
Therapeutic effect of microcurrent therapy in a rat model of secondary lymphedema
Highlight box
Key findings
• Microcurrent therapy promotes angiogenesis, and improves fibrosis in secondary lymphedema.
What is known and what is new?
• Secondary lymphedema is incurable disease and the treatment options remain limited.
• This study tried to investigate the effect of microcurrent therapy in the treatment of secondary lymphedema.
What is the implication, and what should change now?
• Microcurrent therapy may be a novel and non-invasive treatment modality for secondary lymphedema.
Introduction
Lymphedema is a medical condition in which protein-rich fluid accumulates in cutaneous and subcutaneous tissues due to lymphatic system insufficiency. Up to 120 million people worldwide suffer from lymphedema (1,2). It arises from primary lymphatic dysplasia or secondary lymphedema after resection or occlusion of the lymphatic system as a result of lymph node dissection and cancer radiotherapy (2,3). Secondary lymphedema is a clinically incurable disease that occurs commonly after surgical cancer treatment and/or radiation (1). The natural history of secondary lymphedema includes increasing limb swelling, fibrosis, inflammation, and abnormal fat deposition, which increases the risk of recurrent skin infections (4). One of the most common forms of lymphedema treatment is complex decongestive physical therapy (CDT), which consists of manual lymphatic drainage, compression bandaging, self-exercise, and skin care (5). However, lymphedema is a progressive and lifelong disease, and the treatment options for lymphedema remain limited (5,6). Moreover, CDT is labor-intensive and time-consuming, and the chronicity of lymphedema has been suggested as a negative predictive factor for the response to CDT (7).
Microcurrent therapy (MT) is an electrotherapeutic modality that is used to treat musculoskeletal pain. It delivers a very low electric current (<1 mA) across the skin, which is subsensory to the body and does not activate muscle contraction. Very low electric currents have been reported to stimulate adenosine triphosphate (ATP) synthesis, amino acid transportation, and protein synthesis in tissues. Sebastian et al. (8) reported that microcurrent electrical stimulation accelerates cutaneous wound healing by reducing inflammation and enhancing angiogenesis (9). Due to the characteristics of these microcurrent electrical stimulations, MT has been widely used in various studies related to wound healing. Nair reported a significant reduction in wound area and pain score after MT in patients with chronic wounds, such as diabetic foot ulcers, venous leg ulcers, and pressure ulcers (10). More recently, Kwon and Moon reported synergistic regenerative effects of polydeoxyribonucleotide (PDRN) and MT in an animal model for chronic rotator cuff tears. The results showed that combined therapy with PDRN and MT was more effective than PDRN alone in gross morphological and histological analyses (11).
Although lymphedema is associated with inflammation and fibrosis, there have been no studies on the therapeutic efficacy of MT in lymphedema. This study aimed to investigate the therapeutic effect of MT in a rat model for forelimb lymphedema induced by axillary lymph node dissection. This article is presented in accordance with the ARRIVE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-23-94/rc).
Methods
Rat model of forelimb lymphedema
The study protocol was approved by Animal Research Ethics Committee of Daegu Catholic University (No. DCIAFCR-201020-11-Y) in compliance with the national or institutional guidelines for the care and use of animals. Twelve male Sprague-Dawley rats weighing 300 to 350 g were used in this study. The rats were housed in separate cages at room temperature, 40–60% humidity, under a 12-hour light/dark cycle. The rats had free access to water and food. After one week of cage adaptation, a rat model of forelimb lymphedema was created by right axillary lymph node dissection. The rats were anesthetized by an intraperitoneal injection of 40 mg/kg tiletamine hydrochloride, zolazepam hydrochloride (Zoletil, Virbac, Carros, France), and 1.0 to 5.0 mg/kg xylazine (Rompun, Bayer AG, Leverkusen, Germany). A 0.1 mL of 0.5% methylene blue was injected intradermally to the right foot pad. Subsequently, a 10-mm-long incision was made into the dermis across the right axilla, and the stained axillary lymph nodes were removed. Two weeks after surgery, 12 rats were randomly divided into groups that underwent a MT in the lymphedematous forelimb (MT, n=6) and a sham MT in the lymphedematous forelimb (sham MT, n=6). The treated forelimbs were compared with the non-edematous contralateral forelimb of each rat (naive).
