
Efficacy and safety of naldemedine treatment for opioid-induced constipation in gastrointestinal cancer: a retrospective analysis
Highlight box
Key findings
• The response rate was 63.6%, and diarrhea was the predominant gastrointestinal symptom.
What is known and what is new?
• Although patients with gastrointestinal cancer were included in prospective trials, these trials did not focus on gastrointestinal cancer.
• Naldemedine is effective and safe in clinical practice for treating OIC in patients with gastrointestinal cancer.
What is the implication, and what should change now?
• Naldemedine is an effective OIC treatment with manageable side effects that can be used in patients with gastrointestinal cancer. However, further research is required to highlight its role in clinical practice, as this investigation was a retrospective study.
Introduction
Approximately 19.3 million new cancer cases and 10 million cancer deaths occurred globally in 2020 (1). Female breast cancer surpassed lung cancer as the most commonly diagnosed cancer, with an estimated 2.3 million new cases (11.7%), followed by lung (11.4%), colorectal (10.0%), prostate (7.3%), and stomach (5.6%) cancers (2), indicating that gastrointestinal (GI) cancers are among the most prevalent cancers. Cancer treatment includes multidisciplinary therapies such as surgery, drug therapy, and radiation therapy, accompanied by pain management. Opioid analgesics are used to alleviate cancer-induced pain. However, opioid analgesics often cause constipation, nausea, vomiting, and drowsiness. Nausea and drowsiness induce drug tolerance, whereas prolonged constipation (3-5) increases the risk of GI symptoms and delirium, reducing the quality of life and interfering with the use of analgesics (6,7). Therefore, controlling bowel movements is important. Opioid-induced constipation (OIC) is caused by peripheral µ-opioid receptors that inhibit GI peristalsis and promote water absorption from the GI tract (8,9). After the initiation of opioid treatment, OIC leads to altered defecation patterns and habits, and in some patients, it may overlap with fecal impaction with overflow incontinence and opioid-induced bowel dysfunction (reflux, nausea, bloating, etc.). Because µ-opioid receptors are present throughout the GI tract and symptoms are not limited to the colon, OIC is considered an opioid-induced bowel dysfunction, which includes constipation and a series of opioid-induced GI symptoms (10). The symptoms also include hard, dry stools, urinary urgency, sensation of incomplete evacuation, abdominal bloating, abdominal pain, increased stagnation and reflux of gastric contents, dry mouth, nausea, and vomiting (10,11). In a Japanese study, the OIC incidence was reported as 56% using Rome IV—the diagnostic criteria for OIC (12). OIC is treated with conventional constipation medications such as osmotic laxatives; if there is no improvement, naldemedine is recommended (13). The Japanese guidelines recommend peripheral µ-opioid receptor antagonists (PAMORA) for refractory OIC without opioid switching, conventional colorectal-stimulating, or osmotic laxative improvement (14). Naldemedine is a peripheral µ, δ, and κ opioid receptor antagonist that improves OIC. The efficacy and safety of naldemedine have been demonstrated in COMPOSE-4 and COMPOSE-5 trials (15). The other PAMORAs, methylnaltrexone and naloxegol, used in the United States and Europe were designed to act peripherally selectively by modifying naltrexone and naloxone. Methylnaltrexone contains a quaternary ammonium salt and naloxegol has a polyethylene glycol chain, and these unique substructures allow them to act in a peripherally selective manner. Naldemedin, on the other hand, is a new drug that combines high oral absorption and low cerebral translocation by introducing a carboxylic acid as a polar group into an existing naltrindole derivative, making it difficult for the drug to cross the blood-brain barrier (16). Although patients with colorectal cancer were included in the background of COMPOSE-4 and 5 and post-marketing surveillance (17), the analysis did not focus on GI cancer because of the small number of patients.
In the present study, we conducted a multicenter, retrospective analysis focusing on GI cancers to evaluate the effectiveness and clinical safety of naldemedine in patients with cancer who started opioid treatment and received naldemedine in clinical practice. We present this article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1130/rc).
