
Chylothorax associated with primary membranous nephropathy: a case report
Highlight box
Key findings
• We report a case of primary membranous nephropathy presenting with chylothorax that was successfully treated with thoracic duct embolization along with immunosuppressive therapy.
What is known and what is new?
• Chylothorax associated with primary nephrotic syndrome has been reported rarely in adult patients.
• In chylothorax associated with nephrotic syndrome, prompt radiologic intervention for lymphatic leakage should be considered if initial medical therapy is ineffective.
What is the implication, and what should change now?
• This case may help design a therapeutic approach for isolated chylothorax associated with nephrotic syndrome. Further investigations are needed to elucidate the pathogenic mechanism of chylothorax in patients with primary nephrotic syndrome.
Introduction
Chylothorax is a condition in which milky chyle composed of fat particles is released into and accumulates in the pleural cavity due to damage to the lymphatic system, including the thoracic duct. It is associated with a variety of causes, including trauma, cardio-thoracic surgery, malignancy, congestive heart failure, liver cirrhosis, infections, and lymphatic disorders (1). However, due to its rarity, nephrotic syndrome is not well recognized as the cause of chylothorax. Nephrotic syndrome is a clinical syndrome characterized by high-grade proteinuria, generalized edema, hypoalbuminemia, hyperlipidemia, and hypercoagulability (2). Membranous nephropathy (MN) is a common glomerular disease that causes nephrotic syndrome in non-diabetic adults (3). In patients with nephrotic syndrome, the occurrence of chylothorax and/or chyloascites is rare in adults compared to children. Cases of chylothorax associated with nephrotic syndrome in adult patients have rarely been reported since the first description in 1968 (4-12). Therefore, we report a case of primary MN presenting with right-side chylothorax in a patient with bilateral leg edema, along with a literature review. We present this case in accordance with the CARE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-23-101/rc).
Case presentation
A 64-year-old male patient visited the nephrology clinic complaining of bilateral lower extremity edema and dyspnea. The patient had developed edema in both lower extremities 4 weeks prior, which progressed gradually. Shortness of breath during exercise started 2 weeks prior, but there were no complaints of cough, sputum, or chest pain at the time of presentation. He had been taking an angiotensin receptor blocker (candesartan, 16 mg/day) for hypertension, which was diagnosed 6 months before admission. There was no history of diabetes mellitus, chronic liver disease, heart failure, or chest trauma. At the time of admission, vital signs showed a blood pressure of 122/62 mmHg, a pulse of 65 beats/min, a respiration rate of 20 breaths/min, and a body temperature of 36.6 ℃. Physical examination revealed decreased breathing sounds in the right chest as well as moderate pitting edema in the pretibial region of both lower legs. Table 1 shows the results of peripheral blood examinations and serum biochemistry tests at the time of admission. Serum blood urea nitrogen, creatinine (Cr), and estimated glomerular filtration rates were 13.8 mg/dL, 0.8 mg/dL, and 93 mL/min/1.73 m2, respectively. Hypercholesterolemia, hypoalbuminemia, proteinuria, and microscopic hematuria were noted. On a 24-hour urine study, urinary protein excretion was 4,419 mg/day, and the Cr clearance was 101.7 mL/min. Serological markers, such as Venereal Disease Research Laboratory, hepatitis B surface antigen, anti-hepatitis C antibody (Ab), anti-human immunodeficiency virus Ab, anti-nuclear Ab, anti-neutrophil cytoplasmic Ab, and rheumatoid factor, were all negative. Right-side pleurisy was noted on chest computed tomography (CT) (Figure 1A,1B). On abdominal CT, both kidneys appeared unremarkable, with no evidence of ascites, vascular thrombosis, lymphadenopathy, or malignancy (Figure 1C,1D). The pleural fluid aspirated from the thoracentesis was yellowish and milky in appearance (Figure 2), and drainage was maintained after percutaneous catheterization. Pleural fluid analysis led to a diagnosis of lymphocyte-dominant transudate based on the pH of 7.23, 3,200/µL white blood cells (lymphocytes, 95%), pleural fluid-serum protein ratio of 0.35 (1.7/4.8), and pleural fluid-serum lactate dehydrogenase ratio of 0.36 (62/174) (Tables 1,2). Also, biochemical analysis results of the pleural fluid were consistent with the diagnostic criteria of chylothorax (13). The pleural fluid microbiologic results, including bacterial and fungal cultures, acid-fast bacilli smear, and polymerase chain reaction for Mycobacterium tuberculosis, were all negative. Pleural biopsy was not performed. The patient underwent percutaneous kidney biopsy on the third hospital day. A total of 34 glomeruli was identified under a light microscope. No intra-glomerular cell proliferation or abnormalities were observed except for thickening of the glomerular capillary wall (Figure 3A,3B).