Application of MT
Before MT, rat forelimb epilation was performed using a commercial hair remover. Two weeks after axillary lymph node dissection, the MT device (intensity, 25 µA, frequency 8 Hz; Granthe; Cosmic Co., Seoul, Korea) was applied daily for 1 h in each session for two weeks. The probe (cathode) was placed on the distal forelimb, and the reference electrode (anode) was placed on the right axilla. Rats were allowed to ambulate freely in their cages.
Assessment of the forelimb circumference
The circumferences of the carpal joint and 2.5 cm above the carpal joint were measured 3 days and 14 days after surgery, weekly during MT, and 14 days after the last MT. The circumferences were evaluated by wrapping around the limbs with the thread, and measuring its length using a ruler. Each measurement was made two times on each occasion and a mean value was calculated.
Immunohistochemical analysis
The rats were euthanized by carbon dioxide inhalation 14 days after the last MT. Each forelimb was removed, fixed in 4% paraformaldehyde and embedded in paraffin; 4-mm sections were then prepared and deparaffinized. Immunohistochemical staining was performed by a BOND-III automated slide stainer (Leica Biosystems, Wetzlar, Germany) following the manufacturer’s instructions. To evaluate angiogenesis, an anti-pan-endothelial marker [cluster of differentiation (CD) 31, 1:100, Abcam, Cambridge, UK] was applied to the sections, a Bond Polymer Refine Detection kit (Leica Biosystems) was used, and the sections were counterstained with hematoxylin. For the detection of fibrotic tissue, sections were stained with Masson’s trichrome stain. Color images were captured using an automatic digital slide scanner (PANNORAMIC MIDI II; 3D HISTECH Ltd., Budapest, Hungary). Three randomly selected images per slide were obtained at ×200 magnification. The morphometric analysis of the percentage of stained area to the total area was measured as the percentage of total pixels in each image using the threshold technique (ImageJ software) (12). Quantification of the area covered by vessel was determined by measuring the percentage of CD31-positive area. The percentage of blue staining in each image for Masson’s trichrome stain was evaluated by color deconvolution technique, with the dye indicating the presence of collagen fibers in the tissue.
Western blotting
Western blot analysis was used to determine the expression of vascular endothelial growth factor receptor-3 (VEGFR3) and VEGF-C in the forelimbs. Skin samples were homogenized in RIPA buffer (Cell Signaling, #9806; Danvers, MA, USA) and centrifuged at 21,200 ×g at 4 ℃ for 15 min. The supernatants containing equal amounts (40 µg) of protein were separated by SDS-PAGE and the proteins were transferred to nitrocellulose membranes. After blocking with Tris-buffered saline containing 0.1% Tween 20 and 5% skim milk, the membranes were incubated with anti-VEGFR3 (1:1,000; Millipore, CA, USA), anti-VEGF-C (1:1,000; Santa Cruz Biotechnology, Dallas, TX, USA), or anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:5,000; Cell Signaling Technology) antibodies overnight at 4 ℃. The membranes were then incubated with horseradish peroxidase-linked secondary antibodies [Goat Anti-Mouse IgG antibody (HRP), GeneTex, USA] for 1 h at room temperature. The protein bands were visualized using an ECL kit (Thermo Fisher Scientific, Waltham, MA, USA), and densitometric analysis of band intensity was performed using a Chemi-Doc XRS imaging system (Bio-Rad, Hercules, CA, USA). The membranes were then probed with an anti-GAPDH antibody, which was used as the loading control.
Statistical analysis
All statistical analyses were performed using SPSS ver. 19.0 (IBM Corp., Armonk, NY, USA). Characteristics and outcomes were summarized using descriptive analysis. Quantitative variables were presented as means and standard deviations (SDs), and qualitative variables as percentages. Group comparisons of forelimb circumference and immunohistochemical and western blot data were performed using the Kruskal-Wallis test, followed by Dunn’s post hoc test. All tests were two-sided, and P<0.05 was accepted as indicating statistical significance.
Results
Decreased circumference of the forelimb in MT-treated rats
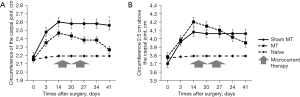
MT was administered two weeks after the axillary lymph node dissection, daily for 2 weeks. Circumference of rat’s forelimb was evaluated at the carpal joint and 2.5 cm above the carpal joint. In the MT group, the circumference at the carpal joint was significantly more attenuated 14 days after the last MIC (D 41) compared to the sham MT group (P=0.021) (Figure 1A). The circumference at 2.5 cm above the carpal joint tended to be reduced 14 days post-MT in the MT group, but no significant difference was observed between the groups (P=0.231) (Figure 1B).
