
Emergent radiotherapy for brain and leptomeningeal metastases: a narrative review※
Introduction
Background
There is no consensus regarding what defines an oncologic emergency, what conditions require emergent radiotherapy (RT), or the appropriate timeframe of initiating emergent RT. Cancer Care Ontario has defined oncologic emergencies as ‘medical conditions arising from a reversible threat to organ function requiring radiation treatment within a few hours of diagnosis (1). Some providers question if emergent indications for RT truly exist, given the delayed responses seen with RT and ability to temporize patients with medical management. Given that data on emergent RT are largely retrospective, physicians must subjectively assess if a delay in treatment initiation may compromise patient outcomes.
A review of emergent RT practice patterns at a Canadian cancer center found that brain metastases (BM) were the second most common indication for emergent treatments (15%) (1). While another retrospective Canadian institutional review found the brain to be the fourth most common organ emergently treated (12.1%) (2). A multicenter patterns of care study at 140 RT centers (university, community, and private practice) in Germany, Austria, and Switzerland identified 3,244 cases of emergent RT. Of these, increased intracranial pressure (ICP) was the third most common indication for emergent RT (11.3%). Seventy percent of these cases had symptomatic improvement, defined as a greater than 25% decrease in symptom intensity (3). As the incidence of central nervous system (CNS) metastases is thought to be rising due to improved systemic therapies prolonging patient survival, emergent presentation of CNS metastasis may be more commonly encountered (4-6). With this background, we set out to perform a review of emergent RT for CNS metastases, specifically focusing on BM and leptomeningeal metastases (LM).
We define the indication for emergent RT of BM and LM as symptomatic metastases despite initiation of standard medical therapies, such as corticosteroids, not better suited for surgical resection, systemic anti-cancer therapy, or supportive care alone. Symptoms may include neurological deficits or signs of mass effect such as headache, nausea, seizure, or altered mentation. We define emergent as requiring treatment initiation within 24–48 hours of symptomatic presentation, including a need to initiate treatment after typical clinical hours or on a weekend or clinic holiday. Thus, a patient with minimal-to-no symptoms while medically managed (e.g., corticosteroids), may not require emergent initiation of RT.
Objectives
- Review the initial work-up and management of BM and LM.
- Define when surgical intervention or systemic anti-cancer therapy may be preferred to emergent RT.
- Define the role of emergent RT for BM, while reviewing appropriate treatment approaches.
- Define the role of emergent RT for LM, while reviewing appropriate treatment approaches.
We present this article in accordance with the Narrative Review reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1276/rc).
Methods
To inform this narrative review, literature searches in PubMed and Google Scholar were conducted in English, Table 1. All publication years were considered, with preference given to articles employing modern RT techniques, when applicable. Full manuscripts and abstracts were considered. Searches were conducted using, but not limited to, combinations of such keywords as ‘emergent radiotherapy’, ‘brain metastases’, and ‘leptomeningeal metastases’. For topics with a paucity of high-quality published evidence, discussion was supplemented by the authors’ expert commentary.
Table 1
| Items | Specification |
|---|---|
| Date of search | April 2022 |
| Databases and other sources searched | PubMed, Google Scholar |
| Search terms used | Combinations of keywords such as, but not limited to: emergent radiotherapy, brain metastases, leptomeningeal metastasis |
| Timeframe | All publication years considered |
| Inclusion criteria | All English language full manuscripts and abstracts were eligible for consideration |
| Selection process | A.B.B. conducted the selection alone and consensus was obtained through all coauthors' review of the manuscript and the list of selected references |
What is the appropriate initial work-up of brain and LM?
Initial evaluation of a cancer patient with neurologic symptoms includes a focused history and physical examination. During the initial examination, the patient must be assessed for signs of increased ICP or herniation, with particular attention given to Cushing’s Triad of widened pulse pressure, bradycardia, and irregular respiration, as well as focal neurologic signs, including cranial nerve deficits. A detailed examination should be performed to distinguish baseline neurological symptoms, including those related to prior therapies (e.g., prior surgery or RT), from new symptoms. New symptoms should be localized to guide initial radiographic examination, which must be compared to prior imaging when available. Serial neurological examinations are essential.