Table 1
| Variables | Value | Reference |
|---|---|---|
| Hematology | ||
| WBC (/mm3) | 5,100 | 3,600–9,600 |
| Hemoglobin (g/dL) | 13.7 | 12.9–16.9 |
| Platelet (×103/μL) | 216 | 140–380 |
| ESR (mm/h) | 33 | 0–10 |
| Biochemistry | ||
| Glucose (mg/dL) | 115 | 70–100 |
| Total protein (g/dL) | 4.8 | 6.5–8.3 |
| LDH (U/L) | 174 | <250 |
| Urinalysis | ||
| RBCs (/HPF) | 3–9 | 0–1 |
| WBCs (/HPF) | 1–4 | 0–3 |
| Proteinuria (g/g Cr) | 3.32 | <0.3 |
| Serology | ||
| IgG (mg/dL) | 1,096 | 700–1,600 |
| IgA (mg/dL) | 274.5 | 70–400 |
| IgM (mg/dL) | 59.1 | 40–230 |
| Anti-thrombin III (%) | 69 | 80–120 |
| D-dimer (μg/mL) | 1.22 | 0–0.55 |
| Protein C (%) | 98.7 | 70–130 |
| Protein S (%) | 78 | 77–143 |
| Pro-BNP (pg/mL) | 375 | 16.9–136.7 |
| Tumor marker | ||
| AFP (ng/mL) | 2.08 | <10 |
| CEA (ng/mL) | 0.907 | <5.2 |
| PSA (ng/mL) | 0.611 | 0.6–4.5 |
WBC, white blood cell; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase; RBC, red blood cell; HPF, high-power field; Cr, creatinine; Ig, immunoglobulin; BNP, B-type natriuretic peptide; AFP, alpha fetoprotein; CEA, carcinoembryonic antigen; PSA, prostate specific antigen.
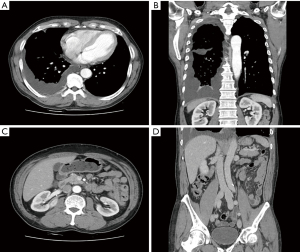
Table 2
| Year reported (reference) | Gender/age (years) | Kidney pathology | Serum | Pleural fluid | Treatment | Clinical outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Albumin (g/dL) | Chol (mg/dL) | TG (mg/dL) | Protein (g/dL) | LDH (U/L) | Chol (mg/dL) | TG (mg/dL) | Site/chyloascites | ||||||
| 1968 (4) | F/61 | MN | 1.5 | 400 | 360 | – | – | 12 | 127 | Both/+ | PD, steroid | Death | |
| 1989 (5) | M/50 | FSGS | 1.9 | 356 | 272 | 1.7 | 125 | 61 | 264 | Rt/+ | PD, pleurodesis | Resolution | |
| 1994 (6) | F/37 | FSGS | 1.5 | 508 | 186 | 1.2 | 30 | 98 | 242 | Rt/+ | – | – | |
| 2001 (7) | M/67 | MN | 1.6 | 437 | 227 | 0.8 | 150 | – | 154 | Rt/+ | PD, pleurodesis | Resolution† | |
| 2005 (8) | M/66 | MPGN | – | – | – | – | – | – | 97 | Rt/+ | – | – | |
| 2007 (9) | F/27 | MN | 1.4 | 280 | 270 | – | – | – | – | Both/+ | – | – | |
| 2009 (10) | M/38 | MCD | 1.6 | 304 | 229 | – | – | – | – | Lt/+ | Steroid, AT | Resolution | |
| 2021 (11) | F/32 | MN | 1.03 | 350 | 460 | 0.7 | – | 80 | 172 | Rt/+ | PD, IST, AT | Resolution | |
| 2022 (12) | M/66 | MN | – | 257 | 173 | –– | 50 | 10 | 167 | Rt/− | PD | Resolution | |
| Present | M/64 | MN | 2.3 | 260 | 138 | 1.7 | 62 | 53 | 274 | Rt/− | PD, IST, TDE | Resolution | |
†, on hemodialysis. Chol, total cholesterol; TG, triglyceride; LDH, lactate dehydrogenase; F, female; MN, membranous nephropathy; +, positive; PD, pleural drainage; M, male; FSGS, focal segmental glomerulosclerosis; Rt, right-sided; MPGN, membranoproliferative glomerulonephritis; MCD, minimal change disease; Lt, left-sided; AT, anticoagulation therapy; IST, immunosuppressive therapy; −, negative or not described; TDE, thoracic duct embolization.