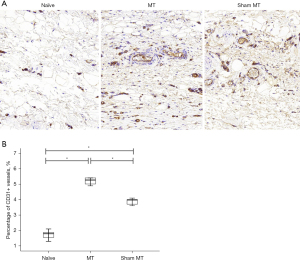
Increased vessel labeling in tissue of MT-treated rats
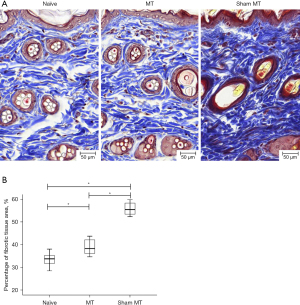
Representative images of the immunohistochemically stained blood vessels are shown in Figure 2A. The vessel density (% area) was significantly higher in the MT group than in the sham MT and in the non-edematous contralateral forelimbs groups (5.33% vs. 3.90% vs. 1.77%) (P<0.05) (Figure 2B). Representative images of fibrotic tissue immunohistochemically stained using Masson’s trichrome are shown in Figure 3A. The extent of fibrotic tissue (% area) was significantly attenuated in the MT group compared to the sham MT group (38.93% vs. 55.88%) (P<0.05) (Figure 3B).
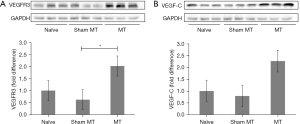
Increased expression of VEGR3 and VEFG-C in MT-treated rats
To identify the protein expression of VEGFR3 and VEGF-C at the tissue level, western blot analysis was performed 2 weeks after the last MT application. The expression of VEGFR3 was 2.02-fold higher for MT group compared for non-edematous contralateral forelimbs group. Expression in the MT group was significantly higher than that in the sham MT group (P=0.007) (Figure 4A). VEGF-C expression was 2.27-fold higher for MT group compared for non-edematous contralateral forelimbs group; however, the difference between the groups was not statistically significant (P=0.051) (Figure 4B).
Discussion
In this study, we assessed the efficacy of MT in the axillary lymph node dissection induced secondary lymphedema in a rat model. Our study showed that the application of MT tended to decrease the circumferences of edematous forelimbs, promote angiogenesis and reduce tissue fibrosis.
Lymphedema is a chronic disease for which there is no curative treatment (13). The initial histological change of lymphedema is characterized by the accumulation of protein-rich interstitial fluid in the subcutaneous and subfascial tissues (14). Chronic lymph stasis stimulates an increase in the numbers of fibroblasts, adipocytes, and keratinocytes in the skin. This condition promotes the progression of the disease (13). The most common form of lymphedema treatment is CDT, which includes manual lymphatic drainage, compression bandaging, self-exercise, and skin care (15). However, in the case of advanced lymphedema, which is characterized by extensive skin fibrosis, the treatment options are still limited and potentially uncomfortable, with visible garments adversely affecting the patient’s quality of life and the ability to perform daily tasks (4).
MT, a form of electrotherapy, has been used in clinical practice to reduce musculoskeletal pain such as shoulder and knee pain (16,17). Its potential mechanism is different from that of transcutaneous electrical nerve stimulation. In contrast to other electrical stimulation methods that bypass cells, microcurrent electrical stimulation mimics the currents generated in the body at the cellular level and is known to exert direct effects on cells (18). Therefore, in recent years, microcurrent electrical stimulation has been used in studies on wound healing (19-21). Previous studies have shown that MT application can accelerate angiogenesis and increase the blood supply to the wound. Bravo et al. (19) clarified that MT plays a critical role in wound healing by increasing fibroblast proliferation, modulating the inflammatory response, and aiding tissue regeneration. Lu et al. (20) demonstrated that a 100 µA microcurrent may support wound healing in keratinocytes of smoking patients by enhancing angiogenesis. In addition, microcurrent electrical stimulation causes cells to produce growth factors that help to sustain proliferating vascular networks (21). Considering the positive effects of MT in wound healing, the aim of the present study was to determine the effectiveness of MT in the treatment of lymphedema.