Computed tomography (CT) and contrast-enhanced magnetic resonance imaging (MRI) are the most valuable imaging modalities. Non-contrast head CT is often the initial examination used to emergently identify hemorrhage, hydrocephalus, and gross mass effect. MRI has enhanced resolution as compared to CT and is required for full characterization of CNS disease burden in order to guide optimal management. When interpreting MRI results in patients with previously irradiated metastases that are newly symptomatic, providers should consider radiation necrosis as a possible etiology (7). In patients with de novo CNS metastases and an unknown primary, systemic imaging should be obtained to identify a primary and to find an accessible site for a biopsy to establish diagnosis. If LM is suspected due to symptoms such as radiculopathies, multiple cranial neuropathies, elevated ICP (e.g., papilledema), unexplained severe headache with nausea and vomiting, or neurological symptoms not clearly explained by focal lesions, contrast-enhanced MRI of the entire craniospinal axis (brain through cauda equina) should be performed. The gold standard for LM diagnosis is identification of malignant cells in cerebrospinal fluid (CSF) via lumbar puncture (LP), but in clinical practice, LP is often omitted when the diagnosis is radiographically and clinically apparent (8). Due to the false-negative rate of single CSF samples, repeated sampling can be considered. More sensitive assays, such as CSF tumor cells, can be considered and are being investigated (9-12). If possible, MRI for LM should be obtained prior to LP or CSF diversion procedure, due to the possibility of these procedures causing artifactual findings. As neither MRI or CSF cytology are fully sensitive for diagnosis of LM, diagnostic criteria are based upon pathology, imaging, and clinical findings (8).
Following identification of clinically significant BM or LM, multidisciplinary consultation involving neurosurgery, radiation oncology, and medical and/or neuro-oncology is recommended. A patient’s goals of care must be elucidated directly or via an alternate decision maker (13), and the option of best supportive care should be discussed. All patients with CNS metastases should have non-urgent referral to palliative care, if not already established. Steroids should be started immediately for symptomatic patients without contraindications, and can be given prior to full workup if clinical suspicion is present. If an etiology of undiagnosed lymphoma is suspected, the clinician and medical oncologist should discuss the benefit of prompt corticosteroid initiation versus risk of obfuscating diagnosis. For patients with moderate to severe symptoms related to mass effect, dexamethasone doses of at least 16 mg per day should be started, given intravenously during the acute phase, whereas 4–8 mg per day can be used for mild symptoms (14). Prophylactic use of anti-epileptic drugs (AED) is not recommended as routine management (15), however, expert consultation should be obtained to discuss use of AED as some providers may advocate for their use in certain clinical situations (e.g., acute hemorrhagic metastasis or large metastases in epileptogenic areas) (16). Other medical interventions, such as the use of hyperventilation or osmotic agents, are outside the scope of this review.
When is surgery the preferred treatment modality?
In scenarios with significant mass effect, hemorrhagic metastases, or increased ICP, surgical intervention is the most immediate and effective method for averting neurologic catastrophe following initial medical management. In emergent situations, surgery is often considered the standard of care unless contraindicated, as it is the only intervention that can immediately prevent impending herniation or severe hydrocephalus. In the setting of hydrocephalus, intervention may consist of a temporary CSF diversion that can be performed at bedside or a CSF shunt, prior to treatment with a different modality. In the setting of mass effect, tumor resection is clearly preferred in medically-operable patients with good performance status and a newly diagnosed solitary BM amenable to safe resection. This recommendation can be extended to patients with a limited number of metastases, particularly when the offending lesion is accessible via a single surgical approach. In the presence of extensive BM, LM, or uncontrolled extracranial disease, immediate surgical intervention to prevent rapid neurologic deterioration may be required for lesions causing significant mass effect. We recommend surgery be strongly considered for tumors in the posterior fossa, as large metastases in the cerebellum represent a life-threatening condition due to the potential for brainstem compression and/or acute hydrocephalus (17). For tumors considered to be highly radiosensitive or chemoresponsive [e.g., small cell lung cancer (SCLC), hematologic malignancy, germ-cell tumors (GCT)], these treatment modalities may be considered based upon a patient’s complete clinical picture. Finally, while not critical to consider in the emergent setting, resection allows for identification of actionable mutations not present in the primary tumor (18), change in receptor subtype, and histologic diagnosis in patients with de novo metastatic disease.
