
Evaluating the impact of the metastatic spinal cord compression coordinator in a regional cancer network: a prospective, non-randomized pilot study
Highlight box
Key findings
• Introduction of MSCC coordinator role led to improved time from imaging to radiotherapy treatment.
What is known and what is new?
• MSCC coordinator role has been previously shown to streamline service, ensure timely decision-making and improve survival outcomes, and is recommended by NICE UK. However, most of the available data are anecdotal or from limited series presented as abstracts in conferences.
• So far, there has been no reported study comparing the outcomes before and after the introduction of this service. This pilot study compared outcomes before and after the introduction of the MSCC coordinator role in 2020 and 2021 respectively.
What is the implication, and what should change now?
• First, our study demonstrates the impact of the MSCC coordinator role and highlights that the benefit remains the same in small centres compared to larger ones. Second, most hospitals reduced their waiting time after the introduction of the MSCC coordinator, and are more likely to meet national standards for time from imaging to MSCC treatment (≤24 hours).
Introduction
Background
Metastatic spinal cord compression (MSCC) is a debilitating and potentially irreversible complication of cancer and was first described in 1925 by Spiller as a cause of progressive paraplegia in cancer patients (1,2). The actual incidence of MSCC is unknown but is estimated to be between 5–10% in patients with malignancy (3,4). It can be caused by any solid tumors however, it is more common in cancers that tend to spread to the spine such as breast, prostate and lung (4).
MSCC is caused by metastatic spread to spine either by causing collapse or compression of the vertebral body but also by direct extension of tumor into the vertebral canal. Cord compression initially leads to reversible changes such as oedema, demyelination and venous congestion. If prolonged, however, leads to vascular injury which in turn causes cord necrosis and irreversible damage to spinal cord (5). Magnetic resonance imaging (MRI) is the standard of care modality for investigating MSCC. It should ideally be performed within 24 hours of presentation as per UK National Institute for Health and Care Excellence (NICE) guidelines (4).
Various prognostic and spinal stability risk factors have been proposed to guide patient selection for surgery and treatment planning. Spinal instability neoplastic score (SINS) (6) is used to identify and assess patients for consideration for surgical intervention. Other scoring systems such as the Tokuhashi score (7) can be used to predict prognosis following MSCC. The goal of treatment in MSCC is multifaceted. It includes pain relief, management of spinal instability, eradication of tumour, reduction of mid-long term neurological deficit, preservation of function and survival benefit.
Standard treatment options for MSCC include either radiotherapy or surgery followed by radiotherapy (6). Surgery is often treatment of choice in patients with unstable spine, single level or oligometastatic disease with good performance status, and requiring tissue sample for disease characterization (8). On the other hand, radiotherapy is preferred in patients who are co-morbid, have widespread disease and due to disease burden have a limited prognosis. A recently emerging and effective treatment is radiosurgery. Under imaging guidance, radiosurgery can deliver a very high dose of radiotherapy to a very small focus with high precision however, there is low level of evidence to show the superiority of stereotactic radiosurgery over conventional fractionated radiation or decompressive surgery in patients with MSCC (9).
Rationale and knowledge gap
One of the factors which affects outcome in MSCC is presence or absence of neurological features prior to treatment (7). Delay in treatment can lead to irreversible neurological damage, subsequent adverse quality of life and burden on health care resources (10). Patients with loss of neurological function for more than 24 hours are unlikely to show improvement and hence not typically offered surgery unless spinal stabilisation is required for pain relief (4). Several studies based on patient experience highlighted the importance of effective communication between teams as one of the main reasons of delay in effective management (4).