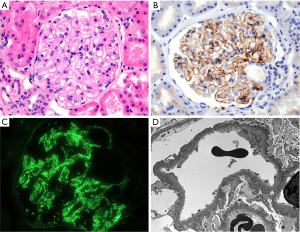
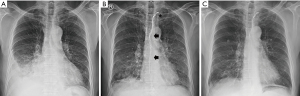
Immunofluorescence microscopy revealed immune deposition to immunoglobulin G (2+), complement 3 (1+), kappa (1+), and lambda (1+) in the glomerular basement membrane (GBM) (Figure 3C). Multiple subepithelial electron-dense deposits were noted with diffuse foot process effacement on electron microscopy (Figure 3D). Based on these findings, the patient was diagnosed with nephrotic syndrome due to MN. The serum concentration of M (muscle)-type anti-phospholipase A2 receptor (anti-PLA2R) Ab was 10.4 RU/mL, which was normal (reference value, <14 RU/mL). The results of serum tumor marker and stool occult blood tests performed to evaluate secondary causes of MN were all negative (Table 1). On day five, medical treatment for chylothorax was initiated while maintaining the pleurisy tube by stopping enteral feeding, starting total parenteral nutrition (TPN), and performing a subcutaneous injection of 0.1 mg of octreotide every 8 hours. Administration of an angiotensin receptor blocker was maintained as a conservative treatment for MN. After that, the chylous pleurisy gradually turned white, and the amount of drainage was appreciably reduced. On day 14, dyspnea and peripheral edema resolved. Concurrently, the serum albumin level was 2.3 g/dL, and the spot urine protein-to-creatinine ratio (PCR) was 2.68. However, on day 25, after non-per os for 3 weeks, the amount of chylous effusion increased again when enteral nutrition of a low-fat diet supplemented with medium-chain triglycerides was resumed. The patient’s anti-PLA2R Ab titer in serum also increased (50.8 RU/mL). On day 30, we instituted specific immunosuppressive therapy (IST) based on the modified Ponticelli regimen for treatment of primary MN. In other words, oral prednisolone (0.5 mg/kg) was administered daily after an intravenous infusion of 1.0 g of methylprednisolone for three consecutive days. The administration of octreotide was discontinued after 1 month due to frequent diarrhea. Dietary modification could no longer be continued due to the marked deterioration of nutritional status and the patient’s poor tolerance. On day 37, the patient was referred to the radiology department and underwent percutaneous lymphangiography and thoracic duct embolization (TDE); lymphangiography was performed by approaching the left inguinal node under ultrasound guidance. The cysterna chyli was punctured to access thoracic duct, and lymphatic leakage was confirmed in the thoracic duct and its tributaries near the mediastinum. TDE was performed after injecting histoacryl glue (1 lipiodol:2 n-butyl-2 cyanoacrylate) under fluoroscopic guidance (14). This procedure significantly reduced chylous drainage from the thoracic cavity, and the right pleurisy on chest X-ray showed remarkable improvement (Figure 4A,4B). The patient’s laboratory findings and nutritional status gradually improved, and he was discharged on day 53. After hospital discharge, IST was maintained for 6 months with alternating monthly cycles of glucocorticoids and oral cyclophosphamide (2.0 mg/kg/day). At 9 months of follow-up, clinical remission of MN was achieved, indicated by a serum albumin level of 3.5 g/dL, a serum Cr level of 0.7 mg/dL, and a urinary PCR of 0.32. No recurrence of chylothorax was found on chest X-ray at that time (Figure 4C).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Publication of this case report and accompanying images was waived from patient consent according to the institutional review board of Daegu Catholic University Medical Center (No. CR-22-070-PRO-001-R).
