
Should endovascular stenting be used routinely as first-line treatment for malignant superior vena cava syndrome?—a critical review in the context of recent advances in oncological treatments
Introduction
Malignant superior vena cava syndrome (SVCS) occurs when a tumour mass compresses on the superior vena cava (SVC), resulting in unique clinical features due to impaired venous return from the upper body to the heart. The SVC is a thinned-wall and distensible vessel that can be easily compressed by tumour masses arising from the anterior and middle mediastinum, lymph nodes from the paratracheal and precarinal stations and lung tumours from the right upper lobe (1). The most common cause of malignant SVCS is non-small cell lung cancer (NSCLC), followed by small cell lung cancer (SCLC) and non-Hodgkin lymphoma (NHL) (2,3). Up to 10% of SCLC patients develop SVCS during their disease course because of its predilection for mediastinal involvement, while SVCS occurs in 2–4% of NSCLC and NHL patients (1). These three cancers together make up more than 90% of all malignant causes for SVCS (3).
Facial, neck and upper limb swelling are the most common symptoms of SVCS (1,2). The severity of symptoms generally correlates with the speed of tumour growth. Compression of the SVC by slow-growing tumours allows time for venous collaterals to form. Some patients may remain completely asymptomatic as a result or spontaneously improve after initial symptoms (2,4). In situations where a more acute obstruction arises from a rapidly enlarging tumour, patients may present with cough, dyspnea, hoarseness and even stridor from nasal and laryngeal edema (5,6). Orthopnea is reported in some patients as lying flat further impairs venous return (1). Cerebral edema may also ensue, leading to neurological symptoms such as headache, dizziness and altered mental status (1,2,6). Fortunately, most patients with malignant SVCS are clinically stable and typically develop symptoms over weeks to months (7). This syndrome is no longer considered a medical emergency as it was in the past. Death directly due to SVCS is rare; the estimated incidence is less than 1% in patients diagnosed with this syndrome (6).
Until the first development of endovascular stenting for SVCS around 35 years ago (8), radiotherapy (RT) was the most commonly used treatment for malignant SVCS, while chemotherapy has been an option for highly chemosensitive tumours (2). Concurrent chemoradiation may produce a higher response rate but is associated with greater toxicities (9). Surgical resection of the tumour followed by venous bypass or reconstruction is theoretically an option (10), but it is rarely performed in patients with stage IV malignancies, and it is not the most definitive oncologic therapy in light of the histologies most associated with SVCS. It may, however, be considered in thymic tumour patients as part of a multimodality treatment (11,12).
Although stenting has gained popularity because of its ability to produce rapid symptomatic improvement, it still has not become a routine first-line treatment for all cancer patients having SVCS. Authors agree that those who present with life-threatening symptoms should be treated promptly with first-line stenting, whereas those with highly chemo- or radiosensitive tumours generally should not (2,6,13,14). In other situations, for example, clinically stable patients with less chemosensitive histologies, whether to proceed with stenting as initial treatment is still an area of debate. This clinical practice review aims to evaluate the existing evidence of endovascular stenting in treating malignant SVCS and discuss the applications of first-line stenting in the context of recent advances in oncological treatments.
Evidence for endovascular stenting in the management of malignant SVCS
Procedure of endovascular stenting
To discuss the emerging indications of first-line stenting, it is first important to understand the details of the procedure. Stenting is performed under conscious sedation with patients’ vital signs closely monitored (13,14). Local anaesthesia is applied at the skin of the vascular access site, most commonly at the internal jugular vein and common femoral vein, but the subclavian and basilic veins are also possible sites (14,15). After confirming the site of stenosis with a venogram, the extent of the stenosis and the patency of brachiocephalic veins and/or proximal and distal SVC are evaluated to define the landing zones for stent placement (13,14). A guidewire is subsequently navigated through the narrowing under X-ray guidance, followed by stent deployment (13-15). While there are no differences in the technical success rates observed in the brands of stents, retrospective studies demonstrated that covered stents provide better long-term patency compared to non-covered stents (16-18).
Some interventionalists routinely give a bolus of heparin during the procedure, but it is not a universal practice, as doing so can increase the risks of bleeding (14,19,20). When extensive thrombus is encountered, thrombolysis or occasionally thrombectomy may first be performed to reduce the length of the stenosis before stent insertion (13,14). This may, in turn, reduce the number of stents needed and the risk of thromboembolism (14). Following stent deployment, a repeat venogram is performed to confirm that obstruction is relieved (13,14). The median procedure time for stenting is around 90 minutes in a retrospective cohort of malignant SVCS patients (21).
Anticoagulation after stenting remains a controversial issue. While some advocate routine administration of anticoagulation to prevent in-stent thrombosis, some only prescribe anticoagulation when SVC thrombosis is present (14,19,20). The type of anticoagulation also varies amongst interventionists. Options include warfarin, heparin, aspirin, clopidogrel or their combinations (19,20). When anticoagulation is prescribed, the duration is not standardized as well, with clinicians prescribing it from three months to lifelong (20).
Practical considerations for obtaining tumour biopsies when stenting is performed
SVCS can be the first presentation of malignancy before a histological diagnosis is obtained. For some patients with a known cancer diagnosis, a rebiopsy may be considered to assess the molecular profile of the tumour to guide systemic treatment selection. Clinicians were historically concerned about increased risks of cardiopulmonary complications and bleeding when biopsies were taken before treating SVCS, especially when procedural sedation was required (22,23). Subsequent studies have shown that both endoscopic transbronchial biopsies and percutaneous trans-thoracic biopsies are safe (24-26).