It has been established in previous studies that incidence of MSCC referrals tend to trend towards a Friday peak (11), however, early care referrals and quicker treatment decisions could lead to a reversal in Friday peak (12,13). This was also confirmed by a large regional, multi-centre retrospective study in the UK presented at American Society of Clinical Oncology (ASCO) 2020 (13). Hospitals which had 7-day acute oncology service and radiology reporting along with a single point of referral (e.g., similar to MSCC coordinator role) had a quicker treatment turnaround and uniform referrals across the week (14). NICE recommends every secondary or tertiary care centre should have an identified lead healthcare professional for MSCC. They will be responsible for implementing the care pathway and coordinating the diagnosis and appropriate management of patients with known or suspected MSCC (4). The MSCC coordinator has been shown to streamline service, ensure timely decision-making and improved survival outcomes (15,16). Recent work from a tertiary centre and a regional cancer network in the UK recommends district general hospitals (DGHs) should consider appointing an MSCC coordinator when designing their service in line with NICE recommendations (14). So far, available evidence is anecdotal or from limited series presented as abstracts in conferences. Hence, this study set out to fill this knowledge gap.
Objective
In this pilot study, data from four DGHs in Kent, UK were analysed to assess the pattern of MSCC referrals. The aim of the study was to assess the impact of the newly introduced role of the MSCC coordinator on the MSCC diagnostic and treatment pathway. The objective of the study was to compare the time from imaging to radiotherapy before and after the introduction of the MSCC coordinator. The hypothesis of this study was that the time from imaging to radiotherapy is shorter for patients with MSCC in 2021 than in 2020. Furthermore, changes in occupational therapy, physiotherapy, hospital palliative and community palliative care referrals were assessed as these have been shown to have positive impact on maintenance and recovery of neurological function as well as symptom control (17,18). We present this article in accordance with the TREND reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1102/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Quality Improvement Project (QIP) (No. 190) and individual consent for this retrospective analysis was waived.
This was a prospective, pilot study and was part of a QIP carried out between April 2020 and August 2021 in Kent, UK. This was done in line with local data governance regulations and ethical approval (QIP approval number 190). Informed consent was not sought as this study involves no more than minimal risks to subjects as described in waiver of informed consent 45CFR 46.116 (19,20). Cancer care in the Kent region is provided under the umbrella of the Kent Oncology Centre (KOC). This includes four main hospital trusts namely (I) Maidstone and Tunbridge Wells NHS Trust (MTW), (II) Dartford and Gravesham NHS Trust (DVH), (III) Medway NHS Foundation Trust (MMH), and (IV) East Kent Hospitals University NHS Foundation Trust (EKH). Data was collected from the local cancer electronic database Kent Oncology Management System (KOMS) and picture archiving and communication system (PACS).
Sample
Data were prospectively collected from 1st April to 30th June 2021. Comparative retrospective data were collected for the period between 1st April to 30th June 2020. This was to compare the impact of the various interventions introduced at the commencement of the prospective study in 2021. The data prospectively collected include: (I) time from diagnostic spine imaging to radiotherapy treatment, and (II) number of hospital palliative care (HPC), occupational/physiotherapy (OPH) and community hospice referrals (CHP). The eligibility criteria for this study are described in Table 1.
Table 1
| Inclusion criteria |
| MRI-confirmed cauda equina or cord compression |
| Imaging done due to suspicion of cauda equina or cord compression |
| Incidental finding on imaging done for routine monitoring |
| Exclusion criteria |
| Impending (but not confirmed) cauda equina or cord compression |
| Imaging done for other indications such as bone or soft tissue metastases |
MSCC, metastatic spinal cord compression; MRI, magnetic resonance imaging.
Study interventions
The role of MSCC coordinator was introduced into the MSCC pathway across the region on the 1st April 2021. This role was carried out by two consultant radiographers in the two radiotherapy centres in the region. One MSCC coordinator was based at Maidstone and Tunbridge Wells NHS Trust covering referrals for west Kent region while the second coordinator was based at East Kent Hospitals University NHS Foundation trust. The main role of the coordinators was to triage MSCC referral phone calls and emails. They ensured patient treatment was commenced as soon as a diagnosis is established by liaising with various multi-disciplinary team members required to expedite emergency radiotherapy treatment.
A number of other key interventions were introduced:
- A dedicated MSCC referral phone number and email address were set up by the information technology (IT) department at the regional hub in Maidstone Hospital.
- The local and regional referral pathways were modified to include the new contact number and email.
- Numerous virtual meetings were held between acute oncology services, palliative care team, medical and clinical oncology teams to encourage uptake and compliance.