Compared to upfront whole brain radiation therapy (WBRT), class I evidence favors the use of surgical resection followed by RT for newly diagnosed solitary BM in the non-emergent setting. As the following studies did not specifically evaluate patients in the emergent setting, our recommendations are extrapolated from such studies combined with the authors’ shared clinical experience. Three randomized studies have compared upfront WBRT to surgical resection (19-21). All three of these randomized studies excluded SCLC and lymphoma, while GCTs, leukemia, and multiple myeloma were excluded on a less consistent basis. One trial excluded patients requiring immediate treatment to prevent acute neurologic deterioration (19). Two studies found a survival benefit to upfront surgery, but noted that the extent of systemic disease and older age were associated with a reduction or absence of surgical benefit (19,21). The third study did not find a difference in median survival by treatment modality (20). The discordant result of this trial may be due to the study containing patients of lower performance status or who more frequently had extensive systemic disease burden, thus leading to increased mortality from systemic progression. These randomized studies and additional observational studies are reviewed in detail elsewhere (22,23).
When upfront surgical resection is performed for solitary BM, adjuvant RT is preferably given 3–4 weeks post-operatively, but is occasionally delayed up to 8 weeks due to patient-specific factors. While class I evidence supports the role of adjuvant WBRT after surgical resection for the endpoint of decreased brain recurrence (24), the role of stereotactic radiosurgery (SRS) has also been evaluated (25,26). As adjuvant RT occurs after the emergent treatment period, a full discussion of these approaches falls outside the scope of this review. Pre-operative radiosurgery has also been studied, but is typically employed in patients in whom symptoms improve on steroids or AEDs to allow radiosurgery planning (27,28).
In the setting of multiple BM, no high-level data exists to guide optimal selection of upfront therapy to maximize patient outcomes, but retrospective studies have examined outcomes for patients with a limited number of BM undergoing surgical resection. One study compared outcomes from solitary BM resection to patients with multiple BM who had resection of all (≤3 BM) or some BM. This study found equivalent survival for patients undergoing resection of solitary BM to those having complete resection of up to 3 BM (29). Another retrospective study compared surgical resection of multiple BM causing significant symptomatic mass effect to solitary symptomatic BM, finding similar performance and survival benefits without increased perioperative complications (30).
While these retrospective studies provide some insight into the utility of resecting a limited number of BM, there is less evidence guiding surgical management in emergent situations, particularly when >3 BM are present. In such situations, the role of surgery is to avert irreversible neurologic catastrophe, as opposed to achieving intracranial control. Subjective clinical decision-making is required to select the most appropriate intervention, but in general, resection is recommended for up to 2–3 symptomatic metastases (31), and surgical intervention is recommended to address CSF outflow obstruction, significant midline shift, and posterior fossa tumors threatening herniation. After the initial neurological emergency is stabilized via surgery, additional tumor-directed treatments can be pursued, including adjuvant RT.
When are systemic therapies the preferred treatment modality for BM in the emergent setting?
No high-level data exists to support the routine use of systemic therapies as upfront management in a neurologic emergency. For treatment of symptomatic BM, recent societal guidelines preferentially recommend local to systemic therapy (32,33). A patient may fail to be a candidate for local therapy in rare situations when intracranial progression or recurrence follows prior local therapies (e.g., recent prior CNS RT), or when extracranial disease progression is life threatening. Emerging data on the intracranial efficacy of some kinase inhibitors (KI) identifies a role for these agents in KI-naïve patients with melanoma or non-small cell lung cancer (NSCLC) when a targetable mutation is present. However, further work is need to guide when these agents should be used preferentially to RT. Multidisciplinary management is essential in making a decision to initiate upfront systemic therapy. In these uncommon emergent situations, decision to initiate systemic therapies is often guided by tumor histology, which will be the focus of this section.