Our results are consistent with those of previous studies. The number of CD31-positive vessels was significantly higher in the MT group than in the sham MT group. In addition, the morphological appearance of the vessels appeared tortuous when lymphedema was induced in the rat forelimb; interestingly, after two weeks of MT, the abnormal morphologic appearance of these tortuous vessels was observed to be somewhat improved in the immunohistochemical analysis by a pathologist. The expressions of VEGFR3 and VEGF-C were higher in the MT group than in the sham MT group. Overexpression of VEGFR3 and its ligand, VEGF-C, induces an increase of lymphatic capillaries density, which improves lymphatic circulation (2,22). Although the mechanisms underlying the effects of MT in lymphedema are not fully understood, it has been suggested that microcurrent electrical stimulation enhances tissue healing via ATP synthesis. Previous studies suggested that MT stimulates ATP synthesis by facilitating the electron transport chain in mitochondria (9). The microcurrent influences the migration of extracellular calcium ions to penetrate the cell membrane, and a higher level of intracellular calcium encourages ATP synthesis. Eventually, protein synthesis is induced by mechanisms that control DNA, thus decreasing inflammation and promoting cellular repair (23).
Additionally, MT may affect the function of cell nuclei, activate genes that adjust collagen breakdown, and induce the relief of fibrosis. Lennox et al. found that after MT, radiation-induced fibrosis in patients with head and neck cancer significantly improved cervical rotational range of motion (23). In a randomized placebo-controlled trial, Kwon and Park used ultrasonographic evaluation to show that in torticollis patients, the thickness and cross-sectional area of the sternocleidomastoid muscles decreased after MT (24). These findings are in accordance with our results. In the current study, MT caused a significant reduction in tissue fibrosis, suggesting that the percentage of Masson’s trichrome density was significantly attenuated in the MT group compared to the sham MT group.
The therapeutic effect of MT is dependent on its intensity. A previous study demonstrated that low-intensity electrotherapy improved the damaged tendons and ligaments healing (11). A recent study demonstrated that intensity of 50 µA was more effective than 500 µA in improving tendon healing in chronic tennis elbow (25). Kwon and Moon found that electrical stimulation with microcurrent (intensity 25 µA, frequency 8 Hz) for 60 min/day for 4 weeks could promote the healing process of a full-thickness rotator cuff tear in a rabbit model (11). Based on previous studies suggesting that low-amperage currents promote tissue regeneration, we also applied a low intensity of 25 µA microcurrent electrical stimulation in a rat model of secondary lymphedema. No adverse effects were observed because MT works at the microampere level and mimics the electrical intensity observed in living tissues.
The present study has several limitations. First, the sample size was insufficient for obtaining statistically significant results. Second, the study was conducted over a relatively short period, and further studies are needed to assess the long-term effects of MT. Third, additional studies are warranted to evaluate the effects of MT at different frequencies, intensities, and durations. Fourth, we did not evaluate lymphatic circulation using lymphography/lymphoscintigraphy according to the changes of forelimb circumference as well as immunohistochemical results. Fifth, in future studies, the quantification of lymphangiogenesis using lymphatic endothelial cell specific markers as podoplanin, LYVE-1, or Prox-1 will be needed.
Conclusions
Our findings indicate that MT promotes angiogenesis and improves fibrosis in secondary lymphedema. These results suggest that MT may be a novel and non-invasive treatment modality for secondary lymphedema.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding: This work was supported by the grant of Research Institute of Medical Science, Catholic University of Daegu (2020).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-23-94/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-23-94/dss
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-23-94/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-23-94/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the Animal Research Ethics Committee of Daegu Catholic University (No. DCIAFCR-201020-11-Y) in compliance with the national or institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dayan JH, Ly CL, Kataru RP, et al. Lymphedema: Pathogenesis and Novel Therapies. Annu Rev Med 2018;69:263-76. [Crossref] [PubMed]