Methods
We evaluated the clinical efficacy and safety of naldemedine administered to patients with GI cancer for the first time during hospitalization at ten Japanese institutions from June 2017 to August 2019. Patients with GI cancer who began opioids during hospitalization and were treated with naldemedine for the first time were included in the study. GI cancers included esophageal, gastric, small bowel, and colorectal cancers. Patients were identified from medical records. The inclusion criteria were as follows: patients who were pathologically or cytologically diagnosed with GI cancer, started naldemedine during hospitalization, and receiving naldemedine in combination with opioids. In addition, the patients who were hospitalized for at least seven days before and after administration of naldemedine and whose number of bowel movements was recorded in the chart were selected for the study. We identified 56 patients with GI cancer who were receiving opioids and who were administered naldemedine for the first time during hospitalization. Twenty-three patients who could not be evaluated for at least seven days before and after the initiation of naldemedine treatment were excluded. Overall, 33 patients evaluated for at least seven days before and after the initiation of naldemedine treatment were included (Figure S1). Patients who discontinued treatment within a week of naldemedine administration are also important in safety analysis, and even if discontinuation occurred within a week, adverse events and defecation frequency in the week after naldemedine initiation were counted and evaluated as a whole. The data for the 33 patients included in this analysis are part of the data in a previously published study (18). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics Committee of Gunma Prefectural Cancer Center (No. 405-31046; approval date: September 27, 2019) and approved by the Institutional Review Board of each participating institution. Because this was a retrospective study, the need for informed consent requirement was waived. However, the opportunity to refuse participation through an opt-out method was guaranteed.
Treatment
Treatment included naldemedine (0.2 mg/dose/day) combined with opioids. It was continued until the attending physician ordered its discontinuation due to toxicity or other reasons or until the patient provided consent for treatment.
Assessment of treatment efficacy and safety
In this study, the number of bowel movements (times/week) was evaluated for seven days before and after naldemedine treatment. Patients who had three or more bowel movements/week and an increase in one or more bowel movements/week from the baseline for seven days after the start of naldemedine administration were defined as responders. The number of stools per week before naldemedine administration was the baseline. Adverse events were assessed using the Common Terminology Criteria for Adverse Events version 5.0.
Statistical analyses
Normality, homoscedasticity, and correspondence between the two groups were verified using the Wilcoxon signed rank test. Results are expressed as 95% CIs, and differences were considered statistically significant with a two-sided P value ≤0.05. All analyses were performed using GraphPad Prism for Windows, version 8.0 (GraphPad Software, San Diego, CA, USA).
Results
Patient characteristics
Details of the enrolled 33 patients with GI cancer are presented in Table 1. There were 19 males (57.6%) and 14 females (42.4%). The median age was 71 years (53–88 years); 13 (39.4%), 7 (21.1%), and 13 (39.4%) patients had a performance status (PS) of 0–1, 2, and 3–4, respectively. Moreover, 25 of the 33 patients died from disease progression during the analysis period; 15 (45.5%) and 14 (42.4%) patients had gastric and colorectal cancer, respectively, accounting for nearly 88% of the total cases. In total, 10 patients (30.3%) received chemotherapy, and 21 (63.6%) received the best supportive care alone. Nineteen patients (57.6%) underwent abdominal surgery before receiving naldemedine. The median body mass index was 19.4 kg/m2, and 4 (12.1%) had diabetes mellitus. Laxatives were used before naldemedine treatment in 25 patients (75.8%). Magnesium oxide was used in 16 patients (48.5%), followed by sennosides in 10 patients (30.3%). Furthermore, 27 patients (81.8%) did not take concomitant anti-nausea medications after starting naldemedine treatment.
