
Clinical characteristics and drug resistance profile of patients with endobronchial tuberculosis in South Korea: single-center experience
Highlight box
Key findings
• Elderly EBTB patients with a history of treatment for TB had a significantly higher proportion of drug-resistant EBTB.
What is known and what is new?
• Although EBTB is a unique form of TB, available data on the drug susceptibility patterns of EBTB patients are scarce.
• Any-resistance occurred in 24 EBTB patients (10.4%), and MDR or XDR occurred in 6 EBTB patients (2.6%). Additionally, the rate of resistance to fluoroquinolones in EBTB patients was higher than that in patients with pulmonary TB.
What is the implication, and what should change now?
• This study was able to identify risk factors for drug-resistant TB in EBTB patients, and these results will help in the care and effective management of these patients.
Introduction
Tuberculosis (TB) remains a major health problem worldwide because it is one of the leading causes of death and is second only to coronavirus disease 2019 (COVID-19) as a leading cause of death from a single infectious agent (1). There were estimated 1.4 million deaths worldwide, and an estimated 9.9 million people had TB in 2020 (1). Previously, South Korea had a high TB burden, with a high mortality rate related to TB (2). Although South Korea is now a country with an intermediate TB burden with an incidence of 39 cases per 100,000 people in 2020 due to various TB control programs (3), the incidence of TB remains the highest among countries of the Organization for Economic Cooperation and Development (2,4). In particular, the incidence of TB among the elderly population has been increasing over the past 10 years. Because South Korea is one of the fastest-aging countries (5), the elderly population accounts for 42% of all TB cases and 82% of TB-related deaths in South Korea (2).
Of the diverse tuberculous infections, endobronchial tuberculosis (EBTB) is defined as a particular form of TB infection of the tracheobronchial tree and shows several different clinical characteristics of TB that occurs in the lung parenchyma (6,7). Initially, EBTB can be mistakenly considered bronchitis, bronchial asthma, or lung malignancy because of non-specific symptoms, including cough, haemoptysis, fever, and dyspnoea, which may lead to exposure to antibiotics such as fluoroquinolone (FQ) prior to accurate diagnosis through bronchoscopic examination. Further, during EBTB, bronchial stenosis and future lung damage can occur as serious complications, despite anti-TB chemotherapy. Compared to pulmonary TB, EBTB is known to have a predilection for young females. However, data on recent clinical characteristics of patients with EBTB are scarce.
Despite significant progress against TB in recent years, some TB patients have received inappropriate management owing to insufficient information regarding drug susceptibility testing (DST) results (8-11). Inadequate drug regimen due to delay or failure to detect drug resistance can lead to an increased TB-related mortality rate and is a major obstacle in controlling TB (8,9). Although the World Health Organization recommends drug regimens guided by DST results (11), data on the DST profile of EBTB are poorly described. Only two previous reports described the DST results of EBTB; one involved only a small number of patients, while the other study performed molecular DST (12,13). Hence, availability of data on the EBTB DST patterns is limited.
Here, this study aimed to elucidate the clinical characteristics of patients with EBTB and evaluate the culture-based DST patterns of EBTB. The DST patterns were compared between patients older and younger than 65 years of age. We present the following article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1218/rc).
Methods
Study population
We retrospectively evaluated patients diagnosed with EBTB at a tertiary referral hospital between January 2013 and December 2019 at the Wonju Severance Christian Hospital (Wonju, South Korea). EBTB was defined as follows: (I) typical bronchoscopic findings, (II) histopathological evidence of inflammation, and (III) microbiological detection of TB using endobronchial biopsy specimen or bronchial washing fluid (6,7). During the study period, 239 patients were diagnosed with EBTB, and patients younger than 20 years (n=3) and those who had no DST data (n=6) were excluded. Finally, 230 patients with EBTB were analysed.
The study was conducted following the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board for Human Research of the Yonsei University Wonju Severance Christian Hospital (No. CR-319156). The requirement for informed consent was waived owing to the retrospective nature of the study.