- Cho HK, Sung WJ, Lee YJ, et al. Two methods of extracorporeal shock-wave therapy in a rat model of secondary lymphedema: a pilot study. J Int Med Res 2021;49:3000605211024473. [Crossref] [PubMed]
- Cohen SR, Payne DK, Tunkel RS. Lymphedema: strategies for management. Cancer 2001;92:980-7. [Crossref] [PubMed]
- Rockson SG, Keeley V, Kilbreath S, et al. Cancer-associated secondary lymphoedema. Nat Rev Dis Primers 2019;5:22. [Crossref] [PubMed]
- Lasinski BB. Complete decongestive therapy for treatment of lymphedema. Semin Oncol Nurs 2013;29:20-7. [Crossref] [PubMed]
- Zasadzka E, Trzmiel T, Kleczewska M, et al. Comparison of the effectiveness of complex decongestive therapy and compression bandaging as a method of treatment of lymphedema in the elderly. Clin Interv Aging 2018;13:929-34. [Crossref] [PubMed]
- Eyigör S, Cinar E, Caramat I, et al. Factors influencing response to lymphedema treatment in patients with breast cancer-related lymphedema. Support Care Cancer 2015;23:2705-10. [Crossref] [PubMed]
- Sebastian A, Syed F, Perry D, et al. Acceleration of cutaneous healing by electrical stimulation: degenerate electrical waveform down-regulates inflammation, up-regulates angiogenesis and advances remodeling in temporal punch biopsies in a human volunteer study. Wound Repair Regen 2011;19:693-708. [Crossref] [PubMed]
- Cheng N, Van Hoof H, Bockx E, et al. The effects of electric currents on ATP generation, protein synthesis, and membrane transport of rat skin. Clin Orthop Relat Res 1982;264-72. [Crossref] [PubMed]
- Nair HKR. Microcurrent as an adjunct therapy to accelerate chronic wound healing and reduce patient pain. J Wound Care 2018;27:296-306. [Crossref] [PubMed]
- Kwon DR, Moon YS. Synergic regenerative effects of polydeoxyribonucleotide and microcurrent on full-thickness rotator cuff healing in a rabbit model. Ann Phys Rehabil Med 2020;63:474-82. [Crossref] [PubMed]
- Caetano GF, Fronza M, Leite MN, et al. Comparison of collagen content in skin wounds evaluated by biochemical assay and by computer-aided histomorphometric analysis. Pharm Biol 2016;54:2555-9. [Crossref] [PubMed]
- Ridner SH. Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Support Care Cancer 2005;13:904-11. [Crossref] [PubMed]
- Korpan MI, Chekman IS, Starostyshyn RV, et al. Lymphedema: clinic-therapeutic aspect. Lik Sprava 2010;11-20. [PubMed]
- The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology 2020;53:3-19. [PubMed]
- Yi D, Lim H, Yim J. Effect of Microcurrent Stimulation on Pain, Shoulder Function, and Grip Strength in Early Post-Operative Phase after Rotator Cuff Repair. Medicina (Kaunas) 2021;57:491. [Crossref] [PubMed]
- Lawson D, Lee KH, Kang HB, et al. Efficacy of microcurrent therapy for treatment of acute knee pain: A randomized double-blinded controlled clinical trial. Clin Rehabil 2021;35:390-8. [Crossref] [PubMed]
- McMakin CR, Oschman JL. Visceral and somatic disorders: tissue softening with frequency-specific microcurrent. J Altern Complement Med 2013;19:170-7. [Crossref] [PubMed]
- Bravo MP, Soares GP, Daniele de Oliveira P, et al. Microcurrent stimulates cell proliferation and modulates cytokine release in fibroblast cells. J Wound Care 2021;30. IIIi-IIIix. [Crossref] [PubMed]
- Lu C, Prahm C, Chen Y, et al. Microcurrent Reverses Cigarette Smoke-Induced Angiogenesis Impairment in Human Keratinocytes In Vitro. Bioengineering (Basel) 2022;9:445. [Crossref] [PubMed]
- Carley PJ, Wainapel SF. Electrotherapy for acceleration of wound healing: low intensity direct current. Arch Phys Med Rehabil 1985;66:443-6. [PubMed]
- Yoon YS, Murayama T, Gravereaux E, et al. VEGF-C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema. J Clin Invest 2003;111:717-25. [Crossref] [PubMed]
- Lennox AJ, Shafer JP, Hatcher M, et al. Pilot study of impedance-controlled microcurrent therapy for managing radiation-induced fibrosis in head-and-neck cancer patients. Int J Radiat Oncol Biol Phys 2002;54:23-34. [Crossref] [PubMed]
- Kwon DR, Park GY. Efficacy of microcurrent therapy in infants with congenital muscular torticollis involving the entire sternocleidomastoid muscle: a randomized placebo-controlled trial. Clin Rehabil 2014;28:983-91. [Crossref] [PubMed]
- Poltawski L, Johnson M, Watson T. Microcurrent therapy in the management of chronic tennis elbow: pilot studies to optimize parameters. Physiother Res Int 2012;17:157-66. [Crossref] [PubMed]