Discussion
MN is characterized by histopathologic renal injury in which the glomerular capillary wall thickens with little or no intra-glomerular cell proliferation or infiltration. It is a glomerular nephropathy that causes nephrotic syndrome in which immune deposits gradually accumulate in the subepithelial space of the GBM, as seen under an electron microscope, eventually resulting in damage to podocytes (3). Primary MN, which accounts for the majority of MN, is an isolated kidney disease caused by autoantibodies specific to endogenous podocyte antigens without an identifiable cause. In particular, the M-type PLA2R is a major target antigen found in about 70–80% of patients with primary MN (3,15). There were no specific abnormalities in serological markers or imaging tests to suggest secondary MN in this patient, but serum anti-PLA2R Ab was negative at the time of admission. Therefore, a kidney biopsy was performed, and nephrotic syndrome due to MN was diagnosed. However, with the persistence of clinical findings indicating nephrotic syndrome and chylothorax, the serum anti-PLA2R Ab titer was elevated to serologic positivity 4 weeks after hospitalization.
The patient was diagnosed with PLA2R-associated MN with immunologic progression and was started on combined IST. Recent studies indicate that serum anti-PLA2R Ab may appear during the course of follow-up evaluations, even if it is initially absent at the onset of MN (15). The reappearance and/or elevation of circulating anti-PLA2R Abs, which were previously negative or present at a lower titer, is a robust predictor of an impending relapse of MN (16).
Chylothorax is a clinical syndrome in which chyle leaks from the thoracic or lymphatic ducts and accumulates in the pleural space. After thoracentesis, if the presence of chylomicrons is confirmed through Sudan III staining of the pleural fluid or lipoprotein analysis, a diagnosis of chylothorax can be made (1). If such a test is not available, the lipid concentration in the pleural fluid is directly measured, and if triglyceride concentration is greater than 110 mg/dL (1.24 mmol/L) and cholesterol concentration is less than 200 mg/dL (5.18 mmol/L), a biochemical diagnosis of chylothorax can be made (1,16). In this case, the first drained pleural fluid after thoracentesis was milky in color, and the concentrations of pleural triglycerides and pleural cholesterol were 274 and 53 mg/dL, respectively. In addition, the pleural fluid-to-serum concentration ratio of triglyceride and cholesterol in this patient was greater than 1 (274/138) and less than 1 (53/260), respectively (Table 2). All these biochemical findings were consistent with chylothorax (17).
The pathogenesis of chylothorax associated with nephrotic syndrome remains unclear; it is explained by the following hypothesis. In patients with nephrotic syndrome, bowel edema is induced by severe hypoalbuminemia, which increases the permeability of lymphatic vessels in the intestinal mucosa or serosa. This progresses to chylomicron leakage from the lymphatics or thoracic duct and the development of chyloascites. Chylous fluid accumulation in the peritoneal cavity causes unidirectional migration of chyle into the pleural space through diaphragmatic lymphatic vessels or diaphragmatic defects due to negative intrathoracic pressure during inspiration (5). Additionally, in patients with nephrotic syndrome, a hypercoagulable state is often induced by loss of anti-thrombin III through the urine and activation of the blood coagulation pathway (2). At this time, thrombosis may occur in the superior vena cava or subclavian vein, and the centripetal movement of the chyle from the thoracic duct may be impeded, resulting in chylothorax (18). When chylothorax is isolated without concomitant chyloascites in a patient with nephrotic syndrome, thrombosis in the thoracic vein or superior vena cava should be investigated as the primary cause (19). A decrease in anti-thrombin III and an increase in D-dimer were found in the serum of this patient with chylothorax alone. However, no evidence suggestive of thoracic duct obstruction or thrombosis of the arteries or veins within the thorax was found on chest CT.