If stenting is indicated for treatment in clinically stable patients, biopsies should be taken before stenting because patients may be started on anticoagulation post-procedure, which increases risks of severe haemorrhage. In patients with life-threatening SVCS symptoms who require urgent stenting, clinicians may consider arranging a biopsy shortly after stenting and defer the start of anticoagulation untill biopsy is obtained. The risk of in-stent thrombosis is likely to be low without anticoagulation during this short period. Common agents used as an antithrombotic during stenting, such as heparin, have a short biological half life of up to 150 minutes (27). Thus, invasive procedures to obtain a biopsy should be safe one to two days after stenting. Ultimately, the relative timing of biopsy in relation to stenting should be assessed individually on a case-by-case basis, balancing the indications and relative urgency of biopsy and those of stenting.
Existing evidence of stenting compared to other SVCS treatments
There is a lack of randomised controlled studies comparing stenting to other treatments for SVCS. Rowell and Gleeson performed the first systematic review of randomized and non-randomized studies evaluating different SVCS treatments for lung cancer patients. Stenting achieved resolution of symptoms (complete or partial) in 24 to 72 hours, whereas chemotherapy or RT required 7 days to 3 weeks. Furthermore, 95% of patients reported symptomatic relief after stenting, but only 59% to 83% of SVCS patients receiving chemotherapy or RT improved. The clinical benefit rates of chemotherapy or RT were higher in patients with SCLC compared to NSCLC, likely related to the greater sensitivity to these treatments in SCLC. Additionally, 10.7% of patients who received stenting developed a recurrent obstruction due to stent thrombosis or tumour ingrowth after a median of 1 to 2 months, but patency was regained with repeat stenting or thrombolysis in most of these patients. This resulted in a primary patency rate of 84.3% and a secondary patency rate of 92.5%. On the other hand, the SVCS relapse rates were up to 18.5% for patients treated with chemotherapy or RT (28).
To date, there is only one randomised controlled phase III study studying the benefit of stenting in SVCS patients. This Japanese study randomised just 32 SVCS and inferior vena cava obstruction patients of various tumour histologies to stenting (test group) or any kind of treatment except stenting (control group). Treatments received by the control group include anticoagulation, diuretics, albumin infusions, physical therapies, chemotherapy, and RT. The cohorts were compared using investigator-designed symptom questionnaires and the standardised quality of life tools EQ-5D and SF-8. Symptom scores were assessed before treatment, on day 1 after treatment then weekly for 4 weeks. Quality of life was evaluated twice before enrollment and then weekly after treatment for 4 weeks. This trial showed that patients who received stenting had significantly greater improvements in symptom scores compared to the control group, but no differences in the quality of life evaluations. However, only 25% of the control arm received RT and/or chemotherapy as treatment for SVCS. This may have overestimated the benefit of stenting compared with the control arm (29).
Three systematic reviews and one literature review on endovascular stenting confirmed the high clinical success rates of stenting in Rowell and Gleeson’s study. The literature review of Léon et al. focused specifically on patients with malignant SVCS (30), whereas the systematic reviews included SVCS patients with both benign and malignant causes (19,20,31). The average clinical success rates were above 90% for all studies (19,20,30,31). Symptom recurrence rates after stenting were around only 10% (19,20). The main causes of recurrence were in-stent thrombosis, tumour in-growth and tumour overgrowth above or below the stent (20). Léon et al. compared the efficacy of different types of stents in patients with malignant SVCS. While clinical effectiveness was not statistically different across the various types of stents, Graft stents and Nitinol stents had significantly higher primary patency rates than Wallstents (Graft stents 96.10%, Nitinol stents 94.87%, Wallstents 83.38%, P=0.01) (30). Repeat interventions were successful in most patients who recurred, giving average secondary patency rates from 94.1% to 96.1% (19,30).
Contraindications and risks of stenting
Stenting can be performed in most patients presenting with SVCS. The technical success of stent deployment is close to 100% (19,20). The only relative technical contraindication is when a patient is not able to lie flat for the procedure (13). In patients with SVC thrombus where thrombolysis is planned, the decision to proceed with the procedure needs to be carefully discussed with patients having high risks of bleeding, for example, those having intact mucosal primaries, hemorrhagic brain metastases, or receiving highly myelosuppressive regimens. Clinicians also need to be careful when selecting patients with cardiovascular comorbidities for stenting, as they may be at risk of cardiac arrhythmias and acute pulmonary edema when the venous return from the SVC to the right atrium is rapidly restored after treatment (32).
Overall, complications of SVC stenting are low. The mean complication rates reported in the four reviews ranged from 5.8% to 8.3% (19,20,30,31). A large proportion of studies reported no complications (10 out of 32 in Azizi et al. and 25 out of 54 in Aung et al.) (19,20). Early complications include local pain, hematoma, local wound infection, whereas less common side effects include cardiac arrhythmias, acute pulmonary edema, and respiratory distress. Long-term complications include stent thrombosis, stent migration, bleeding events from anticoagulation, and thromboembolic events. Procedure-related mortality was uncommon, ranging from 0.6% to 3.0% (19,20,30,31). The most common cause of death is SVC perforation resulting in cardiac tamponade and hemoptysis (19,30). It should be highlighted that the pooled complication rates presented in the systematic reviews also included patients with benign SVCS. The risks of major complications may be higher in a patient series with malignant SVCS only, as cancer patients can be more prone to bleeding and thrombosis (33,34).