For CNS metastases from gestational trophoblastic neoplasia (GTN) or GCTs, chemotherapy is generally the preferred non-emergent approach. Based on review of retrospective data and expert opinion, systemic therapy should generally serve as the frontline treatment of GTN with BM, with the use of RT being limited to cases of resistant/recurrent disease requiring palliation, or in the context of clinical trials. If the patient has symptoms from mass effect of the metastasis, an urgent neurosurgical consultation should be sought (34,35). For GCTs, chemotherapy is preferred when BM are identified at initial diagnosis, while multimodal therapy tailored to a patient’s unique situation is preferred in the case of relapsed or resistant disease (36).
Lung primaries are the most common source of BM (37). Although there is a weak recommendation for using targeted therapies in the treatment of BM from NSCLC in the non-emergent setting (32), data are limited in the emergent setting. For epidermal growth factor receptor mutant NSCLC treated with osimertinib, a subgroup analysis within a phase 3 trial demonstrated a progression-free survival benefit for patients with CNS metastases (38), and a phase 2 study demonstrated a CNS response in at least half of patients who had RT-naïve CNS metastases (39). Icotinib can also be considered for patients with BM (32). Second generation anaplastic lymphoma kinase (ALK) inhibitors have shown clear benefit for patients with BM in phase 3 studies (40,41). A phase 2 study of the third generation ALK and ROS1 inhibitor lorlatinib demonstrated substantial intracranial activity, including in patients that had progressed on prior ALK-targeted therapy (42). In a combined analysis of phase 1 and phase 2 studies for entrectinib in patients with ROS1 fusion-positive NSCLC, 11 of 20 patients with CNS metastases obtained an intracranial response (43). Currently, a weak recommendation exists regarding the use of alectinib, brigatinib, and ceritinib prior to local therapy for BM (32). A final consideration is the use of pembrolizumab, which can be considered for NSCLC patients with programmed death-ligand 1 expression, who are receiving pemetrexed and a platinum agent (32). For SCLC, systemic therapies can be effective for CNS disease, but focal therapy with radiation should precede chemotherapy due to the radiosensitivity of this histology.
The treatment of breast cancer is highly dependent upon receptor subtype. Triple negative breast cancers have a high rate of CNS involvement in the metastatic setting, but currently lack systemic therapies approved in the setting of BM. Metastatic HER2-amplified breast cancer also frequently involves the CNS. A phase 2 study of capecitabine combined with lapatinib found objective CNS response in 66% of patients (44). A phase 2 study of trastuzumab plus pertuzumab in patients with progressive BM showed clinical benefit in 68% of patients (45). Additionally, a retrospective analysis of trastuzumab emtansine (TDM1) suggested improved survival as compared to capecitabine-lapatinib for patients with HER2-amplified CNS metastases (46). From the authors’ personal experience, we advise caution when managing a patient with a high burden of CNS disease on TDM1 due to the potential for rapid cell lysis leading to worsening neurologic status. While single arm studies, multiple studies have demonstrated high intracranial response rates to trastuzumab deruxtecan (47-49). Currently, the combination of tucatinib, trastuzumab, and capecitabine is weakly recommended for patients that have progressed on prior HER2-directed therapy and have asymptomatic BMs (32). Regarding hormone-positive breast cancers, cyclin-dependent kinase 4/6 inhibitors have significantly improved progression free survival, but their potential use in CNS disease is limited. Phase 2 data on abemaciclib showed an intracranial clinical benefit rate of 24% (50). In a phase 2 study of the novel therapeutic paclitaxel trevatide used in recurrent BM or LM without regard to receptor subtype, substantial CNS treatment effect was shown, with potentially prolonged survival when treating LM (51).
Melanoma has a propensity for CNS metastasis. Phase 2 data supports the use of combined immune checkpoint blockade with ipilimumab/nivolumab in patients with untreated melanoma BM, with intracranial clinical benefit in 56% of the 94 study patients (52). However, the concurrent use of high-dose steroids reduces the benefit of immunotherapy (53), making immunotherapy a poor treatment option in the emergent setting. Agents targeted at mutations in the mitogen-activated protein kinase pathway have demonstrated clinical benefit in melanoma. Most notably, a phase 2 study of combination dabrafenib and trametinib in patients harboring BRAFV600E mutant melanoma with radiation-naïve BM showed an intracranial response in greater than 50% of patients (54). Combination encorafenib and binimetinib has also demonstrated a significant intracranial response (55). Randomized data are needed to guide optimal decision-making for patients with melanoma BM, but these phase 2 data suggest a promising potential for systemic therapies. Given the uncertain role of upfront molecular marker targeted therapy in the emergent setting, when the goal is rapid relief of neurologic symptoms, the risk of deferring local therapy has to be carefully weighed against the benefit of this strategy. In general, if any of the lesions is larger than 2 cm and/or if there are neurologic deficits or neurologic symptoms, emergent local therapy, namely, surgery or RT, should be considered.
