible With Rome IV Criteria in a Population-based Study. Clin Gastroenterol Hepatol 2018;16:1252-1259.e5. [Crossref] [PubMed]
- Vivier H, Ross EJ, Cassisi JE. Classification of gastrointestinal symptom patterns in young adults. BMC Gastroenterol 2020;20:326. [Crossref] [PubMed]
- Ross EJ, Vivier H, Cassisi JE, et al. Gastrointestinal health: An investigation of mediating effects on mood and quality of life. Health Psychol Open 2020;7:2055102920974524. [Crossref] [PubMed]
- Clevers E, Lutin E, Cornelis J, et al. Gastrointestinal symptoms in office workers are predicted by psychological distress and short sleep duration. J Psychosom Res 2020;138:110230. [Crossref] [PubMed]
- Wainberg ML, Scorza P, Shultz JM, et al. Challenges and Opportunities in Global Mental Health: a Research-to-Practice Perspective. Curr Psychiatry Rep 2017;19:28. [Crossref] [PubMed]
- Garakani A, Murrough JW, Freire RC, et al. Pharmacotherapy of Anxiety Disorders: Current and Emerging Treatment Options. Front Psychiatry 2020;11:595584. [Crossref] [PubMed]
- Malhi GS, Mann JJ. Depression. Lancet 2018;392:2299-312. [Crossref] [PubMed]
- Zhuang SM, Chen F. Chinese Adolescents and Youth With Methamphetamine Dependence: Prevalence and Concurrent Psychological Problems. Nurs Res 2016;65:117-24. [Crossref] [PubMed]
- Meza JI, Bath E. One Size Does Not Fit All: Making Suicide Prevention and Interventions Equitable for Our Increasingly Diverse Communities. J Am Acad Child Adolesc Psychiatry 2021;60:209-12. [Crossref] [PubMed]
- Schäfer JÖ, Naumann E, Holmes EA, et al. Emotion Regulation Strategies in Depressive and Anxiety Symptoms in Youth: A Meta-Analytic Review. J Youth Adolesc 2017;46:261-76. [Crossref] [PubMed]
- Iorfino F, Scott EM, Carpenter JS, et al. Clinical Stage Transitions in Persons Aged 12 to 25 Years Presenting to Early Intervention Mental Health Services With Anxiety, Mood, and Psychotic Disorders. JAMA Psychiatry 2019;76:1167-75. [Crossref] [PubMed]
- Ng QX, Soh AYS, Loke W, et al. Systematic review with meta-analysis: The association between post-traumatic stress disorder and irritable bowel syndrome. J Gastroenterol Hepatol 2019;34:68-73. [Crossref] [PubMed]
- Ross EJ, Cassisi JE, Joseph D, et al. Cross-lagged analyses between gastrointestinal symptoms, psychological distress, and disability in emerging adults. Appl Psychol Health Well Being 2022;14:920-36. [Crossref] [PubMed]
- Labanski A, Langhorst J, Engler H, et al. Stress and the brain-gut axis in functional and chronic-inflammatory gastrointestinal diseases: A transdisciplinary challenge. Psychoneuroendocrinology 2020;111:104501. [Crossref] [PubMed]
- Mei M, Xu H, Wang L, et al. Current practice and awareness of pediatric off-label drug use in Shanghai, China -a questionnaire-based study. BMC Pediatr 2019;19:281. [Crossref] [PubMed]
- Kalinowska P, Marcinowicz L. Job satisfaction among family nurses in Poland: A questionnaire-based study. Nurs Open 2020;7:1680-90. [Crossref] [PubMed]
- Alzahrani SH, Saeedi AA, Baamer MK, et al. Eating Habits Among Medical Students at King Abdulaziz University, Jeddah, Saudi Arabia. Int J Gen Med 2020;13:77-88. [Crossref] [PubMed]
- Chen IH, Lin CY, Zheng X, et al. Assessing Mental Health for China's Police: Psychometric Features of the Self-Rating Depression Scale and Symptom Checklist 90-Revised. Int J Environ Res Public Health 2020;17:2737. [Crossref] [PubMed]
- Yu Y, Wan C, Huebner ES, et al. Psychometric properties of the symptom check list 90 (SCL-90) for Chinese undergraduate students. J Ment Health 2019;28:213-9. [Crossref] [PubMed]
- Perna A, Maccora D, Rossi S, et al. High Prevalence and Gender-Related Differences of Gastrointestinal Manifestations in a Cohort of DM1 Patients: A Perspective, Cross-Sectional Study. Front Neurol 2020;11:394. [Crossref] [PubMed]
- Yoon K, Kim N. Roles of Sex Hormones and Gender in the Gut Microbiota. J Neurogastroenterol Motil 2021;27:314-25. [Crossref] [PubMed]
- Meleine M, Matricon J. Gender-related differences in irritable bowel syndrome: potential mechanisms of sex hormones. World J Gastroenterol 2014;20:6725-43. [Crossref] [PubMed]
- Morris MJ, Lucero PF, Zanders TB, et al. Diagnosis and management of chronic lung disease in deployed military personnel. Ther Adv Respir Dis 2013;7:235-45. [Crossref] [PubMed]