- The new contact number was listed on the local Induction mobile phone app (21) (mainly used by junior doctors and nurses).
- The media and communications teams of the four Hospital trusts helped with publicity of the new changes in the referral pathway via their trust intranet and newsletters.
Study outcomes
The primary outcome measure was to assess whether these interventions will lead to reduced time from radiological diagnosis of MSCC to receiving radiotherapy. Secondary outcomes included number of HPC, OPH and CHPs, where appropriate, as a result of introduction of these interventions.
Statistical analyses
Shapiro-Wilk was used to assess normality of data and unpaired T-test to compare referral numbers between 2020 vs. 2021. Mann-Whitney U test was used to analyse the datasets. Python package (SciPy) version 1.8.0 was used. Analyses was carried out on an individual patient level. P≤0.05 was considered significant. P values were reported to 2 decimal places.
Results
The descriptive statistics of the patients in each time period, as well as their times from MSCC confirmatory imaging to treatment, are summarized in Table 2 below. The most common malignancy subtypes in the 2020 collection period are urological, whereas in the 2021 it was lung. In both periods, the majority of the radiotherapy treatments were performed with emergency intent, with the rest being palliative. There were no adverse events or unintended side effects reported during the study.
Table 2
| Variable | 2020 | 2021 | P value |
|---|---|---|---|
| Collection period (1st April to 30th June for both years), n | |||
| Number of patients in dataset | 58 | 24 | |
| Number of patients with Imaging or treatment dates missing | 18 | 4 | 0.31 |
| Number of patients in final dataset | 40 | 20 | |
| Primary diagnosis, n (%) | 0.39 | ||
| Type of malignancy | |||
| Urological | 13 (32.5) | 3 (15.0) | |
| Breast | 4 (10.0) | 4 (20.0) | |
| Haematological | 3 (7.5) | 3 (15.0) | |
| Lung | 8 (20.0) | 5 (25.0) | |
| Skin | 1 (2.5) | 0 (0.0) | |
| Lower GI | 4 (10.0) | 0 (0.0) | |
| Upper GI | 1 (2.5) | 2 (10.0) | |
| Other | 6 (15.0) | 3 (15.0) | |
| Treatment intent | 0.47 | ||
| Emergency | 31 (77.5) | 13 (75.0) | |
| Palliative | 9 (22.5) | 7 (25.0) | |
| Imaging to treatment times | |||
| Number of patients treated in 1 day or less, n (%) | 15 (37.5) | 13 (65.0) | 0.02 |
| Median time to treat (days) [95% CI] | 2 [1–4] | 1 [1–2] | 0.04 |
| Mean time to treat (days) | 3.88 | 2.15 | |
| Longest time to treat (days) | 23 | 11 |
GI, gastrointestinal.
In an exploratory analysis, following the introduction of the MSCC coordinator role regionally, there was a significant reduction in the median time from imaging to treatment in the management MSCC patients (P=0.045). In comparison to the 2020 period [median time 2 days (95% CI: 1–4 days)], there was a shorter median time to treatment in 2021 median time 1 day (95% CI: 1–2 days), with more patients being treated within 24 hours as per NICE guidelines.
Differences within hospitals
Most hospitals reduced their waiting times following the introduction of MSCC coordinators. In terms of proportion of patients being treated within 24 hours, only East Kent Hospitals saw a reduction in how many patients were seen within the target; all other hospitals saw an increase in the same metric. However, it should be noted that many hospitals have had small sample sizes in the collection period following intervention, making this analysis less likely to be truly representative for them. East Kent only had 1 patient in the period following intervention, and so the deterioration may be due to chance. This is demonstrated in Table 3. Four patients in the pre-intervention phase and 7 patients in the post-intervention phase had missing data for their hospital, so were not included in this analysis.
Table 3
| Hospital | Before intervention | Following intervention | |||
|---|---|---|---|---|---|
| Number of patients | Proportion treated within 1 day | Number of patients | Proportion treated within 1 day | ||
| DVH | 4 | 25% | 2 | 50% | |
| EK | 12 | 42% | 1 | 0% | |
| MMH | 7 | 14% | 2 | 50% | |
| MTW | 13 | 54% | 8 | 87% | |
DVH, Dartford and Gravesham NHS Trust; EK, East Kent; MMH, Medway NHS Foundation Trust; MTW, Maidstone and Tunbridge Wells NHS Trust.