While in-stent thrombosis can be salvaged with thrombolysis or thrombectomy, and risks can be mitigated with long-term anticoagulation, stent migration into the right atrium is a fearful long-term complication, as fatal outcomes have been reported (35-37). Open heart surgery was previously required to treat this complication, but advances in endovascular techniques have allowed successful retrieval and repeat stenting with less morbidity (38). The risks of stent migration can be minimised by careful evaluation of the venous anatomy before the procedure and by choosing appropriate lengths of stents to bypass the obstruction by experienced operators (38). The decision to proceed with stenting should be carefully evaluated in patients with tumours that have a high chance of completely resolving after oncological treatments as they have an increased risk of stent migration (38).
Table 1 summarises the benefits, relative technical contraindications and risks of stenting for the treatment of SVCS.
Table 1
| Benefits of stenting | Relative technical contraindications of stenting | Patient groups with higher risks of complications | Complications of stenting |
|---|---|---|---|
| Rapid symptom resolution compared to RT and chemotherapy | Patients who cannot lie flat | Patients with high bleeding risks | Short term: |
| • Local pain | |||
| • Hematoma | |||
| High clinical success rates (>90%) | Patients with cardiovascular comorbidities | • Local wound infection | |
| • Cardiac arrhythmias | |||
| • Acute pulmonary edema | |||
| Low risks of complications (<10%) and procedure-related deaths (<1%) | Patients having tumours with high chances of complete response after oncological treatments | Long-term: | |
| • Stent thrombosis | |||
| • Stent migration | |||
| Restenosis can be successfully salvaged with repeat stenting | • Bleeding events from anticoagulation | ||
| • Thromboembolic events |
SVCS, superior vena cava syndrome; RT, radiotherapy.
Advances in oncological treatments and considerations in first-line stenting
While the procedure of SVC stenting has not changed much since its initial development, RT techniques and systemic treatments have vastly evolved in the last two decades. Therefore, there is a need to review whether these changes would affect clinicians’ decision to consider stenting as an initial treatment.
Advances in radiation techniques
The benefit of radiation in treating patients with SVCS was first reported in the 1970s (39). At that time, RT was prescribed to the mediastinum using simple parallel opposed fields under X-ray guidance. Initial success was reported using a starting dose of 4 Gy for 3 daily fractions, then 1.5 Gy daily fractions to a total dose of 30 Gy (39). Subsequently, multiple dose schedules were used in retrospective series including 30 Gy in 10 daily fractions, 20 Gy in 5 daily fractions and 8 to 10 Gy in a single fraction (40,41). No randomised studies were performed to demonstrate the superiority of one radiation schedule over another. Armstrong et al. showed that a higher radiation dose was associated with higher rates of clinical response (42).
RT techniques have undergone major changes in the last few decades with the development of computerised tomography (CT) guided simulation and computerised inverse planning using intensity-modulated RT (IMRT). These allow radiation doses to conform to irregular, concave treatment targets better while minimising dose to the organs at risk (43). In a retrospective study of NSCLC patients with SVCS, T staging, extent of disease and presence of metastatic disease were not associated with poorer survival in multivariate analysis. Performance status was the most critical prognostic factor for overall survival (44). Therefore, SVCS patients having localised disease should be offered radical treatment with advanced radiation techniques if they have a good performance status (44).
IMRT is increasingly used for RT planning for stage III NSCLC, as clinical studies have shown that it results in lower rates of severe pneumonitis and cardiac doses, better compliance to chemotherapy and better preservation of quality of life compared to 3D-conformal RT (45,46). This is of increasing importance as patients treated with chemoradiation are surviving longer with the integration of consolidative durvalumab as standard of care (47). They are, hence, more susceptible to normal tissue complications such as pulmonary fibrosis and ischemic heart disease in the long term (48). Tighter planning target volume margins for IMRT and treatment planning with 4D-CT and on-treatment image guidance with cone beam CT are recommended to ensure that treatment targets are covered at different positions of the breathing cycle (49). A dedicated quality assurance program is also indicated to ensure treatment accuracy (49).
For the above reasons, advanced modalities have the potential to require patients to be on the treatment couch for longer period of time. For stage III NSCLC patients presenting with SVCS, stenting before RT has the benefit of immediate symptomatic relief and allows patients to go through these sophisticated planning procedures without compromising safety. Additionally, stenting allows patients to be treated more safely with cisplatin-based chemotherapy, which requires a large volume of saline hydration to reduce risks of nephrotoxicity (50). Stent migration is of less concern for stage III NSCLC patients receiving concurrent chemoradiation as complete response rates after treatment are very low. In the PACIFIC trial, the complete response rate after chemoradiation was only 2.2%, while 47.1% of patients achieved stable disease. After being treated with durvalumab, only 28.5% of patients further responded (51).