When should emergent RT be considered for BM, and what treatment approach should be used?
For patients not requiring rapid decompression, with BM that are too widespread to be surgically addressed, or when surgical morbidity is deemed prohibitive (e.g., tumor in eloquent cortex), upfront radiation should be considered. In the emergent setting, treatment should be initiated as 2D- or 3D-conformal WBRT as opposed to more advanced techniques, such as WBRT with hippocampal avoidance (HA-WBRT) or SRS. While various appropriate dose-fractionations exist for WBRT, we generally recommend 30 Gy in 10 or 20 Gy in 5 treatment fractions. The use of a 10 vs. 5 fraction treatment course may be guided by prognosis, although supportive care alone should be explored as an alternative option in the setting of poor prognosis (33). Additional consideration for use of WBRT may be given to histological subtypes that are radiosensitive and/or prone to occult micrometastatic disease (e.g., SCLC). WBRT may be contraindicated in patients with significant mass effect or obstruction, severe cerebral edema, active hemorrhage, or if a large lesion in the posterior fossa is threatening herniation. Unlike surgery, the therapeutic benefit of RT is not immediate, thus there is no consensus definition of situations requiring emergent RT, and this decision is left to the subjective discretion of the primary physician. Due to a lack of prospective studies regarding the use of emergent RT for BM, these recommendations are based on expert opinion and extrapolation from non-emergent settings.
In the emergent setting, parallel-opposed or 3D-conformal WBRT is the preferred modality. Partial brain irradiation (PBI) could be considered in select cases, but carries an increased risk of out-of-field failures and can complicate field matching with future RT courses, and is thus not recommended as standard therapy. A prescription of 20 Gy in 5 fractions should be considered if using PBI. Mounting evidence supports the use of SRS in the non-emergent setting due to studies suggesting improved survival and neurocognitive outcomes when SRS is used for a limited number of BM as compared to WBRT (56-58). However, patients requiring emergent RT for BM typically have significant neurologic symptoms, making treatment with SRS unsafe given the risk of acutely worsening a patient’s symptoms during a critical period. HA-WBRT is employed to reduce the cognitive toxicity of WBRT (59), but the complexity of treatment planning would delay emergent initiation of care. Instead, memantine can be considered for use as a neuroprotectant during RT (60).
Various acceptable dose-fractionations exist for WBRT, although none have been validated for use in the emergent setting. Two of the most common used dose-fractionations, 30 Gy in 10 or 20 Gy in 5 treatment fractions, were established as standard practice four decades ago (61). Attempts to improve functional or survival outcomes by using protracted treatment courses or twice daily treatments to a higher total dose have not demonstrated clinical benefit (62-64). Shorter treatment courses of 10 Gy in 1 or 12 Gy in 2 fractions have also been investigated and can be beneficial in addressing neurologic symptoms, but provide lower rates of complete symptom improvement and have a less durable response (65). To improve the therapeutic ratio of WBRT, studies have attempted to combine radiosensitizers to WBRT, but have not shown a benefit (66,67) and are not currently recommended for clinical use (32). There is insufficient evidence to support selection of dose-fractionation based upon histology (22,68,69). In the emergent setting, shorter treatment schedules may be favored due to worse prognosis and higher rates of early treatment cessation among hospitalized patients (70).
The choice to emergently initiate WBRT may be influenced by a patient’s current systemic treatments or prior history of radiation. Limited high-level evidence exists regarding the combination of systemic therapy with intracranial radiation. A review of commonly used systemic therapies for solid tumors identified the majority to be safe, while identifying a significant risk of neurotoxicity when combining cranial RT with gemcitabine, erlotinib, or vemurafenib (71).