Table 1
| Characteristics | Patient (N=33) |
|---|---|
| Sex, n (%) | |
| Male | 19 (57.6) |
| Female | 14 (42.4) |
| Median age at treatment (years), range | 71 [53–88] |
| Performance status, n | |
| 0/1/2/3/4 | 3/10/7/9/4 |
| Primary tumor, n (%) | |
| Esophageal cancer | 3 (9.10) |
| Stomach cancer | 15 (45.5) |
| Small bowel cancer | 1 (3.00) |
| Colorectal cancer | 14 (42.4) |
| Therapy before and during naldemedine administration, n (%)*, ** | |
| Chemotherapy | 10 (30.3) |
| Radiotherapy | 1 (3.0) |
| Chemoradiotherapy | 1 (3.0) |
| Surgery | 0 |
| Best supportive care alone, n (%) | 21 (63.6) |
| Central nervous system metastases, n | |
| Yes | 2 |
| No | 31 |
| Cancerous peritonitis, n | |
| Yes | 8 |
| No | 25 |
| Gastrointestinal obstruction, n | |
| Yes | 0 |
| No | 33 |
| History of abdominal surgery before starting naldemedine administration, n | |
| Yes | 19 |
| No | 14 |
| History of radiation to the abdomen and pelvic region before starting naldemedine administration, n | |
| Yes | 2 |
| No | 31 |
| Presence of diabetes mellitus, n | |
| Yes | 4 |
| No | 29 |
| BMI (kg/m2) | |
| <22/≥22, n | 22/11 |
| Median BMI, range | 19.4 (13.7–34.8) |
| Discontinuation within 7 days, n | |
| Yes | 6 |
| No | 27 |
| Presence of laxatives before starting naldemedine administration, n | |
| Yes | 25 |
| No | 8 |
| Other laxatives continued after starting naldemedine administration, n | |
| Yes | 24 |
| No | 9 |
| Regular use of antiemetic medication after initiation of naldemedine administration, n | |
| Yes | 6 |
| No or unknown | 27 |
| Irregular use of antiemetic agents after starting naldemedine administration, n | |
| Yes | 7 |
| No or unknown | 26 |
| Survival status at data-cutoff date, n | |
| Death | 25 |
| Alive | 8 |
| Period to death from initiation of naldemedine administration | |
| Median period (days), range | 32 [7–522] |
*, within 3 weeks before starting naldemedine administration; **, total number of patients. BMI, body mass index.
Table 2 describes the use of opioids, laxatives, and antiemetics; 30 mg/day was the median oral morphine equivalent, and less than 20 mg/day was administered to 19 patients (57.6%). Nineteen patients (57.6%) received oxycodone. The number of days between opioid initiation and concomitant naldemedine use was less than eight days in seven patients (21.2%) and 8–29 days in 17 patients (51.5%).
Table 2
| Opioids, laxatives, and antiemetics | N (%) |
|---|---|
| Daily dose of opioids*, mg | |
| <20 | 19 (57.6) |
| 20–49 | 6 (18.2) |
| 50–99 | 6 (18.2) |
| ≥100 | 2 (6.1) |
| Regular use of opioids | |
| Oxycodone | 19 (57.6) |
| Morphine | 2 (6.1) |
| Fentanyl | 6 (18.2) |
| Hydromorphone | 3 (9.1) |
| Others | 2 (6.1) |
| No regular use | 1 (3.0) |
| Days from first opioid administration to initial naldemedine use | |
| <4 | 4 (12.1) |
| 4–7 | 3 (9.1) |
| 8–29 | 17 (51.5) |
| 30–99 | 7 (21.2) |
| ≥100 | 2 (6.1) |
| Drugs of concomitant laxatives** | |
| Magnesium oxide | 16 (48.5) |
| Sennoside | 10 (30.3) |
| Bisacodyl | 6 (18.2) |
| Lubiprostone | 3 (9.1) |
| Sodium picosulfate hydrate | 3 (9.1) |
| Sodium hydrogen carbonate, sodium dihydrogen phosphate suppository | 2 (6.1) |
| Others | 1 (3.0) |
| Drugs of concomitant antiemetic (regular and abbreviated use)** | |
| Metoclopramide | 3 (9.1) |
| Domperidone | 3 (9.1) |
| Prochlorperazine | 4 (12.1) |
| Olanzapine | 3 (9.1) |
| Others | 0 |
| No use | 27 (81.8) |
*, oral morphine equivalent to regular opioids; **, total number of patients.
Treatment efficacy
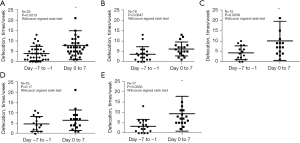
As displayed in Figure 1, 21 (63.6%, 95% CI: 46.6–77.9%) patients were responders, and 12 were non-responders. The median number of bowel movements during seven days before and after naldemedine treatment was 3 (range, 0–13) and 7 (range, 1–39), respectively, in the overall population, with a significant increase in defecation frequency after naldemedine administration (Wilcoxon signed rank test, P=0.0013, Figure 2A). Moreover, the number of bowel movements in the upper (esophagus and stomach) and lower GI tracts (small and colorectal) were also analyzed separately. The median number of bowel movements during the seven days before and after naldemedine treatment was three (range, 0–13) and 5.5 (range, 2–13) in the upper GI tract, respectively, showing a trend toward increased defecation frequency after naldemedine administration (Wilcoxon signed rank test, P=0.0647, Figure 2B). The median number of bowel movements during the seven days before and after naldemedine treatment was four (range, 0–10) and seven (range, 1–39), respectively, in the lower GI tract, with a significant increase in defecation frequency after naldemedine administration (Wilcoxon signed rank test, P=0.0056, Figure 2C). Moreover, the number of bowel movements at lower (<30 mg/day of morphine equivalent) and higher opioid dosages (≥30 mg/day of morphine equivalent) were also analyzed separately. The median number of bowel movements during the seven days before and after naldemedine treatment was 4 (range, 0–11) and 6 (range, 1–21) at lower opioid dosages, respectively, without a significant increase in defecation frequency after naldemedine administration (Wilcoxon signed rank test, P=0.17, Figure 2D). The median number of bowel movements during the seven days before and after naldemedine treatment was 3 (range, 0–13) and seven (range, 2–39), respectively, at higher opioid dosages, with a significant increase in defecation frequency after naldemedine administration (Wilcoxon signed rank test, P=0.0033, Figure 2E).