Bronchoscopic procedures
All bronchoscopy procedures were performed by attending physicians during EBTB diagnosis. All procedures were performed transnasally or transorally under local anaesthesia. Midazolam (0.07 mg/kg) was delivered intravenously when needed to achieve adequate sedation prior to the procedure. In all patients, complete airway inspection was performed, and bronchial washing and biopsy were performed for suspected endobronchial lesions.
The EBTB site involved was classified as the trachea, main bronchus, right bronchus intermedius (RBI), or lobar bronchus. EBTB with two or more bronchial levels involved was defined as multiple-level involvement, which was described separately for each lesion. The subtype of cases with two or more endobronchial involvement was classified according to the main lesion site. Bronchoscopic findings were classified into the following subtypes: actively caseating, edematous-hyperaemic, fibrostenotic, tumorous, granular, ulcerative, and non-specific bronchitis (12).
Microbiologic, histopathologic, and radiologic evaluation
All bronchial washing fluid specimens were stained using the Ziehl-Neelsen method for the examination of acid-fast bacilli (AFB) on smears and cultured using both solid (3% Ogawa medium; Korean Institute of Tuberculosis, Cheongju, Korea) and liquid (BACTEC 960 Mycobacterial Growth Indicator Tube; Becton Dickinson, Sparks, MD, USA) media (14). Positive AFB stains were described as 1+ to 4+ (1+, 1–9 AFB/100 fields; 2+, 1–9 AFB/10 fields; 3+, 1–9 AFB/field; and 4+, >9 AFB/field) (14,15).
The bronchoscopic biopsy specimen was evaluated by a pathologist using microscopy. The histopathologic findings were chronic granulomatous inflammation, acute or chronic inflammation, erosion, ulcer, or caseous necrosis (16,17).
Chest radiography and computed tomography (CT) were performed prior to bronchoscopy evaluation and cavitary and involved lesions were evaluated in all patients.
DST and definitions
TB isolates from bronchial washing fluid or endobronchial biopsy samples obtained prior to treatment were sent to the Korean Institute of Tuberculosis (Cheongju, South Korea), a supranational TB reference laboratory. Phenotypic DST was performed using the absolute concentration method with Lowenstein-Jensen medium (18). The drugs tested for resistance and their critical concentrations were as follows: isoniazid (INH) 0.2 and 1.0 µg/mL, rifampicin (RFP) 40 µg/mL, ethambutol (EMB) 2.0 µg/mL, rifabutin (RBT) 20 µg/mL, streptomycin (SM) 10 µg/mL, amikacin (AMK) 40 µg/mL, kanamycin (KM) 40 µg/mL, capreomycin (CAP) 40 µg/mL, ofloxacin (OFL) 2.0 µg/mL, levofloxacin (LFX) 2.0 µg/mL, moxifloxacin (MFX) 2.0 µg/mL, prothionamide (PTH) 40 µg/mL, cycloserine (CS) 30 µg/mL, and para-aminosalicylic acid (PAS) 1.0 µg/mL. The susceptibility to pyrazinamide (PZA) was determined using the pyrazinamidase test (15,18).
TB isolates were classified as all-susceptible if they were susceptible to all the drugs tested. Drug resistance was defined as the resistance to one or more drugs. Mono-resistance was defined as resistance to only one drug and susceptibility to others. Poly-drug resistance was defined as resistance to multiple drugs, including either INH or RFP, but not both INH and RFP (19). Multidrug resistant TB (MDR-TB) refers to TB isolates resistant to at least two key first-line anti-TB drugs: INH and RFP. Extensively drug-resistant TB (XDR-TB) is defined as MDR-TB plus resistance to at least one of the FQ and one of the second-line injectable agents (KM, AMK, and CAP) used in MDR-TB treatment regimens (20).
Statistical analysis
Descriptive analyses were conducted in this study. Data are presented as the median with interquartile range (IQR) for continuous variables or as the number of patients with percentages of the total for categorical variables. Categorical data were compared using Pearson’s chi-square test or Fisher’s exact test, as appropriate, and a two-sided P value <0.05 was considered to indicate statistical significance. All analyses were performed using IBM SPSS Statistics for Windows, version 23.0 (IBM Co., Chicago, IL, USA).