Stereotactic body radiation therapy (SBRT), which was first developed in the 1990s, has revolutionised the field of radiation oncology. Indications of its use continue to expand in both localised and metastatic settings. SBRT is characterised by patient immobilisation and control of organ motion to give ablative doses of radiation to treatment targets in a single or few fractions (52). SBRT has become the standard of care for medically inoperable early-stage NSCLC after multiple phase trials demonstrated its benefit over conventionally fractionated RT (53). This technique was initially used in peripheral lesions, as a phase II study showed that centrally located tumours had an 11-fold higher risk of severe toxicity compared to peripheral tumours (54). With improved techniques in image guidance and modifications of radiation schedules, many patients with central tumours, defined as lesions within 2 cm of the proximal bronchial tree, can be treated safely with hypofractionation to a high dose per fraction as well (55-57). SBRT also has increased applications in patients with metastatic disease to provide local control to oligometastatic lung and mediastinal lymph node metastases following the publication of the SABR-COMET trial, which showed a significant survival benefit from the addition of SBRT compared to standard of care alone (58). For patients with SVCS caused by small tumours in the mediastinum, SBRT can be a feasible definitive treatment option in well-selected patients.
Similar to IMRT, SBRT often requires a longer planning and treatment time per fraction compared to 3D-conformal RT to ensure high accuracy. Patients also need to have stable or controlled breathing cycles during treatment to avoid geographical misses of the treatment target (59). This may be difficult to achieve for dyspneic SVCS patients who have trouble lying flat for extended periods. Stabilising patients’ symptoms with stenting first is often preferred to ensure that patients can have a reproducible set up every fraction, and to prevent exacerbation of symptoms from the radiation-induced edema caused by high doses of RT (41). If stenting was not performed but eventually required due to symptom exacerbation between fractions, the SBRT treatment often needs to be replanned to account for the dosimetric changes after stent insertion. This would prolong patients’ overall treatment time.
Currently, there is one case report in the literature of a patient with SVCS treated with SBRT, an elderly gentleman with a localised squamous cell carcinoma of the lung located at the right paratracheal region. He was treated with SBRT to a dose of 50 Gy in 5 alternate-day fractions. He achieved symptomatic improvement already after the second fraction, and this benefit from SBRT persisted at follow-up three months after treatment (60). This patient likely had a slow-growing tumour, as evidenced by the well-formed venous collaterals in his diagnostic CT. This allowed him to tolerate the SBRT set-up and treatment without prior stent insertion. In more symptomatic patients, this could be challenging.
Stenting compliments the sophisticated RT techniques—including IMRT, SBRT and even proton therapy—when radical treatment is planned for SVCS patients. The sequence of stenting followed by RT appears safe with no additional complications in a retrospective case series, but most patients were treated with palliative radiation doses of up to 40 to 50 Gy (61). Prospective studies are required to evaluate its safety when treating with hypofractionation radiation regimens to high total doses.
Advances in systemic treatments
SVCS patients with tumours that are chemo- or radiosensitive, such as lymphomas, germ cell tumours and small cell lung cancer, have been conventionally treated with chemotherapy or radiation instead of endovascular stenting (3,13). This is especially the case for patients planning for radical intent treatments, as the long-term risks of having stents in situ are higher. Stenting is most commonly reserved as salvage after initial treatments have failed (13). It is unclear, to date, if this paradigm may shift with the development of immunotherapy in chemotherapy-refractory tumours.
Aggressive non-Hodgkin B-cell lymphoma patients who are resistant to first-line chemo-immunotherapy and salvage autologous hematopoietic transplant historically have had an extremely poor prognosis. If these patients developed SVCS, stenting would be a logical treatment option given their short-term survival and resistance to conventional therapies. Promising treatment results of chimeric antigen receptor T-cell (CAR-T) therapy may change this approach. CAR-T therapy involves harvesting T-cells from patients via leukapheresis for genetic engineering to target tumour cells and then infuse them back into patients. In the phase I/II ZUMA-1 study investigating the anti-CD19 CAR-T therapy axicabtagene ciloleucel (axi-cel), the objective response rate in chemotherapy-refractory lymphoma patients was 83%, with 58% achieving a complete response (62). At a median follow-up of 51.1 months, the 4-year overall survival rate was 44%, suggesting that a proportion of patients in this poor prognostic group may achieve long-term survival with CAR-T therapy (63). It is important to note that CAR-T therapy requires a cell-manufacturing process that takes a median of 15 to 33 days (64). Bridging therapy can be indicated for SVCS patients planned for CAR-T therapy to avoid symptomatic progression. As prolonged myelosuppression is not uncommon with CAR-T therapy (65), stenting is not preferred before treatment to minimise risks of wound complications. RT is an effective and safe bridging treatment for this patient group (66).
Immunotherapy has also produced promising long-term results in advanced NSCLC, but with different response patterns than CAR-T therapy in chemotherapy-refractory lymphoma. For NSCLC patients without driver mutations, failure of platinum-based chemotherapy historically carried a poor prognosis. Second-line chemotherapy with docetaxel is associated with high rates of toxicity, and most patients succumbed to their disease within 1 year (67). With the development of immune checkpoint inhibitors (ICIs), 10–20% of patients can achieve long-term survival with up to five years of follow-up (68,69).
However, these drugs carry a low overall response rate of only 10% to 25% despite the ability to produce long durations of disease control in advanced NSCLC (68,69). Tumours in a small proportion of patients may even progress in size due to immune cell infiltration before subsequent treatment response, a phenomenon referred to as pseudoprogression (70). Patients with SVCS treated with ICIs may not be able to achieve symptomatic relief, and thus other modalities are required for initial treatment. Given the time required for treatment response for RT, high-dose steroids are commonly used until patients have symptomatic improvement. Administering high-dose steroids with immunotherapy is counter-intuitive, and studies have shown that their concurrent administration is associated with poorer treatment outcomes (71). For this reason, giving RT to SVCS patients who are planned for immunotherapy can delay the use of this highly effective systemic treatment. Upfront stenting, on the other hand, avoids this issue while providing rapid relief of SVCS symptoms.