A total of nine cases of chylothorax has been reported since the first description of the condition in 1968 in adults (aged ≥18 years) with nephrotic syndrome (Table 2). An analysis of these nine cases found a male-to-female ratio of 1.25:1, and the average age at the time of diagnosis was 42.7 years (range, 27–67 years). MN was the most common cause of nephrotic syndrome, as witnessed in 5 cases (55.6%). In four cases, nephrotic syndrome and chylothorax appeared simultaneously (4,9-11), but there also have been cases where chylothorax was found 1 month or 5 years after onset of nephrotic syndrome (7,12). Thus, the elapsed time between onset of nephrotic syndrome and development of chylothorax did not show a consistent pattern. All cases had severe hypoalbuminemia (<2.0 g/dL) and hypercholesterolemia (>240 mg/dL). Eight patients had accompanying chyloascites. Chylothorax frequently occurred on the right side (six cases), but bilateral chylothorax was reported in two cases. The average concentration of triglycerides in the pleural fluid was 174.7 mg/dL (range, 97–264 mg/dL), which met the diagnostic criteria of chylothorax (>110 mg/dL) in six cases (Table 2). Our patient was a 64-year-old male with nephrotic syndrome due to primary MN. The serum albumin concentration (2.3 g/dL) at the time of chylothorax presentation was higher than the average (1.5 g/dL) of previously reported cases. In this patient, isolated chylothorax without chyloascites occurred, which was not consistent with the clinical manifestations of most of the previous cases.
Correction of underlying disease is a first-line treatment for chylothorax. In addition, supportive therapy for metabolic complications, such as malnutrition, electrolyte imbalance, and immune suppression due to continued loss of chylous fluid, has been suggested (17). Surgical therapy is recommended if chylous drainage is more than 1.5 L/day, if drainage is more than 1 L/day for 5 days, or if drainage persists for more than 2 weeks despite medical therapy (17). A surgical approach is considered if a patient’s nutritional status deteriorates rapidly during conservative treatment (1). As per previous cases of chylothorax in adult patients with nephrotic syndrome, repeated thoracentesis or percutaneous catheter drainage of pleurisy, administration of diuretics, and intravenous infusion of albumin were performed as primary treatments in most cases. There was also a case in which chylothorax and renal vein thrombosis co-occurred, where chylothorax improved after IST and chronic anticoagulation (11). Although chemical pleurodesis with tetracycline was attempted in two cases, its therapeutic efficacy was difficult to determine, as it resulted in complete resolution of chylothorax and dependence on regular hemodialysis after resurgence of chylothorax, respectively (4,7). In this case, generalized edema and respiratory distress were alleviated through percutaneous drainage of chylous effusion and combined diuretic and albumin therapy. Octreotide, a synthetic analogue of somatostatin that may reduce the amount of chylous fluid absorbed into the thoracic duct by inhibiting intestinal chyle production, was also administered (1). However, the nutritional status of the patient rapidly deteriorated during conservative medical therapy, such as alimentary abstinence for 3 weeks and the combination of TPN and other medications. Therefore, we performed intranodal lymphangiography to assess the leakage of lymphatic fluid, followed by TDE (14,20). During the follow-up period, more than 9 months after TDE, there was no relapse of chylous pleurisy or any sequelae related to the radiologic procedure, and the nutritional status of the patient improved remarkably. The precise mechanism of intrathoracic lymphatic injury in this patient was not clear. However, similar to the case described by Xu et al., it was presumed that disruption of the lymphatic vessel wall occurred due to tissue edema resulting from severe hypoalbuminemia, swelling of the lymphatics, and increased pressure in the thoracic duct, causing leakage of chylous fluid into the adjacent pleural cavity (12).