There is often a reluctance to provide a second course of WBRT due to the risk of neurotoxicity including symptomatic radiation necrosis. While no consensus opinion exists regarding reirradiation, multiple retrospective studies have shown acceptable toxicity profiles when using appropriate patient selection (72-76). It is generally recommended to provide at least a 6-month interval between courses of RT, while conducting reirradiation with a lower dose per fraction, such as 20–25 Gy in 10 fractions (77).
What is the appropriate role of emergent RT for LM?
Survival for patients with LM is poor with a median survival of months (78-80). While median survival for LM varies with primary tumor location and histology, poor functional status at emergent presentation of LM portends a dismal prognosis and support care alone should be considered (8). As with BM, initial emergent treatment follows the standard principle of medical management with steroids and AEDs if indicated, and surgical intervention for elevated ICP. If further emergent management is required following initial medical and surgical intervention, additional local therapy is favored over chemotherapy. For local therapy, multiple RT approaches exist, with proper treatment design dependent upon clinical symptoms and imaging. In the emergent setting, typically in patients with poor-risk LM, involved-field radiotherapy (IFRT) to symptomatic sites is frequently used and supported by the National Comprehensive Cancer Network (NCCN) guidelines (81). However, it must be acknowledged that leptomeningeal disease is a diffuse process, and focal RT is a temporizing measure.
Based on retrospective studies subject to patient-selection bias, use of systemic and intrathecal chemotherapy is associated with prolonged survival in patients with LM (82,83). We currently lack high-level data on the efficacy of novel targeted and immune therapies in treating LM as patients with LM are typically excluded from early clinical trial cohorts. However, multiple case reports and retrospective series have demonstrated a benefit in patients with LM (84-87). Novel peptide-drug conjugates have shown promise in early studies (51). Regarding treatment with chemotherapy in the emergent setting, patients are unlikely to tolerate or quickly benefit from these therapies, and typically require focal therapy. Further, the use of intrathecal therapy may be complicated by CSF flow blocks, which are common among patients with LM, and difficult to assess in the emergent setting (88).
RT is an essential part of multidisciplinary management in patients with LM. It is supported in guidelines for both good-risk and poor-risk patients (81). RT should be considered for palliation, stabilization, and prevention of neurologic symptoms. RT should also be considered for improvement of CSF flow obstruction. RT can be delivered comprehensively via craniospinal irradiation (CSI), or be limited to symptomatic sites via IFRT. In a phase II trial comparing proton CSI to photon IFRT in patients with LM from NSCLC or breast cancer, proton CSI improved CNS disease control and CNS progression-free survival (89). Limited hematologic and gastrointestinal toxicities are associated with proton CSI due to the sparing of the majority of the vertebral column and anterior organs (89,90), as can be seen in Figure 1. However, the complexity of treatment planning limits the utility of proton CSI in the emergent setting. We do not advise routine use of photon CSI in the emergent setting, given the significant risk of myelosuppression and palliative intent of CSI. In exceptional circumstances, initiation of CSI with initial fields limited to areas of emergent concern can be considered. Comprehensive CNS RT may also be considered in patients with CNS leukemia and negative bone marrow, as the CNS may act as a sanctuary location for the disease (91), but initial treatment in the emergent setting would generally be limited to the symptomatic target. Generally, we advise withholding CSI for non-emergent management of certain tumors, and advise use of proton CSI (8,89,92-94).

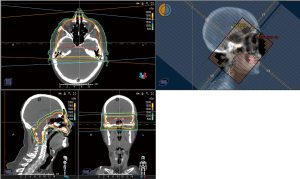
For emergent scenarios, particularly in patients with poor-risk disease, IFRT should be considered. IFRT may include WBRT, treatment of the skull base, or focal spine RT. WBRT has not been consistently associated with improved survival in retrospective studies of LM patients, but may reduce some neurologic symptoms (95-100). WBRT should be considered in the emergent setting for patients with concurrent BM, extensive nodular intracranial LM, or symptomatic linear intracranial LM (8). When treating LM with WBRT, traditional WBRT treatment fields should be extended to include the spinal cord down to the caudal aspect of the second cervical vertebral body, and should cover areas of CSF flow including the cribriform plate, optic nerves, and cranial nerve foramen and canals (101). For patients with LM confined to the base of skull, individual cranial nerves, or with pure cranial neuropathies, RT may be limited to the skull base, as can be seen in Figure 2. Focal spine RT can be used to treat well circumscribed, symptomatic lesions, particularly lesions that are bulky, obstructing CSF flow, or encasing spinal roots. In the setting of CSF flow obstruction, focal RT may restore CSF flow in approximately 30% of patients with spinal and 50% with intracranial CSF flow blocks, and may assist subsequent efficacy of intra-CSF therapy (102,103). Focal RT can also be used to treat cauda equina syndrome and would typically target the lumbosacral vertebrae, as can be seen in Figure 3.