Another important development in the treatment of advanced NSCLC is tyrosine kinase inhibitors (TKIs). Patients newly diagnosed with NSCLC are routinely tested for driver mutations to see if they can benefit from these effective treatments before considering options of cytotoxic chemotherapy or immunotherapy. In the first-line setting, TKIs provide high response rates of 80% or greater with a durable disease control of 1.5 to 3 years for the more common epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK) mutations (72,73). SVCS patients with driver mutations may first be treated with TKIs and observed closely for response in symptoms. Since the time to tumour response with TKIs can be up to 3 weeks or more (74), some patients with more intensive symptoms may need additional treatments while awaiting TKI treatment response. With a higher rate of pneumonitis observed when TKIs are given concurrently with RT (75,76), some authors recommend withholding them during thoracic RT and only resuming after completion (75). For patients with potentially life-threatening extrathoracic metastases, stenting can be a better option than RT as it provides rapid symptomatic relief and avoids interruption of TKI.
The benefits of novel systemic treatments, however, have not consistently been observed with certain tumour subtypes such as platinum-resistant small cell lung cancer (77). Maximising quality of life is of utmost importance in patients with poor prognoses. Clinicians should select treatments that have the least side effects and only require short hospital visits. Stenting provides a rapid resolution of symptoms through a single-day procedure, which is desirable for patients with only a few weeks to months to live. As seen in systematic reviews, the procedure is associated with a low incidence of complications, and stenting is often better tolerated and symptom response is more predictable compared to chemotherapy and RT. Additionally, it will not cause a transient flare of SVCS symptoms, as in the case of chemotherapy or RT (41,78,79). Based on the patient selection described above, long-term complications of stenting such as migration are often of a less concern as well since these patients may succumb to their disease before the complications arise.
With ongoing innovations in anticancer treatments, clinicians should regularly review the literature for the response rates, time to response and survival benefits of planned treatments rather than solely relying on tumour sensitivities to chemotherapy and radiation when deciding whether to proceed with stenting as first-line treatment for SVCS.
Limitations of current studies to assess patient outcomes and future directions in SVCS research
Conclusions from existing systematic reviews on SVCS treatments are mostly based on retrospective single-centre cohort studies that carry risks of publication bias (19,20,28). Due to their retrospective nature, most investigators measure the clinical benefit of SVCS treatments by reviewing whether patients reported their symptoms improved at follow-up. It is difficult to clearly document if there were any partially resolved symptoms, which might significantly impair patients’ quality of life. Also, the time to treatment effect could not be evaluated with this approach.
Nicholson et al. prospectively used an investigator-designed scoring system based on 10 SVCS symptoms and measured changes in scores before and after 48 hours of stenting (41). Likewise, Wilson et al. designed a patient-reported symptom diary card to assess the efficacy of treatments on a daily basis during treatment (80). While these measures could better assess patients’ changes in physical symptoms, they did not inquire about the effects of SVCS on patients’ emotional well-being and functional status, which were likely affected given the changes in appearance and mobility from upper body swelling due to SVCS.
The goal of SVCS treatment is for maximal symptomatic relief to maintain quality of life rather than preventing life-threatening complications as they are very rare. Looking into the future, efforts should be focused to devise a patient-reported outcome tool through patient and clinician interviews and validate it against standardised quality of life tools such as the EORTC QLQ-C30 or the 36-item Short Form Survey (SF-36). Such a scoring system will provide researchers with a standardised tool when planning prospective clinical trials and allow for better comparisons of treatment options during and after treatments. It will also identify the specific quality of life issues that SVCS patients have so clinicians can plan treatments tailored to their needs.
Since treatment strategies for SVCS depend greatly on patients’ tumour histology and oncological prognoses, future comparative studies should recruit patients of specific tumour subgroups, for example, stage III NSCLC planning for radical chemoradiation, to guide the optimal sequence of treatments. International collaboration in SVCS research is, therefore, highly important to expedite patient recruitment.