Conclusions
Patients with nephrotic syndrome such as primary MN may rarely exhibit refractory chylothorax without chylous ascites, increasing the risk of serious metabolic complications, such as severe malnutrition. Therefore, upon confirming the diagnosis of chylothorax associated with primary nephrotic syndrome, prompt radiologic assessment and appropriate intervention for lymphatic leakage should be considered in addition to specific IST for underlying glomerulopathy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-23-101/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-23-101/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Publication of this case report and accompanying images was waived from patient consent according to the institutional review board of Daegu Catholic University Medical Center (No. CR-22-070-PRO-001-R).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Riley LE, Ataya A. Clinical approach and review of causes of a chylothorax. Respir Med 2019;157:7-13. [Crossref] [PubMed]
- Busuioc RM, Mircescu G. Nephrotic Syndrome Complications - New and Old. Part 2. Maedica (Bucur) 2022;17:404-14. [PubMed]
- Ronco P, Beck L, Debiec H, et al. Membranous nephropathy. Nat Rev Dis Primers 2021;7:69. [Crossref] [PubMed]
- Lindenbaum J, Scheidt SS. Chylous ascites and the nephrotic syndrome. Report of a case, associated with renal vein thrombosis. Am J Med 1968;44:830-6. [Crossref] [PubMed]
- Moss R, Hinds S, Fedullo AJ. Chylothorax: a complication of the nephrotic syndrome. Am Rev Respir Dis 1989;140:1436-7. [Crossref] [PubMed]
- Voudiclari S, Sonikian M, Kallivretakis N, et al. Chylothorax and nephrotic syndrome. Nephron 1994;68:388. [Crossref] [PubMed]
- Lin SH, Lin YF, Shih YL. An unusual complication of nephrotic syndrome: chylothorax treated with hemodialysis. Nephron 2001;87:188-9. [Crossref] [PubMed]
- Chen YC, Kuo MC, Chen HC, et al. Chylous ascites and chylothorax due to the existence of transdiaphragmatic shunting in an adult with nephrotic syndrome. Nephrol Dial Transplant 2005;20:1501-2. [Crossref] [PubMed]
- Colak HB, Alicil T, Tekce H, et al. Chylous ascites and chylothorax due to membranous nephropathy. Clin Nephrol 2007;67:333-4. [Crossref] [PubMed]
- Lin WY, Lin GM, Wu CC. Coexistence of non-communicated chylothorax and chylous ascites in nephrotic syndrome. Nephrology (Carlton) 2009;14:700. [Crossref] [PubMed]
- Singh SK, Chauhan A, Swain B. A rare case of nephrotic syndrome with chylothorax. J Family Med Prim Care 2021;10:3498-501. [Crossref] [PubMed]
- Xu Y, Shi J, Xu S, et al. Primary nephrotic syndrome complicated by chylothorax: a case report. Ann Palliat Med 2022;11:2523-8. [Crossref] [PubMed]
- Bediwy AS, Al-Biltagi M, Saeed NK, et al. Pleural effusion in critically ill patients and intensive care setting. World J Clin Cases 2023;11:989-99. [Crossref] [PubMed]
- Lee EW, Shin JH, Ko HK, et al. Lymphangiography to treat postoperative lymphatic leakage: a technical review. Korean J Radiol 2014;15:724-32. [Crossref] [PubMed]
- van de Logt AE, Fresquet M, Wetzels JF, et al. The anti-PLA2R antibody in membranous nephropathy: what we know and what remains a decade after its discovery. Kidney Int 2019;96:1292-302. [Crossref] [PubMed]
- Wang YN, Feng HY, Nie X, et al. Recent Advances in Clinical Diagnosis and Pharmacotherapy Options of Membranous Nephropathy. Front Pharmacol 2022;13:907108. [Crossref] [PubMed]
- Ur Rehman K, Sivakumar P. Non-traumatic chylothorax: diagnostic and therapeutic strategies. Breathe (Sheff) 2022;18:210163. [Crossref] [PubMed]
- Bernet-Buettiker V, Waldvogel K, Cannizzaro V, et al. Antithrombin activity in children with chylothorax. Eur J Cardiothorac Surg 2006;29:406-9. [Crossref] [PubMed]
- Hanna J, Truemper E, Burton E. Superior vena cava thrombosis and chylothorax: relationship in pediatric nephrotic syndrome. Pediatr Nephrol 1997;11:20-2. [Crossref] [PubMed]
- Expert Panel on Vascular Imaging and Interventional Radiology. ACR Appropriateness Criteria® Chylothorax Treatment Planning. J Am Coll Radiol 2017;14:S118-26. [Crossref] [PubMed]