Safety
We examined adverse events, and those judged to be causally related to naldemedine administration are listed in Table 3. In the overall population (Table 3, Part a), diarrhea was the predominant GI symptom, with 13 (39.4%) patients experiencing grade 1 adverse events. Other grade 1 adverse events were negligible: abdominal pain in two patients, nausea in two patients, and anorexia in one patient. No patient experienced grade 4 or higher adverse events. Moreover, adverse events in the upper (esophagus and stomach) and lower GI tracts (small and colorectal; Table 3, Part b,c) and at lower (<30 mg/day of morphine equivalent) and higher opioid dosages (≥30 mg/day of morphine equivalent) were also demonstrated separately (Table 3, Part d,e).
Table 3
| Adverse events* | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| a. Adverse events in the overall population (N=33) | ||||
| Diarrhea | 13 | 3 | 0 | 0 |
| Abdominal pain | 2 | 0 | 0 | – |
| Nausea | 2 | 0 | 0 | – |
| Anorexia | 1 | 0 | 0 | 0 |
| Vomiting | 0 | 0 | 0 | 0 |
| Fatigue | 1 | 0 | 0 | – |
| b. Adverse events in upper gastrointestinal patients (N=18) | ||||
| Diarrhea | 7 | 3 | 0 | 0 |
| Abdominal pain | 1 | 0 | 0 | – |
| Nausea | 2 | 0 | 0 | – |
| Anorexia | 1 | 0 | 0 | 0 |
| Vomiting | 0 | 0 | 0 | 0 |
| Fatigue | 1 | 0 | 0 | – |
| c. Adverse events in lower gastrointestinal patients (N=15) | ||||
| Diarrhea | 6 | 0 | 0 | 0 |
| Abdominal pain | 1 | 0 | 0 | – |
| Nausea | 0 | 0 | 0 | – |
| Anorexia | 0 | 0 | 0 | 0 |
| Vomiting | 0 | 0 | 0 | 0 |
| Fatigue | 0 | 0 | 0 | – |
| d. Adverse events at lower opioid dosages (<30 mg/day of morphine equivalent) (N=16) | ||||
| Diarrhea | 3 | 2 | 0 | 0 |
| Abdominal pain | 2 | 0 | 0 | – |
| Nausea | 1 | 0 | 0 | – |
| Anorexia | 1 | 0 | 0 | 0 |
| Vomiting | 0 | 0 | 0 | 0 |
| Fatigue | 1 | 0 | 0 | – |
| e. Adverse events at higher opioid dosages (≥30 mg/day of morphine equivalent) (N=17) | ||||
| Diarrhea | 10 | 1 | 0 | 0 |
| Abdominal pain | 0 | 0 | 0 | – |
| Nausea | 1 | 0 | 0 | – |
| Anorexia | 0 | 0 | 0 | 0 |
| Vomiting | 0 | 0 | 0 | 0 |
| Fatigue | 0 | 0 | 0 | – |
*, adverse events were graded using the Common Terminology Criteria for Adverse Events version 5.0.
Clinical factors influencing treatment response
Finally, multivariate logistic regression analysis was performed to assess the relationship between naldemedine efficacy and various clinical factors, as shown in Table 4. There were no statistically significant differences in the efficacy of naldemedine with respect to age, PS, or daily opioid dose in oral morphine equivalents.