Conclusions
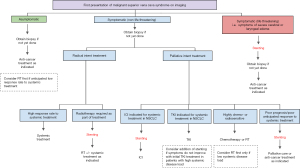
With an improved understanding of cancer biology, novel systemic treatments and advanced radiation techniques have improved survival outcomes for malignancies. This literature review highlights the implications of recent developments in oncological treatments on the decision to offer initial stenting for SVCS. A multitude of factors including response rates to treatment, the durability of treatment response, the technique of radiation employed and patients’ overall prognoses affect the decision to choose stenting as first-line treatment. We propose a pathway to guide clinical decision making based on these observations (Figure 1). Until more prospective studies are available to confirm the efficacy and safety of these treatment strategies, an individualised approach with multidisciplinary input from medical oncologists, haematologists, radiation oncologists and interventional radiologists is required to ensure SVCS patients receive a sequence of treatments that not only provide quick symptomatic relief but also durable disease control and the lowest possible treatment-related morbidity.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1293/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1293/coif). CBS serves as the Editor-in-Chief of Annals of Palliative Medicine. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wan JF, Bezjak A. Superior vena cava syndrome. Hematol Oncol Clin North Am 2010;24:501-13. [Crossref] [PubMed]
- Straka C, Ying J, Kong FM, et al. Review of evolving etiologies, implications and treatment strategies for the superior vena cava syndrome. Springerplus 2016;5:229. [Crossref] [PubMed]
- Ostler PJ, Clarke DP, Watkinson AF, et al. Superior vena cava obstruction: a modern management strategy. Clin Oncol (R Coll Radiol) 1997;9:83-9. [Crossref] [PubMed]
- Klein-Weigel PF, Elitok S, Ruttloff A, et al. Superior vena cava syndrome. Vasa 2020;49:437-48. [Crossref] [PubMed]
- Baker GL, Barnes HJ. Superior vena cava syndrome: etiology, diagnosis, and treatment. Am J Crit Care 1992;1:54-64. [Crossref] [PubMed]
- Yu JB, Wilson LD, Detterbeck FC. Superior vena cava syndrome--a proposed classification system and algorithm for management. J Thorac Oncol 2008;3:811-4. [Crossref] [PubMed]
- Patriarcheas V, Grammoustianou M, Ptohis N, et al. Malignant Superior Vena Cava Syndrome: State of the Art. Cureus 2022;14:e20924. [Crossref] [PubMed]
- Charnsangavej C, Carrasco CH, Wallace S, et al. Stenosis of the vena cava: preliminary assessment of treatment with expandable metallic stents. Radiology 1986;161:295-8. [Crossref] [PubMed]
- Lepper PM, Ott SR, Hoppe H, et al. Superior vena cava syndrome in thoracic malignancies. Respir Care 2011;56:653-66. [Crossref] [PubMed]
- Lanuti M, De Delva PE, Gaissert HA, et al. Review of superior vena cava resection in the management of benign disease and pulmonary or mediastinal malignancies. Ann Thorac Surg 2009;88:392-7. [Crossref] [PubMed]
- Kawaida T, Tanabe H, Kotani M, et al. Open surgical treatment of superior vena cava syndrome due to invasive thymoma. SAGE Open Med Case Rep 2022;10:2050313X221138652.
-
da Rosa GRS Takizawa N Schimidt D Tratamento cirúrgico da síndrome da veia cava superior causado por timoma invasivo. Rev Bras Cir Cardiovasc 2010 . doi: . - Watkinson AF, Yeow TN, Fraser C. Endovascular stenting to treat obstruction of the superior vena cava. BMJ 2008;336:1434-7. [Crossref] [PubMed]
- Uberoi R. Quality assurance guidelines for superior vena cava stenting in malignant disease. Cardiovasc Intervent Radiol 2006;29:319-22. [Crossref] [PubMed]
- Kalra M, Sen I, Gloviczki P. Endovenous and Operative Treatment of Superior Vena Cava Syndrome. Surg Clin North Am 2018;98:321-35. [Crossref] [PubMed]
- Wang ZS, Li CW, Li JX, et al. Covered versus uncovered stent insertion for malignant superior vena cava obstruction. Minim Invasive Ther Allied Technol 2020;29:353-8. [Crossref] [PubMed]
- Gwon DI, Ko GY, Kim JH, et al. Malignant superior vena cava syndrome: a comparative cohort study of treatment with covered stents versus uncovered stents. Radiology 2013;266:979-87. [Crossref] [PubMed]
- Haddad MM, Simmons B, McPhail IR, et al. Comparison of Covered Versus Uncovered Stents for Benign Superior Vena Cava (SVC) Obstruction. Cardiovasc Intervent Radiol 2018;41:712-7. [Crossref] [PubMed]
- Azizi AH, Shafi I, Zhao M, et al. Endovascular therapy for superior vena cava syndrome: A systematic review and meta-analysis. EClinicalMedicine 2021;37:100970. [Crossref] [PubMed]
- Aung EY, Khan M, Williams N, et al. Endovascular Stenting in Superior Vena Cava Syndrome: A Systematic Review and Meta-analysis. Cardiovasc Intervent Radiol 2022;45:1236-54. [Crossref] [PubMed]
- Leung ST, Sung TH, Wan AY, et al. Endovascular stenting in the management of malignant superior vena cava obstruction: comparing safety, effectiveness, and outcomes between primary stenting and salvage stenting. Hong Kong Med J 2015;21:426-34. [Crossref] [PubMed]