Results
Baseline characteristics and incidence of EBTB
The clinical characteristics of 230 patients with EBTB are presented in Table 1. The median age was 74.0 years (IQR, 59.0–80.0 years) and the proportion of female patients was 65.2% (150/230). Of 230 patients, 34 (14.8%) had a history of previous TB treatment, and the most common comorbidity was diabetes (21.3%, n=49), followed by chronic liver disease (3.9%, n=9), and chronic kidney disease (3.5%, n=8).
Table 1
| Characteristic | Total (N=230) |
|---|---|
| Age, years | 74 [59–80] |
| Sex, female | 150 (65.2) |
| Body mass index, kg/m2 | 21.4 (19.7–23.6) |
| Smoking history | |
| Never-smoker | 161 (70.0) |
| History of previous TB treatment | 34 (14.8) |
| Comorbidities | |
| Diabetes | 49 (21.3) |
| Chronic liver disease | 9 (3.9) |
| Chronic kidney disease | 8 (3.5) |
| Respiratory symptoms* | |
| Cough or sputum | 145 (63.0) |
| Dyspnea on exertion | 42 (18.3) |
| Haemoptysis | 11 (4.8) |
| Asymptomatic | 61 (26.5) |
| AFB stain of bronchial washing fluid† | |
| 0 | 71 (30.9) |
| 1+ | 75 (32.6) |
| 2+ | 43 (18.7) |
| 3+ | 29 (12.6) |
| 4+ | 12 (5.2) |
Values are presented as median (interquartile range) or count (percentage). *, cases are duplicated; †, positive AFB stains were described as 1+ to 4+ (1+, 1–9 AFB/100 fields; 2+, 1–9 AFB/10 fields; 3+, 1–9 AFB/field; and 4+, >9 AFB/field). EBTB, endobronchial tuberculosis; TB, tuberculosis; AFB, acid-fast bacilli.
Respiratory symptoms, including cough or sputum (63.0%, 145/230), dyspnea (18.3%, 42/230), and haemoptysis (4.8%, 11/230), were observed in patients with EBTB, while 61 patients (26.5%) did not have any respiratory symptoms. Of the 230 patients, 159 (69.1%) had a positive AFB smear of bronchial washing fluid. Smears 1+ and 4+ were observed in 75 (32.6%) and 12 (5.2%) patients, respectively. Pulmonary parenchymal tuberculosis was combined in 191 (83.0%) EBTB patients.
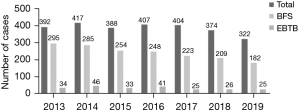
The annual number of EBTB cases is shown in Figure 1. Between January 2013 and December 2019, 2,704 patients were diagnosed with pulmonary TB, and bronchoscopic evaluation was conducted in 1,696 patients (62.7%). Overall, patients diagnosed with EBTB were 14.1% (239/1,696) and 8.8% (239/2,704) of patients with pulmonary TB who underwent bronchoscopic evaluation and those newly diagnosed with pulmonary TB during the study period, respectively. There were no complications related to bronchoscopy procedure.
Bronchoscopic and radiologic features
Bronchoscopic and radiological findings are summarised in Table 2. The most common subtype of bronchoscopic findings was active caseating (54.4%, 125/230), followed by edematous-hyperaemic (16.1%, 37/230), fibrostenotic (14.8%, 34/230), non-specific bronchitis (7.8%, 18/230), ulcerative (3.9%, 9/230), tumorous (1.7%, 4/230), and granular (1.3%, 3/230). Chest CT scans were obtained from 222 (97%) patients and cavitary lesions were observed in 13.5% (30/222).