Table 4
| Variables | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Age (years) | |||
| <75/≥75 | 0.56 | 0.10–2.86 | 0.48 |
| Performance status | |||
| 0–2/≥3 | 4.35 | 0.82–34.5 | 0.08 |
| Daily opioid dose in oral morphine equivalents | |||
| <30/≥30 | 2.16 | 0.45–11.1 | 0.33 |
Discussion
In this study, we investigated the defecation frequency and adverse events in patients with GI cancer receiving opioids who were hospitalized for at least seven days before and after the start of naldemedine administration.
Naldemedine efficacy was assessed through changes in defecation frequency before and after the start of naldemedine treatment. Eligible patients for COMPOSE-4 had an Eastern Cooperative Oncology Group performance status of ≤2, a cancer type that did not directly affect GI function, and a cancer condition expected to remain stable throughout the study. The response rate in this study was 63.6% (95% CI: 46.6–77.9%), comparable to those of the studies on COMPOSE-4 (71.1%) and naloxegol (73%) (19,20). Therefore, the current analysis confirmed that naldemedine might be effective even in patients with GI cancer with OIC. However, the COMPOSE-4 assessment methodology defined spontaneous bowel movement with a feeling of complete evacuation (CSBM); responders as participants with three or more CSBMs per week and an increase by at least one CSBM per week from the baseline. The proportion of participants with spontaneous bowel movements (SBMs) or CSBMs per week and changes from the baseline in the average SBM or CSBM frequency per week, estimated as the least squares mean, were assessed (CSBM refers to bowel evacuation with a sensation of complete bowel evacuation not induced by rescue laxatives). Therefore, the results should be interpreted considering the different methods used in previous studies and the current one. In addition, as shown in Figure 2A, naldemedine was administered in combination with opioids to patients who had maintained bowel movements during the week prior to naldemedine administration. Although the current analysis was retrospective and initiation of naldemedine treatment is recommended after the diagnosis of OIC, it is thought that actual cases of GI tumors and prophylactic use of naldemedine exist in clinical practice. In this sense, the patients enrolled in the current study are cases with GI tumors, and preventing constipation in advance may be an option to prevent serious events such as GI perforation. In addition, changes in defecation frequency were evaluated for upper and lower GI tumors and opioid dosages (<30/≥30 mg/day of morphine equivalent). Defecation frequency was statistically increased in patients with lower GI tumors and higher opioid dosages, while there was a trend toward increased frequency in patients with upper GI tumors. Notably, these analyses were exploratory and based on a small number of patients.
Adverse events included grade 1 and grade 2 diarrhea in 39% and 9% of patients, respectively, without any grade 3 and 4 adverse events. Interestingly, no other GI symptoms were observed at grade 2–4 severity. In prospective clinical trials in cancer patients with OIC, the most common adverse events were diarrhea and abdominal pain, with incidence rates ranging from 19.6–39.7% and 1.7%, respectively. (15,21). In our analyzed cohort, the occurrence of diarrhea and abdominal pain was 27.5% and 0%, respectively, which is comparable to that of the randomized phase III studies. Naldemedine was safe and showed comparable results to other studies on carcinomas (15,21), although some issues specific to GI tumors might cause problems with drug absorption and dosing. Although the analyzed cohort included patients with a PS ≥3, no serious adverse events were observed, demonstrating the safety of naldemedine administration in patients with GI cancer in clinical practice. In addition, adverse events were evaluated for upper and lower GI tumors and opioid dosages (<30/≥30 mg/day of morphine equivalent). Mild diarrhea was the most common adverse event in each patient group; however, specific adverse events for each population could not be identified due to the low number of cases. It is important to note that when administering naldemedine, adverse events include diarrhea and abdominal pain, with severe diarrhea being reported as the most serious. Prior to the administration of naldemedine, it must be confirmed that the patient has no GI obstruction or history of GI perforation to prevent such events.