- Chaudhary K, Gupta A, Wadhawan S, et al. Anesthetic management of superior vena cava syndrome due to anterior mediastinal mass. J Anaesthesiol Clin Pharmacol 2012;28:242-6. [Crossref] [PubMed]
- Pullerits J, Holzman R. Anaesthesia for patients with mediastinal masses. Can J Anaesth 1989;36:681-8. [Crossref] [PubMed]
- Wong MK, Tam TC, Lam DC, et al. EBUS-TBNA in patients presented with superior vena cava syndrome. Lung Cancer 2012;77:277-80. [Crossref] [PubMed]
- Boily-Daoust C, Plante A, Adam C, et al. Performance and safety of diagnostic procedures in superior vena cava syndrome. ERJ Open Res 2021;7:00392-2020. [Crossref] [PubMed]
- Koegelenberg CF, Bolliger CT, Plekker D, et al. Diagnostic yield and safety of ultrasound-assisted biopsies in superior vena cava syndrome. Eur Respir J 2009;33:1389-95. [Crossref] [PubMed]
- Hirsh J, Anand SS, Halperin JL, et al. Guide to anticoagulant therapy: Heparin: a statement for healthcare professionals from the American Heart Association. Circulation 2001;103:2994-3018. [Crossref] [PubMed]
- Rowell NP, Gleeson FV. Steroids, radiotherapy, chemotherapy and stents for superior vena caval obstruction in carcinoma of the bronchus: a systematic review. Clin Oncol (R Coll Radiol) 2002;14:338-51. [Crossref] [PubMed]
- Takeuchi Y, Arai Y, Sone M, et al. Evaluation of stent placement for vena cava syndrome: phase II trial and phase III randomized controlled trial. Support Care Cancer 2019;27:1081-8. [Crossref] [PubMed]
- Léon D, Rao S, Huang S, et al. Literature Review of Percutaneous Stenting for Palliative Treatment of Malignant Superior Vena Cava Syndrome (SVCS). Acad Radiol 2022;29:S110-20. [Crossref] [PubMed]
- Kordzadeh A, Askari A, Hanif MA, et al. Superior Vena Cava Syndrome and Wallstent: A Systematic Review. Ann Vasc Dis 2022;15:87-93. [Crossref] [PubMed]
- Nguyen NP, Borok TL, Welsh J, et al. Safety and effectiveness of vascular endoprosthesis for malignant superior vena cava syndrome. Thorax 2009;64:174-8. [Crossref] [PubMed]
- Mantha S. Bleeding Disorders Associated with Cancer. Cancer Treat Res 2019;179:191-203. [Crossref] [PubMed]
- Lip GY, Chin BS, Blann AD. Cancer and the prothrombotic state. Lancet Oncol 2002;3:27-34. [Crossref] [PubMed]
- Anand G, Lewanski CR, Cowman SA, et al. Superior vena cava stent migration into the pulmonary artery causing fatal pulmonary infarction. Cardiovasc Intervent Radiol 2011;34:S198-201. [Crossref] [PubMed]
- Dinkel HP, Mettke B, Schmid F, et al. Endovascular treatment of malignant superior vena cava syndrome: is bilateral wallstent placement superior to unilateral placement? J Endovasc Ther 2003;10:788-97. [Crossref] [PubMed]
- Sobrinho G, Aguiar P. Stent placement for the treatment of malignant superior vena cava syndrome - a single-center series of 56 patients. Arch Bronconeumol 2014;50:135-40. [Crossref] [PubMed]
- Taylor JD, Lehmann ED, Belli AM, et al. Strategies for the management of SVC stent migration into the right atrium. Cardiovasc Intervent Radiol 2007;30:1003-9. [Crossref] [PubMed]
- Davenport D, Ferree C, Blake D, et al. Radiation therapy in the treatment of superior vena caval obstruction. Cancer 1978;42:2600-3. [Crossref] [PubMed]
- Ampil F, Caldito G, Previgliano C. Palliative radiotherapy for superior vena caval obstruction by lung cancer: A major issue about timing and a minor issue about efficacy. Ann Thorac Med 2012;7:170-1. [Crossref] [PubMed]
- Nicholson AA, Ettles DF, Arnold A, et al. Treatment of malignant superior vena cava obstruction: metal stents or radiation therapy. J Vasc Interv Radiol 1997;8:781-8. [Crossref] [PubMed]
- Armstrong BA, Perez CA, Simpson JR, et al. Role of irradiation in the management of superior vena cava syndrome. Int J Radiat Oncol Biol Phys 1987;13:531-9. [Crossref] [PubMed]
- Taylor A, Powell ME. Intensity-modulated radiotherapy--what is it? Cancer Imaging 2004;4:68-73. [Crossref] [PubMed]
- Rodrigues G, Chu K. Prognostic Indicators of Outcome in Superior Vena Cava Obstruction in Lung Cancer. Int J Radiat Oncol Biol Phys 2007;69:S185. [Crossref]
- Movsas B, Hu C, Sloan J, et al. Quality of Life Analysis of a Radiation Dose-Escalation Study of Patients With Non-Small-Cell Lung Cancer: A Secondary Analysis of the Radiation Therapy Oncology Group 0617 Randomized Clinical Trial. JAMA Oncol 2016;2:359-67. [Crossref] [PubMed]
- Chun SG, Hu C, Choy H, et al. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J Clin Oncol 2017;35:56-62. [Crossref] [PubMed]
- Spigel DR, Faivre-Finn C, Gray JE, et al. Five-year survival outcomes with durvalumab after chemoradiotherapy in unresectable stage III NSCLC: An update from the PACIFIC trial. J Clin Oncol 2021;39:8511. [Crossref]
- Simone CB 2nd. Thoracic Radiation Normal Tissue Injury. Semin Radiat Oncol 2017;27:370-7. [Crossref] [PubMed]
- Chan C, Lang S, Rowbottom C, et al. Intensity-modulated radiotherapy for lung cancer: current status and future developments. J Thorac Oncol 2014;9:1598-608. [Crossref] [PubMed]