Table 2
| Characteristic | Total (N=230) |
|---|---|
| Bronchoscopic finding | |
| Actively caseating type | 125 (54.4) |
| Edematous-hyperaemic type | 37 (16.1) |
| Fibrostenotic type | 34 (14.8) |
| Non-specific bronchitis type | 18 (7.8) |
| Ulcerative type | 9 (3.9) |
| Tumorous type | 4 (1.7) |
| Granular type | 3 (1.3) |
| Bronchoscopic evaluation-site involved* | |
| Vocal cord | 3 (1.3) |
| Trachea | 10 (4.3) |
| Right main bronchus | 11 (4.8) |
| Right bronchus intermedius | 13 (5.7) |
| Right upper lobe bronchus | 68 (30.0) |
| Right middle lobe bronchus | 48 (20.9) |
| Right lower lobe bronchus | 20 (8.7) |
| Left main bronchus | 21 (9.1) |
| Left upper lobe bronchus | 47 (20.4) |
| Left lower lobe bronchus | 27 (11.7) |
| Cavitary lesion in CT image‡ | 30 (13.0) |
Values are presented as count (percentage). *, EBTB with multiple-level involvement was counted for each involvement. ‡, data were obtained from 222 of 230 patients. EBTB, endobronchial tuberculosis; CT, computed tomography.
Drug susceptibility profiles
The results of DST are presented in Table 3. The rate of resistance to any anti-TB drug was 10.4% (24/230). The proportion of any-drug resistance was higher in elderly patients (≥65 years) (12.5%, 20/159) than that in young patients (<65 years) (5.6%, 4/71).
Table 3
| Category | Total (N=230) | Age ≥65 (n=159) | Age <65 (n=71) | |||
|---|---|---|---|---|---|---|
| Previously treated (n=21) | Treatment-naïve (n=138) | Previously treated (n=13) | Treatment-naïve (n=58) | |||
| Any resistance | 24 (10.4) | 4 (19.0) | 16 (11.6) | 1 (7.7) | 3 (5.2) | |
| INH | 17 (7.4) | 4 (19.0) | 11 (8.0) | 1 (7.7) | 1 (1.7) | |
| RFP | 7 (3.0) | 3 (14.3)* | 3 (2.2) | 0 | 1 (1.7) | |
| EMB | 3 (1.3) | 2 (9.5)** | 1 (0.7) | 0 | 0 | |
| PZA | 3 (1.3) | 2 (9.5)** | 1 (0.7) | 0 | 0 | |
| SM | 8 (3.5) | 2 (9.5) | 4 (2.9) | 0 | 2 (3.4) | |
| LFX | 6 (2.6) | 1 (4.8) | 4 (2.9) | 0 | 1 (1.7) | |
| PTH | 2 (0.9) | 0 | 2 (1.4) | 0 | 0 | |
| PAS | 1 (0.4) | 0 | 1 (0.7) | 0 | 0 | |
| Mono-resistance | 11 (4.8) | 0 | 8 (5.8) | 1 (7.7) | 2 (3.4) | |
| INH | 5 (2.2) | 0 | 4 (2.9) | 1 (7.7) | 0 | |
| RFP | 1 (0.4) | 0 | 1 (0.7) | 0 | 0 | |
| LFX | 3 (1.3) | 0 | 2 (1.4) | 0 | 1 (1.7) | |
| SM | 2 (0.9) | 0 | 1 (0.7) | 0 | 1 (1.7) | |
| Poly-resistance | 8 (3.5) | 1 (4.8) | 6 (4.3) | 0 | 1 (1.7) | |
| INH + SM only | 4 (1.7) | 1 (4.8) | 2 (1.4) | 0 | 1 (1.7) | |
| INH + SM + EMB | 1 (0.4) | 0 | 1 (0.7) | 0 | 0 | |
| INH + PTH only | 1 (0.4) | 0 | 1 (0.7) | 0 | 0 | |
| INH + LFX + PTH | 1 (0.4) | 0 | 1 (0.7) | 0 | 0 | |
| LFX + PAS | 1 (0.4) | 0 | 1 (0.7) | 0 | 0 | |
| Multi-drug resistance or extensively drug-resistance | 6 (2.6) | 3 (14.3)*** | 2 (1.4) | 0 | 1 (1.7) | |
| Multi-drug resistance | 5 (2.2) | 2 (9.5) | 2 (1.4) | 0 | 1 (1.7) | |
| INH + RFP | 2 (0.9) | 1 (4.8) | 1 (0.7) | 0 | 0 | |
| INH + RFP + PZA | 1 (0.4) | 0 | 1 (0.7) | 0 | 0 | |
| INH + RFP + EMB + PZA | 1 (0.4) | 1 (4.8) | 0 | 0 | 0 | |
| INH + RFP + SM | 1 (0.4) | 0 | 0 | 0 | 1 (1.7) | |
| Extensively drug-resistance: INH + RFP + EMB + PZA + LFX + SM | 1 (0.4) | 1 (4.8) | 0 | 0 | 0 | |
Values are presented as count (percentage). *, P=0.031; **, P=0.046; ***, P=0.017. EBTB, endobronchial tuberculosis; INH, isoniazid; RFP, rifampicin; EMB, ethambutol; PZA, pyrazinamide; SM, streptomycin; LFX, levofloxacin; PTH, prothionamide; PAS, para-aminosalicylic acid.