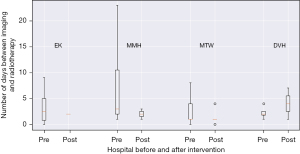
In a further exploratory analysis, median time from imaging to treatment reduced in all hospital trusts except for DVH. DVH was also the only hospital trust where a patient in the post intervention period had to wait longer than the patient with the longest wait in the pre-collection period. The delay in treatment in this patient was due to need for repeated communication between the tertiary neurosurgical centre in London and the local hospital in Kent. Due to the complexity of this case, it had to be discussed in the weekly neuro-oncology multi-disciplinary team meeting which usually happens on a Friday. During this waiting period the patient was commenced on high dose steroids (dexamethasone) and analgesia. In comparison, all other hospital trusts reduced their maximum waiting time. This is demonstrated in Figure 1.
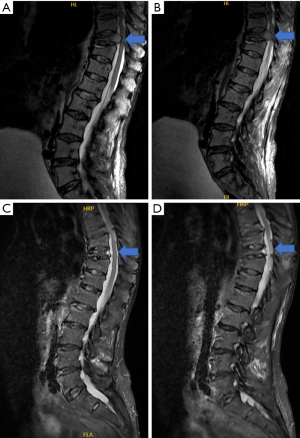
During the pilot period, there were 7 referrals each made to: (I) HPC, (II) physiotherapy and occupational health and (III) hospice palliative care teams respectively. Figure 2 shows MRI scan of a patient treated in the new pathway with good response and with preservation of function.
Discussion
Key findings
This pilot study compared time between confirmation of MSCC on imaging to radiotherapy treatment before and after the introduction of the MSCC coordinator role in 2020 and 2021 respectively. Our analysis showed a 1-day median time between imaging and radiotherapy (mean 2.8 days, range, 0–10 days) in 2021, compared to a median time of 2 days (mean 4.1 days, range, 0–22 days), P=0.04 in 2020. Three out of 4 participating hospitals reduced their waiting times following the introduction of the MSCC coordinator. The median time in days between the cohorts pre- and post-intervention were 2.5 vs. 2 days in EKH, 1 vs. 1 day in MTW, 3 vs. 2 days in MMH, 2 vs. 4 days in DVH.
Strengths and limitations
Our findings need to be interpreted in light of some inevitable methodological constraints. This prospective pilot study was conducted across a small patient cohort. Although we used the data from four hospital trusts in Kent, UK, this pilot study collected data from only limited number of patients (n=24) recruited in the first three months post-intervention. This could have led to less-representative results, for instance in Dartford and Gravesham NHS Trust, which had four patients in the initial period but only two in the post-intervention phase. Furthermore, the number of patients included in the 2021 (n=20) and 2020 (n=40) cohorts were different because we only included the patients referred through the MSCC coordinator pathway in order to evaluate the impact of this new role and to compare study outcomes for similar time period for both years. The time between symptom onset to confirmation of MSCC on imaging could not be evaluated. Though it might be possible to measure the time between presentation and investigations/imaging, it is more complex to determine the onset of symptoms reliably, especially in patients who were incidentally diagnosed with MSCC on interval CT scans. Another reason is due to high incidence of low back pain (one third of general population) (22), this might delay symptom awareness and subsequent presentation by cancer patients (4,22). Furthermore, several patients with MSCC do not have symptoms at the onset of their condition, leading to delays in investigations and subsequent diagnosis.
Despite these methodological limitations, our results have important implications for clinical practice. First, our study demonstrates the impact of the MSCC coordinator role and highlights that there is no significant difference between different centres, with the benefit remaining the same in small centres compared to larger ones (15). Second, most hospitals reduced their waiting time after the introduction of the MSCC coordination, which means the centres involved in this study are more likely to meet national standards for time from imaging to MSCC treatment (≤24 hours) as a result of this intervention.