There is limited data on IFRT dose fractionation, but dosing typically ranges from 20–40 Gy in 5–20 fractions. Conventional dosing of 30 Gy in 10 fractions and 20 Gy in 5 fractions is commonly used for WBRT when treating LM (97,104). A retrospective review of patients with CNS involvement from myeloma treated with RT failed to show a dose-response relationship, but found total doses of at least 20 Gy to associate with improved response in patients with cranial nerve involvement (69). In patients previously treated with resection and SRS, salvage WBRT can be an effective therapy (105). Concurrent treatment with WBRT and intrathecal methotrexate should be avoided due to the increased risk of leukoencephalopathy (106-108), but if given concurrently, a lower dose-per-fraction of 2 Gy should be considered (109). Given the paucity of data informing dose-fractionation for LM, we recommend a similar strategy for selection of dose-fractionation as proposed for BM, while considering a lower dose-per fraction in the presence of concomitant intrathecal therapy.
Conclusions
Emergent management of BM and LM requires multidisciplinary care coordination and informed discussion regarding a patient’s goals of care. Proper work-up and initial medical management is essential, followed by surgical evaluation to determine if intervention is required to address mass effect or divert CSF due to elevated ICP. Subsequent management decisions related to supportive care, systemic therapy, and RT are largely driven by tumor histology and patient factors, due to the paucity of high-level data in the emergent setting. While there is a role for upfront systemic therapy for some entities such as GTN and germ cell tumors, the upfront use of KI in melanoma and NSCLC with targetable mutations is an emerging paradigm. When treating with RT in the emergent setting, shorter treatment schedules and limited treatment fields may be favored due to worse prognosis and higher rates of early treatment cessation among hospitalized patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Palliative Medicine, for the series “Radiotherapy for Oncologic Emergencies”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1276/rc
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1276/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1276/coif). The series “Radiotherapy for Oncologic Emergencies” was commissioned by the editorial office without any funding or sponsorship. S.S.L. served as the unpaid Guest Editor of the series and serves as an unpaid Associate Editor-in-Chief of Annals of Palliative Medicine from February 2022 to January 2024. S.T.C. has received honoraria from Varian, and honoraria and travel support from Blue Earth Diagnostics; S.T.C reports the following: grants, contracts, consulting fees, honoraria, travel, and accommodation expenses with Roche, AstraZeneca, Medac, Dr. Sennewald Medizintechnik, Elekta, Accuray, BMS, Brainlab, Daiichi Sankyo, Icotec AG, Carl Zeiss Meditec AG, HMG Systems Engineering; NOA board member; DEGRO board member. M.F. has received research grants, consultancy fees, and honoraria from Elekta, Varian; Ex Officio Board Member ISRS. L.M.H. reports grant funding to her institution from Biomimetix. S.S.L. reports research support from Elekta, Kuni Foundation; travel support from JASTRO; leadership position with Hutchinson Center, American College of Radiology, Radiosurgery Society. J.P. reports lectures and payments from Varian and ICOTEC; manuscript writing and educational events for Novocure. K.J.R. reports research funding to her institution from Accuray, Canon Elekta; consulting fees from ICOTEC; travel support from ICOTEC, Brainlab, Accuray, Elekta; payments from BioMimetix, MSKCC, University of Maryland; a patent under development for radiogenomics in collaboration with Canon. A.S. reports the following: Elekta AB grants, fees, honoraria, travel support; Varian grants, honoraria, travel support; Astra Zeneca fees, honoraria; Seagen fees, honoraria; Brainlab honoraria, travel support; Vice President of ISRS; Co-Chair of the AO Spine Knowledge Forum; Member of the Elekta MR Linac Research Consortium. J.H.S. reports serving on scientific advisory board to NovoCure and serves on board of trustees for ABR and is treasurer for IRRF. Y.D.T. reports being a faculty lecturer for the ASTRO annual meeting; leadership positions with PCG, PTCOG, ASTRO. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
※Special series on Radiotherapy for Oncologic Emergencies.