Resistance to INH occurred most frequently (n=17, 7.4%), followed by SM (n=8, 3.5%) and LFX (n=6, 2.6%). Among elderly patients, the resistance rate to RFP was significantly higher in previously treated patients (14.3%, 3/21) than that in treatment-naïve patients (2.2%, 3/138) (P=0.031). Resistance rates to EMB and PZA were also significantly higher in previously treated patients (9.5%, 2/21) than that in treatment-naïve patients (0.7%, 1/138) (P=0.046). Mono-resistance was observed in 11 of 230 patients (4.8%), most of whom were treatment-naïve and elderly (n=8, 5.8%), and the rate of INH mono-resistance was 2.2% (5/230). Poly-drug resistance was observed in 8 out of 230 patients (3.5%). MDR and XDR were identified in 5 (2.2%) and 1 (0.4%) patient, respectively. The proportion of MDR and XDR was significantly higher in previously treated elderly patients (14.3%, 3/21) than that in treatment-naïve elderly patients (1.4%, 2/138) (P=0.017).
Clinical characteristics of six patients with MDR- or XDR-TB
The clinical features of the patients with TB (five MDR- and one XDR-TB) are presented in Table S1. Except one patient, all others were elderly (≥65 years) and the median age was 76.0 years. Of the six patients with MDR- or XDR-TB, four (66.6%) were females and three (50.0%) had previous history of TB treatment. Except one patient, all others had a positive AFB smear result. Of the six patients, two (33.3%) had cavitary lesions on CT scan, and bronchoscopic findings showed that four (66.6%) and the remaining two (33.3%) patients had EBTB involving the upper lobe bronchus and the main bronchus, respectively.
Discussion
In this study, we evaluated the clinical characteristics of EBTB patients and their drug susceptibility profile. Any-resistance occurred in 24 patients (10.4%), and MDR or XDR occurred in 6 patients (2.6%). Previously treated elderly patients had a significantly higher proportion of resistance to RFP (14.3% vs. 2.2%), EMB (9.5% vs. 0.7%), and PZA (9.5% vs. 0.7%) than that of treatment-naïve patients. Further, MDR and XDR occurred more frequently in previously treated elderly patients (14.3%) than that in treatment-naïve patients (1.4%).
The clinical characteristics of patients with EBTB were comparable to those previously reported. The median age of patients with EBTB was relatively higher (74 years) than that reported in previous studies (48 years) (21). Regarding bronchoscopic findings, the most common type was the active caseating (54.4%) which was consistent with results from previous studies (12,21-23). However, Ozkaya et al. reported the most common type as the edematous hyperaemic subtype (24). Differences in demographic, racial, and geographic characteristics could affect the differences in common types of bronchoscopic findings (25,26).