Comparison with similar research
The MSCC coordinator role has been previously shown to streamline service, ensure timely decision-making and improve survival outcomes, and is recommended by NICE UK (4). However, most of the available data are anecdotal or from limited series presented as abstracts in conferences. So far, there has been no reported study comparing the outcomes before and after the introduction of this service. Our study represents the first prospective data, albeit pilot study demonstrating the impact of the coordinator on MSCC pathway across a regional cancer network in the UK. The introduction of the service led to improved radiotherapy treatment times and improved engagement with critical medical support services to improve patient care and recovery.
Explanations of findings
The longest time to treatment was substantially reduced from 23 to 11 days since the introduction of the new role. Additionally, the percentage of patients treated within 24 hours significantly increased, with a figure of 37% vs. 65% (P=0.044) after the introduction of this role, which is critical to achieving the best possible neurological and functional outcomes for patients (4). Furthermore, during the pilot period from 1st April to 30th June 2021, there were 7 referrals each made to (I) HPC, (II) physiotherapy and occupational health and (III) hospice palliative care teams respectively.
Following treatment for MSCC, many patients are often discharged to their primary cancer care team, community palliative care, or hospice. Therefore, accessing clinical follow-up data on patients discharged to the community palliative or hospice teams was challenging. In addition, some people had moved out of area to be closer to relatives for rehabilitation or end-of-life care. Therefore, we were unable to assess survival outcomes in this study.
Implications and actions needed
Obstacles such as availability of funding, awareness of this service by new staff members during induction and training have to be resolved. This will be quite crucial for the wider adoption of the service. Furthermore, the service needs to be expanded to allow direct access referral from the community healthcare teams for patients who are diagnosed by general practitioners or community allied healthcare professionals. Adequate funding will ensure availability of cross-cover MSCC coordinator staff to support the service during annual or sick leave. This will help ensure continuity and maintain standard of care.
In future, it will be key to perform a contemporaneous comparison between 2021 subgroups that went through the MSCC coordinator versus those who did not, to further evaluate the impact of the new intervention. Further periodic data collection is required in order to assess the long-term impact of these interventions on the MSCC pathway across the region. Evaluation of the pathway for complex neurosurgical referrals to tertiary centres needs to be carried out to further reduce waiting times and expedite treatment decisions. Embedding these new changes across the region for every new generation of junior doctors and trainees is required to ensure sustained impact and improvement in quality of care across the region. Finally, the health economic impact and detailed patient-reported outcomes should be investigated and may be part of future prospective studies based on our initial findings.
Conclusions
Our study showed that the introduction of a MSCC coordinator role led to improved time from imaging to referral for radiotherapy treatment. The new service led to engagement with rehabilitative and palliative services. Future work should be done to assess the long-term impact of this role on utilization of support services and patient recovery.
Acknowledgments
Special thanks to Jane Webb, computer scientist at Maidstone Hospital who helped with electronic medical records data collection.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Stergios Boussios, Elie Rassy, Matin Sheriff and Aruni Ghose) for the series “Medical Oncology: Challenges in 2022” published in Annals of Palliative Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1102/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1102/dss
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1102/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1102/coif). The series “Medical Oncology: Challenges in 2022” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by ethics board of Quality Improvement Project (QIP) (No.: 190) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Spiller W. Rapidly progressive paralysis associated with carcinoma. Arch Neurol Psychiatr 1925;13:471-7. [Crossref]