- Norton GR, Norton PJ, Asmundson GJ, et al. Neurotic butterflies in my stomach: the role of anxiety, anxiety sensitivity and depression in functional gastrointestinal disorders. J Psychosom Res 1999;47:233-40. [Crossref] [PubMed]
- Suarez K, Mayer C, Ehlert U, et al. Psychological stress and self-reported functional gastrointestinal disorders. J Nerv Ment Dis 2010;198:226-9. [Crossref] [PubMed]
- Lee EY, Mun MS, Lee SH, et al. Perceived stress and gastrointestinal symptoms in nursing students in Korea: A cross-sectional survey. BMC Nurs 2011;10:22. [Crossref] [PubMed]
- Zhang B, Verne ML, Fields JZ, et al. Intestinal Hyperpermeability in Gulf War Veterans With Chronic Gastrointestinal Symptoms. J Clin Gastroenterol 2019;53:e298-302. [Crossref] [PubMed]
- Li X, Kan EM, Lu J, et al. Combat-training increases intestinal permeability, immune activation and gastrointestinal symptoms in soldiers. Aliment Pharmacol Ther 2013;37:799-809. [Crossref] [PubMed]
- Wang C, Liu J, Shi X, et al. Prevalence of Helicobacter pylori Infection in Military Personnel from Northeast China: A Cross-Sectional Study. Int J Gen Med 2021;14:1499-505. [Crossref] [PubMed]
- Huerta-Franco MR, Banderas JW, Allsworth JE. Ethnic/racial differences in gastrointestinal symptoms and diagnosis associated with the risk of Helicobacter pylori infection in the US. Clin Exp Gastroenterol 2018;11:39-49. [Crossref] [PubMed]
- Peppas S, Pansieri C, Piovani D, et al. The Brain-Gut Axis: Psychological Functioning and Inflammatory Bowel Diseases. J Clin Med 2021;10:377. [Crossref] [PubMed]
- Abautret-Daly Á, Dempsey E, Parra-Blanco A, et al. Gut-brain actions underlying comorbid anxiety and depression associated with inflammatory bowel disease. Acta Neuropsychiatr 2018;30:275-96. [Crossref] [PubMed]
- Li JX. Pain and depression comorbidity: a preclinical perspective. Behav Brain Res 2015;276:92-8. [Crossref] [PubMed]
- Weltens N, Iven J, Van Oudenhove L, et al. The gut-brain axis in health neuroscience: implications for functional gastrointestinal disorders and appetite regulation. Ann N Y Acad Sci 2018;1428:129-50. [Crossref] [PubMed]
- Nishimura Y, Fukuda Y, Okonogi T, et al. Dual real-time in vivo monitoring system of the brain-gut axis. Biochem Biophys Res Commun 2020;524:340-5. [Crossref] [PubMed]
- Hao WZ, Li XJ, Zhang PW, et al. A review of antibiotics, depression, and the gut microbiome. Psychiatry Res 2020;284:112691. [Crossref] [PubMed]
- Wei Y, Melas PA, Wegener G, et al. Antidepressant-like effect of sodium butyrate is associated with an increase in TET1 and in 5-hydroxymethylation levels in the Bdnf gene. Int J Neuropsychopharmacol 2014;18:pyu032. [Crossref] [PubMed]
- Ballou S, Katon J, Singh P, et al. Chronic Diarrhea and Constipation Are More Common in Depressed Individuals. Clin Gastroenterol Hepatol 2019;17:2696-703. [Crossref] [PubMed]
- Lu J, Shi L, Huang D, et al. Depression and Structural Factors Are Associated With Symptoms in Patients of Irritable Bowel Syndrome With Diarrhea. J Neurogastroenterol Motil 2020;26:505-13. [Crossref] [PubMed]
- Cunningham NR, Cohen MB, Farrell MK, et al. Concordant parent-child reports of anxiety predict impairment in youth with functional abdominal pain. J Pediatr Gastroenterol Nutr 2015;60:312-7. [Crossref] [PubMed]
- Aggarwal Dutta R, Ely SL, Cunningham NR. The Utility of an Anxiety Screening Measure in Youth With Functional Abdominal Pain Disorders and Clinical Characteristics Associated With Presence of Anxiety. Clin J Pain 2021;37:616-22. [Crossref] [PubMed]
- Beyazit F, Sahin B. Effect of Nausea and Vomiting on Anxiety and Depression Levels in Early Pregnancy. Eurasian J Med 2018;50:111-5. [Crossref] [PubMed]
- Liu J, Lv C, Wu D, et al. Subjective Taste and Smell Changes in Conjunction with Anxiety and Depression Are Associated with Symptoms in Patients with Functional Constipation and Irritable Bowel Syndrome. Gastroenterol Res Pract 2021;2021:5491188. [Crossref] [PubMed]
- Perry-Parrish C, Copeland-Linder N, Webb L, et al. Mindfulness-Based Approaches for Children and Youth. Curr Probl Pediatr Adolesc Health Care 2016;46:172-8. [Crossref] [PubMed]
- Chong RIH, Yaow CYL, Loh CYL, et al. Vitamin D supplementation for irritable bowel syndrome: A systematic review and meta-analysis. J Gastroenterol Hepatol 2022;37:993-1003. [Crossref] [PubMed]