Although EBTB was known to have a predilection for young women previously, this study showed that EBTB occurred commonly in elderly patients (16,22). In South Korea, TB was a major infectious disease in the 1940s, and its incidence was high until 2016 (2,27). By the time the development of anti-TB medication regimens (including full use of RFP) was completed in the 1980s (27-29), many patients treated prior to the 1980s were exposed to inadequate anti-TB regimens. Now, South Korea is one of the fastest ageing countries worldwide (5), and distribution of new and recurrent TB by age revealed the highest incidence in elderly patients (27,30). Moreover, TB among elderly patients accounts for approximately 80% of TB-related deaths in South Korea (31). Regarding the DST results, elderly patients were more likely to have any-drug resistance than that of young patients (12.6% vs. 5.6%), regardless of their previous history of TB. Moreover, previously treated elderly patients had 10 times higher rate of MDR-/XDR-TB than that of treatment-naïve elderly patients (14.3% vs. 1.4%). When compared with previous studies based on culture-confirmed TB and phenotypic DST results collected during approximately the same period (2010–2019 and 2015–2018) in South Korea, 6.0% and 4.1% of TB cases, respectively, were MDR-TB (18,29). A similar trend was observed when analysing our results with respect to drug resistance. In contrast, the LFX resistance rate of EBTB was significantly higher than that in a previous study on pulmonary TB [2.6% (6/230) vs. 0.7% (38/5,221), P=0.0097] (31). This may be due to exposure to fluoroquinolones prior to an accurate diagnosis through bronchoscopic examination. To the best of our knowledge, this study showed the largest number of drug susceptibility patterns in EBTB.
This study had some limitations. First, because this study was performed retrospectively, all patients did not undergo bronchoscopic examinations which could cause an underestimation of the incidence of EBTB. Although the diagnostic evaluation did not proceed according to a uniform protocol in patients with suspected pulmonary TB, almost all study patients underwent CT and bronchoscopic evaluation with culture and biopsy. Further prospective studies with systematic evaluation of the incidence and prevalence of EBTB are warranted. Second, there was limited generalisability due to the single-center nature of the study. However, we evaluated a considerable number of EBTB cases compared to that reported in previous studies. Lastly, our study was conducted in a high-income country with an intermediate TB burden, which may limit the application of our results to other countries with differing incomes and TB burdens.
Conclusions
Elderly EBTB patients with a history of treatment for TB had a significantly higher proportion of drug-resistant TB and these patients should be carefully assessed using DST results.
Acknowledgments
This study was presented by poster at the 24th Congress of the Asian Pacific Society of Respirology (APSR) 2019.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1218/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1218/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1218/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board for Human Research of the Yonsei University Wonju Severance Christian Hospital (No. CR-319156). The requirement for informed consent was waived owing to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee SH. Active Case Finding in the Elderly Tuberculosis in South Korea. Tuberc Respir Dis (Seoul) 2019;82:261-3. [Crossref] [PubMed]
- Cho KS. Tuberculosis control in the Republic of Korea. Epidemiol Health 2018;40:e2018036. [Crossref] [PubMed]
- Son E, Jeon D. Current situation of tuberculosis and national strategic plan for tuberculosis control in Korea. J Korean Med Assoc 2021;64:316-23. [Crossref]
- Lee H, Kim J, In H, et al. Characteristics and trends in deaths from tuberculosis in the Republic of Korea, 2001-2020. Korea Disease Control and Prevention Agency (KDCA) 2021:3408-12.
- Kim KW, Kim OS. Super Aging in South Korea Unstoppable but Mitigatable: A Sub-National Scale Population Projection for Best Policy Planning. Spat Demogr 2020;8:155-73. [Crossref] [PubMed]
- Hoheisel G, Chan BK, Chan CH, et al. Endobronchial tuberculosis: diagnostic features and therapeutic outcome. Respir Med 1994;88:593-7. [Crossref] [PubMed]
- Shahzad T, Irfan M. Endobronchial tuberculosis-a review. J Thorac Dis 2016;8:3797-802. [Crossref] [PubMed]
- Rapid communication: key changes to the treatment of drug-resistant tuberculosis. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO.