- Loblaw DA, Laperriere NJ, Mackillop WJ. A population-based study of malignant spinal cord compression in Ontario. Clin Oncol (R Coll Radiol) 2003;15:211-7. [Crossref] [PubMed]
- Bach F, Larsen BH, Rohde K, et al. Metastatic spinal cord compression. Occurrence, symptoms, clinical presentations and prognosis in 398 patients with spinal cord compression. Acta Neurochir (Wien) 1990;107:37-43. [Crossref] [PubMed]
- Metastatic spinal cord compression. Available online: https://www.nice.org.uk/guidance/cg75/evidence/cg75-metastatic-spinal-cord-compression-full-guideline-2 (Accessed 21st Dec, 2022)
- Robson P. Metastatic spinal cord compression: a rare but important complication of cancer. Clin Med (Lond) 2014;14:542-5. [Crossref] [PubMed]
- Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila Pa 1976) 2010;35:E1221-9. [Crossref] [PubMed]
- Tokuhashi Y, Matsuzaki H, Oda H, et al. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 2005;30:2186-91. [Crossref] [PubMed]
- Loblaw A, George KJ, Misra V. Surgical and Radiotherapeutic Management of Malignant Extradural Spinal Cord Compression. Clin Oncol (R Coll Radiol) 2020;32:745-52. [Crossref] [PubMed]
- Boussios S, Cooke D, Hayward C, et al. Metastatic Spinal Cord Compression: Unraveling the Diagnostic and Therapeutic Challenges. Anticancer Res 2018;38:4987-97. [Crossref] [PubMed]
- Shah S, Kutka M, Lees K, et al. Management of Metastatic Spinal Cord Compression in Secondary Care: A Practice Reflection from Medway Maritime Hospital, Kent, UK. J Pers Med 2021;11:110. [Crossref] [PubMed]
- Koiter E, Poortmans P, Cloin B. Always on a Friday: referral pattern for metastatic spinal cord compression. Radiother Oncol 2013;107:259-60. [Crossref] [PubMed]
- Kinnaird W, Adeleke S, Lin R, et al. Radiotherapy Referral Patterns for Metastatic Spinal Cord Compression. Clin Oncol (R Coll Radiol) 2020;32:545. [Crossref] [PubMed]
- Adeleke SM, Kinnaird W, Lin R, et al. 394P Reversing the trend of Friday peak for metastatic spinal cord compression referrals. Ann Oncol 2020;31:S408. [Crossref]
- Adeleke S, Hakim R, Dean L, et al. Reversing the Friday peak in metastatic cord compression referrals: Not as simple as previously thought? J Clin Oncol 2021;39:e14050. [Crossref]
- Richards L, Misra V, Verma R, et al. Metastatic Spinal Cord Compression (MSCC) – Collaborative Work between the Tertiary Cancer Centre and the Specialist Spinal Centre Since the Introduction of the MSCC Coordinator Service Has Seen a Marked Increase in Surgical Rates, with 20% of Patients Who Presented with MSCC in the First 24 Months Having Spinal Surgery. This Has Resulted in Improved Survival Rates for MSCC Patients in Greater Manchester and Cheshire. Spine J 2017;17:S30-1. [Crossref]
- Richards L, Misra V, Shanahan C. O-9 The development of a centralized metastatic spinal cord compression coordinator service. BMJ Supportive & Palliative Care 2019;9:A3-4.
- Eriks IE, Angenot EL, Lankhorst GJ. Epidural metastatic spinal cord compression: functional outcome and survival after inpatient rehabilitation. Spinal Cord 2004;42:235-9. [Crossref] [PubMed]
- Ruff RL, Adamson VW, Ruff SS, et al. Directed rehabilitation reduces pain and depression while increasing independence and satisfaction with life for patients with paraplegia due to epidural metastatic spinal cord compression. J Rehabil Res Dev 2007;44:1-10. [Crossref] [PubMed]
- IRB Waiver or Alteration of Informed Consent for Clinical Investigations Involving No More Than Minimal Risk to Human Subjects. Guidance for Sponsors, Investigators, and Institutional Review Boards. Available online: https://www.fda.gov/files/about%20fda/published/IRB-Waiver-or-Alteration-of-Informed-Consent-for-Clinical-Investigations-Involving-No-More-Than-Minimal-Risk-to-Human-Subjects---Printer-Friendly.pdf (Accessed 21st Dec 2022)
- 2018 Requirements (2018 Common Rule). Available online: https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/revised-common-rule-regulatory-text/index.html#46.116 (Accessed 21st Dec 2022)
- Induction healthcare software. Available online: https://app.induction-app.com (Accessed on 21st Dec 2022)
- Low back pain and sciatica in over 16s: assessment and management. Available online: https://www.nice.org.uk/guidance/NG59 (Accessed 21st Dec 2022)