In the present analysis, 39.4% of patients had PS ≥3. However, in the COMPOSE-4 and -5 prospective phase III clinical studies of naldemedine in cancer patients with OIC, patient eligibility criteria were PS ≤2 (15). Therefore, the effectiveness and clinical safety of naldemedine treatment in most patients receiving this agent in the clinical setting have not been investigated in prospective clinical studies. Although naldemedine is used in many clinical settings, this study was limited to inpatients because it is impossible to assess defecation volume accurately. Limiting the study to inpatients makes the data more reliable because physicians, nurses, pharmacists, and other health care professionals assessed the defecation status. Notably, hospitalization for at least seven days before and after initiation of naldemedine administration was required to compile and examine adequate data. Moreover, these findings should be interpreted with caution because the patients in this study were poor PS patients who used opioids and required inpatient care or treatment for complications. Patients in this study had a low PS of 2–4 and short survival. Differences in the patient background between this analysis and the COMPOSE-4 and -5 randomized phase III trials should be considered. This study analyzed malignancies, including GI cancers, and its results were comparable to that of the randomized phase III trial of naldemedine in terms of adverse events and effectiveness. However, it was biased toward patients who were able to receive the drug orally without ileus. Nevertheless, naldemedine used to treat OIC was well tolerated by patients with GI cancers. As per our multivariate logistic regression analysis, none of the clinical factors we examined (age, PS, or daily opioid dose in oral morphine equivalents) were significant with reference to the efficacy of naldemedine. These findings were consistent with those of previous studies that have reported that the efficacy of naldemedine in patients with OIC is independent of the baseline characteristics of patients (22,23).
A multicenter retrospective study previously published by us evaluated the efficacy and safety of naldemedine for OIC in 149 patients with various cancer types (18). With regard to efficacy, the share of responders was 65.7%, with a significant increase in defecation frequency one week before and after naldemedine administration. On the other hand, with regard to safety, diarrhea was the most common adverse event (48.4%), but most events were grade 1. The results suggest that the efficacy and safety of naldemedine in clinical practice are comparable to those determined in prospective studies. In this subanalysis focusing on efficacy and safety in GI cancers, these parameters were comparable to those in the overall population, and there were no new signals of reduced efficacy or toxicities specific to GI tumors.
This study had several limitations. First, it was a retrospective study with a small number of patients. However, all participants were hospitalized patients with GI cancer whose stool frequency was carefully monitored by a healthcare professional for at least seven days before and after naldemedine treatment. Second, objective assessments, such as the Bristol Stool Form Scale (24), Bowel Function Index (25), and defecation diaries, were unavailable in this study. Thus, we lacked objective validity in the assessment. To ensure that naldemedine administration led to defecation, data were limited to inpatients rather than numerous outpatients. Assessment required hospitalization for at least seven days before and after the start of naldemedine administration to compile sufficient data. Records were based on data from multiple medical staff (physicians, pharmacists, and nurses) and were thus reliable. Third, the decision to initiate or discontinue naldemedine administration was left to the discretion of individual physicians and varied subjectively.
Conclusions
In conclusion, naldemedine is an effective and feasible OIC treatment for patients with GI cancer with fewer side effects. However, this investigation was a retrospective study, and further validation in clinical practice is required.
Acknowledgments
We thank Dr. Mie Kotake, Dr. Kyoichi Kaira, and Dr. Shiro Koizuka for their assistance in preparing this manuscript. We also thank Editage (www.editage.jp) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1130/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1130/dss
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1130/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1130/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Ethics Committee of Gunma Prefectural Cancer Center (No. 405-31046; approval date: September 27, 2019) and approved by the Institutional Review Board of each participating institution. Because this was a retrospective study, the need for informed consent requirement was waived. However, the opportunity to refuse participation through an opt-out method was guaranteed.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization (WHO). Global health estimates: deaths by cause, age, sex, by country and by region, 2000-2019. 2020. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Campora E, Merlini L, Pace M, et al. The incidence of narcotic-induced emesis. J Pain Symptom Manage 1991;6:428-30. [Crossref] [PubMed]