- Mase SR, Chorba T. Treatment of Drug-Resistant Tuberculosis. Clin Chest Med 2019;40:775-95. [Crossref] [PubMed]
- Ramalho DMP, Miranda PFC, Andrade MK, et al. Outcomes from patients with presumed drug resistant tuberculosis in five reference centers in Brazil. BMC Infect Dis 2017;17:571. [Crossref] [PubMed]
- Nguyen TNA, Anton-Le Berre V, Bañuls AL, et al. Molecular Diagnosis of Drug-Resistant Tuberculosis; A Literature Review. Front Microbiol 2019;10:794. [Crossref] [PubMed]
- Chung HS, Lee JH. Bronchoscopic assessment of the evolution of endobronchial tuberculosis. Chest 2000;117:385-92. [Crossref] [PubMed]
- Zhang Q, Zhang Q, Sun BQ, et al. GeneXpert MTB/RIF for rapid diagnosis and rifampin resistance detection of endobronchial tuberculosis. Respirology 2018;23:950-5. [Crossref] [PubMed]
- Leylabadlo HE, Kafil HS, Yousefi M, et al. Pulmonary Tuberculosis Diagnosis: Where We Are? Tuberc Respir Dis (Seoul) 2016;79:134-42. [Crossref] [PubMed]
- Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med 2000;161:1376-95. [Crossref] [PubMed]
- Kashyap S, Solanki A. Challenges in endobronchial tuberculosis: from diagnosis to management. Pulm Med 2014;2014:594806. [Crossref] [PubMed]
- Siow WT, Lee P. Tracheobronchial tuberculosis: a clinical review. J Thorac Dis 2017;9:E71-7. [Crossref] [PubMed]
- Lee T, Lee SJ, Jeon D, et al. Additional Drug Resistance in Patients with Multidrug-resistant Tuberculosis in Korea: a Multicenter Study from 2010 to 2019. J Korean Med Sci 2021;36:e174. [Crossref] [PubMed]
- Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. Geneva: World Health Organization, 2014.
- WHO consolidated guidelines on tuberculosis: module 4: treatment: drug-resistant tuberculosis treatment. Geneva: World Health Organization; 2020.
- Jung SS, Park HS, Kim JO, et al. Incidence and clinical predictors of endobronchial tuberculosis in patients with pulmonary tuberculosis. Respirology 2015;20:488-95. [Crossref] [PubMed]
- Mohd Esa NY, Othman SK, Mohd Zim MA, et al. Bronchoscopic Features and Morphology of Endobronchial Tuberculosis: A Malaysian Tertiary Hospital Experience. J Clin Med 2022;11:676. [Crossref] [PubMed]
- Pathak V, Shepherd RW, Shojaee S. Tracheobronchial tuberculosis. J Thorac Dis 2016;8:3818-25. [Crossref] [PubMed]
- Ozkaya S, Bilgin S, Findik S, et al. Endobronchial tuberculosis: histopathological subsets and microbiological results. Multidiscip Respir Med 2012;7:34. [Crossref] [PubMed]
- Lee M, Han J, Kim YR, et al. Multidrug-resistant tuberculosis in South Korea: a retrospective analysis of national registry data in 2011-2015. Int J Tuberc Lung Dis 2019;23:850-7. [Crossref] [PubMed]
- Na HJ, Eom JS, Lee G, et al. Exposure to Mycobacterium tuberculosis during Flexible Bronchoscopy in Patients with Unexpected Pulmonary Tuberculosis. PLoS One 2016;11:e0156385. [Crossref] [PubMed]
- Song JH, Huh K, Chung DR. Modern History of Tuberculosis in Korea. Infect Chemother 2019;51:414-26. [Crossref] [PubMed]
- Murray JF, Schraufnagel DE, Hopewell PC. Treatment of Tuberculosis. A Historical Perspective. Ann Am Thorac Soc 2015;12:1749-59. [Crossref] [PubMed]
- Lee EG, Min J, Kang JY, et al. Age-stratified anti-tuberculosis drug resistance profiles in South Korea: a multicenter retrospective study. BMC Infect Dis 2020;20:446. [Crossref] [PubMed]
- Youn HM, Shin MK, Jeong D, et al. Risk factors associated with tuberculosis recurrence in South Korea determined using a nationwide cohort study. PLoS One 2022;17:e0268290. [Crossref] [PubMed]
- Kim H, Mok JH, Kang B, et al. Trend of multidrug and fluoroquinolone resistance in Mycobacterium tuberculosis isolates from 2010 to 2014 in Korea: a multicenter study. Korean J Intern Med 2019;34:344-52. [Crossref] [PubMed]