- Crona DJ, Faso A, Nishijima TF, et al. A Systematic Review of Strategies to Prevent Cisplatin-Induced Nephrotoxicity. Oncologist 2017;22:609-19. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Guckenberger M, Andratschke N, Alheit H, et al. Definition of stereotactic body radiotherapy: principles and practice for the treatment of stage I non-small cell lung cancer. Strahlenther Onkol 2014;190:26-33. [Crossref] [PubMed]
- Videtic GMM, Donington J, Giuliani M, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: Executive Summary of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol 2017;7:295-301. [Crossref] [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [Crossref] [PubMed]
- Rowe BP, Boffa DJ, Wilson LD, et al. Stereotactic body radiotherapy for central lung tumors. J Thorac Oncol 2012;7:1394-9. [Crossref] [PubMed]
- Lindberg K, Grozman V, Karlsson K, et al. The HILUS-Trial-a Prospective Nordic Multicenter Phase 2 Study of Ultracentral Lung Tumors Treated With Stereotactic Body Radiotherapy. J Thorac Oncol 2021;16:1200-10. [Crossref] [PubMed]
- Lodeweges JE, van Rossum PSN, Bartels MMTJ, et al. Ultra-central lung tumors: safety and efficacy of protracted stereotactic body radiotherapy. Acta Oncol 2021;60:1061-8. [Crossref] [PubMed]
- Palma DA, Olson R, Harrow S, et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J Clin Oncol 2020;38:2830-8. [Crossref] [PubMed]
- Tsang MW. Stereotactic body radiotherapy: current strategies and future development. J Thorac Dis 2016;8:S517-27. [Crossref] [PubMed]
- McKenzie JT, McTyre E, Kunaprayoon D, et al. Stereotactic body radiotherapy for superior vena cava syndrome. Rep Pract Oncol Radiother 2013;18:179-81. [Crossref] [PubMed]
- Lanciego C, Pangua C, Chacón JI, et al. Endovascular stenting as the first step in the overall management of malignant superior vena cava syndrome. AJR Am J Roentgenol 2009;193:549-58. [Crossref] [PubMed]
- Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med 2017;377:2531-44. [Crossref] [PubMed]
- Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol 2019;20:31-42. [Crossref] [PubMed]
- Lin X, Lee S, Sharma P, et al. Summary of US Food and Drug Administration Chimeric Antigen Receptor (CAR) T-Cell Biologics License Application Approvals From a Statistical Perspective. J Clin Oncol 2022;40:3501-9. [Crossref] [PubMed]
- Cordeiro A, Bezerra ED, Hirayama AV, et al. Late Events after Treatment with CD19-Targeted Chimeric Antigen Receptor Modified T Cells. Biol Blood Marrow Transplant 2020;26:26-33. [Crossref] [PubMed]
- Pinnix CC, Gunther JR, Dabaja BS, et al. Bridging therapy prior to axicabtagene ciloleucel for relapsed/refractory large B-cell lymphoma. Blood Adv 2020;4:2871-83. [Crossref] [PubMed]
- Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000;18:2095-103. [Crossref] [PubMed]
- Borghaei H, Gettinger S, Vokes EE, et al. Five-Year Outcomes From the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non-Small-Cell Lung Cancer. J Clin Oncol 2021;39:723-33. [Crossref] [PubMed]
- Herbst RS, Garon EB, Kim DW, et al. Five Year Survival Update From KEYNOTE-010: Pembrolizumab Versus Docetaxel for Previously Treated, Programmed Death-Ligand 1-Positive Advanced NSCLC. J Thorac Oncol 2021;16:1718-32. [Crossref] [PubMed]
- Onesti CE, Frères P, Jerusalem G. Atypical patterns of response to immune checkpoint inhibitors: interpreting pseudoprogression and hyperprogression in decision making for patients' treatment. J Thorac Dis 2019;11:35-38. [Crossref] [PubMed]
- Petrelli F, Signorelli D, Ghidini M, et al. Association of Steroids use with Survival in Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers (Basel) 2020;12:546. [Crossref] [PubMed]
- Mok T, Camidge DR, Gadgeel SM, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol 2020;31:1056-64. [Crossref] [PubMed]
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 2020;382:41-50. [Crossref] [PubMed]
- Imai H, Shukuya T, Takahashi T, et al. Comparison of the time-to-response between radiotherapy and epidermal growth factor receptor--tyrosine kinase inhibitors for advanced non-small cell lung cancer with EGFR mutation. Anticancer Res 2013;33:3279-84. [PubMed]
- Jia W, Gao Q, Wang M, et al. Overlap time is an independent risk factor of radiation pneumonitis for patients treated with simultaneous EGFR-TKI and thoracic radiotherapy. Radiat Oncol 2021;16:41. [Crossref] [PubMed]
- Jia W, Guo H, Jing W, et al. An especially high rate of radiation pneumonitis observed in patients treated with thoracic radiotherapy and simultaneous osimertinib. Radiother Oncol 2020;152:96-100. [Crossref] [PubMed]
- Spigel DR, Vicente D, Ciuleanu TE, et al. Second-line nivolumab in relapsed small-cell lung cancer: CheckMate 331 Ann Oncol 2021;32:631-41. [Crossref] [PubMed]
- Maddox AM, Valdivieso M, Lukeman J, et al. Superior vena cava obstruction in small cell bronchogenic carcinoma. Clinical parameters and survival. Cancer 1983;52:2165-72. [Crossref] [PubMed]
- Kane RC, Cohen MH. Superior vena caval obstruction due to small-cell anaplastic lung carcinoma. Response to chemotherapy. JAMA 1976;235:1717-8. [Crossref] [PubMed]
- Wilson P, Bezjak A, Asch M, et al. The difficulties of a randomized study in superior vena caval obstruction. J Thorac Oncol 2007;2:514-9. [Crossref] [PubMed]