- Hardy J, Daly S, McQuade B, et al. A double-blind, randomised, parallel group, multinational, multicentre study comparing a single dose of ondansetron 24 mg p.o. with placebo and metoclopramide 10 mg t.d.s. p.o. in the treatment of opioid-induced nausea and emesis in cancer patients. Support Care Cancer 2002;10:231-6. [Crossref] [PubMed]
- Nosek K, Leppert W, Nosek H, et al. A comparison of oral controlled-release morphine and oxycodone with transdermal formulations of buprenorphine and fentanyl in the treatment of severe pain in cancer patients. Drug Des Devel Ther 2017;11:2409-19. [Crossref] [PubMed]
- Wiffen PJ, Wee B, Moore RA. Oral morphine for cancer pain. Cochrane Database Syst Rev 2016;4:CD003868. [PubMed]
- Wiffen PJ, Wee B, Derry S, et al. Opioids for cancer pain - an overview of Cochrane reviews. Cochrane Database Syst Rev 2017;7:CD012592. [PubMed]
- Suzuki T, Sawada T, Kawai K, et al. Pharmacological profile of TAN-452, a novel peripherally acting opioid receptor antagonist for the treatment of opioid-induced bowel syndromes. Life Sci 2018;215:246-52. [Crossref] [PubMed]
- Mori T, Shibasaki Y, Matsumoto K, et al. Mechanisms that underlie μ-opioid receptor agonist-induced constipation: differential involvement of μ-opioid receptor sites and responsible regions. J Pharmacol Exp Ther 2013;347:91-9. [Crossref] [PubMed]
- Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg 2001;182:11S-8S. [Crossref] [PubMed]
- Brock C, Olesen SS, Olesen AE, et al. Opioid-induced bowel dysfunction: pathophysiology and management. Drugs 2012;72:1847-65. [Crossref] [PubMed]
- Tokoro A, Imai H, Fumita S, et al. Incidence of opioid-induced constipation in Japanese patients with cancer pain: A prospective observational cohort study. Cancer Med 2019;8:4883-91. [Crossref] [PubMed]
- Crockett SD, Greer KB, Heidelbaugh JJ, et al. American Gastroenterological Association Institute Guideline on the Medical Management of Opioid-Induced Constipation. Gastroenterology 2019;156:218-26. [Crossref] [PubMed]
- Mawatari H, Shinjo T, Morita T, et al. Revision of Pharmacological Treatment Recommendations for Cancer Pain: Clinical Guidelines from the Japanese Society of Palliative Medicine. J Palliat Med 2022;25:1095-114. [Crossref] [PubMed]
- Katakami N, Harada T, Murata T, et al. Randomized Phase III and Extension Studies of Naldemedine in Patients With Opioid-Induced Constipation and Cancer. J Clin Oncol 2017;35:3859-66. [Crossref] [PubMed]
- Inagaki M, Kume M, Tamura Y, et al. Discovery of naldemedine: A potent and orally available opioid receptor antagonist for treatment of opioid-induced adverse effects. Bioorg Med Chem Lett 2019;29:73-7. [Crossref] [PubMed]
- Takata K, Nakazawa M, Honda K, et al. Post-marketing surveillance of the safety and effectiveness of naldemedine in the management of opioid-induced constipation in patients with cancer pain in Japan. Support Care Cancer 2022;30:3943-54. [Crossref] [PubMed]
- Nishiba H, Imai H, Fujita Y, et al. Efficacy and Safety of Naldemedine for Patients with Cancer with Opioid-Induced Constipation in Clinical Practice: A Real-World Retrospective Study. J Clin Med 2022;11:2672. [Crossref] [PubMed]
- Katakami N, Harada T, Murata T, et al. Randomized phase III and extension studies: efficacy and impacts on quality of life of naldemedine in subjects with opioid-induced constipation and cancer. Ann Oncol 2018;29:1461-7. [Crossref] [PubMed]
- Lemaire A, Pointreau Y, Narciso B, et al. Effectiveness of naloxegol in patients with cancer pain suffering from opioid-induced constipation. Support Care Cancer 2021;29:7577-86. [Crossref] [PubMed]
- Katakami N, Oda K, Tauchi K, et al. Phase IIb, Randomized, Double-Blind, Placebo-Controlled Study of Naldemedine for the Treatment of Opioid-Induced Constipation in Patients With Cancer. J Clin Oncol 2017;35:1921-8. [Crossref] [PubMed]
- Kubota R, Fukumura K, Wajima T. Population Pharmacokinetics and Exposure-Response Relationships of Naldemedine. Pharm Res 2018;35:225. [Crossref] [PubMed]
- Osaka I, Ishiki H, Yokota T, et al. Safety and efficacy of naldemedine in cancer patients with opioid-induced constipation: a pooled, subgroup analysis of two randomised controlled studies. ESMO Open 2019;4:e000527. [Crossref] [PubMed]
- Müller-Lissner S, Bassotti G, Coffin B, et al. Opioid-Induced Constipation and Bowel Dysfunction: A Clinical Guideline. Pain Med 2017;18:1837-63. [PubMed]
- Rentz AM, Yu R, Müller-Lissner S, et al. Validation of the Bowel Function Index to detect clinically meaningful changes in opioid-induced constipation. J Med Econ 2009;12:371-83. [Crossref] [PubMed]