References
- Mitera G, Swaminath A, Wong S, et al. Radiotherapy for oncologic emergencies on weekends: examining reasons for treatment and patterns of practice at a Canadian cancer centre. Curr Oncol 2009;16:55-60. [Crossref] [PubMed]
- Yeo R, Campbell T, Fairchild A. Is Weekend Radiation Therapy Always Justified? J Med Imaging Radiat Sci 2012;43:38-42. [Crossref] [PubMed]
- Christian E, Adamietz IA, Willich N, et al. Radiotherapy in oncological emergencies--final results of a patterns of care study in Germany, Austria and Switzerland. Acta Oncol 2008;47:81-9. [Crossref] [PubMed]
- Sundermeyer ML, Meropol NJ, Rogatko A, et al. Changing patterns of bone and brain metastases in patients with colorectal cancer. Clin Colorectal Cancer 2005;5:108-13. [Crossref] [PubMed]
- Bouffet E, Doumi N, Thiesse P, et al. Brain metastases in children with solid tumors. Cancer 1997;79:403-10. [Crossref] [PubMed]
- Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep 2012;14:48-54. [Crossref] [PubMed]
- Vellayappan B, Tan CL, Yong C, et al. Diagnosis and Management of Radiation Necrosis in Patients With Brain Metastases. Front Oncol 2018;8:395. [Crossref] [PubMed]
- Le Rhun E, Weller M, Brandsma D, et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol 2017;28:iv84-99. [Crossref] [PubMed]
- Lin X, Fleisher M, Rosenblum M, et al. Cerebrospinal fluid circulating tumor cells: a novel tool to diagnose leptomeningeal metastases from epithelial tumors. Neuro Oncol 2017;19:1248-54. [Crossref] [PubMed]
- Wijetunga NA, Boire A, Young RJ, et al. Quantitative cerebrospinal fluid circulating tumor cells are a potential biomarker of response for proton craniospinal irradiation for leptomeningeal metastasis. Neurooncol Adv 2021;3:vdab181. [Crossref] [PubMed]
- Malani R, Fleisher M, Kumthekar P, et al. Cerebrospinal fluid circulating tumor cells as a quantifiable measurement of leptomeningeal metastases in patients with HER2 positive cancer. J Neurooncol 2020;148:599-606. [Crossref] [PubMed]
- Diaz M, Singh P, Kotchetkov IS, et al. Quantitative assessment of circulating tumor cells in cerebrospinal fluid as a clinical tool to predict survival in leptomeningeal metastases. J Neurooncol 2022;157:81-90. [Crossref] [PubMed]
- DeMartino ES, Dudzinski DM, Doyle CK, et al. Who Decides When a Patient Can't? Statutes on Alternate Decision Makers. N Engl J Med 2017;376:1478-82. [Crossref] [PubMed]
- Ryken TC, Kuo JS, Prabhu RS, et al. Neurosurgery 2019;84:E189-91. [Crossref] [PubMed]
- Walbert T, Harrison RA, Schiff D, et al. SNO and EANO practice guideline update: Anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Neuro Oncol 2021;23:1835-44. [Crossref] [PubMed]
- Boire A. Metastasis to the Central Nervous System. Continuum (Minneap Minn) 2020;26:1584-601. [Crossref] [PubMed]
- Fujimaki T. Surgical treatment of brain metastasis. Int J Clin Oncol 2005;10:74-80. [Crossref] [PubMed]
- Brastianos PK, Carter SL, Santagata S, et al. Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer Discov 2015;5:1164-77. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990;322:494-500. [Crossref] [PubMed]
- Mintz AH, Kestle J, Rathbone MP, et al. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer 1996;78:1470-6. [Crossref] [PubMed]
- Vecht CJ, Haaxma-Reiche H, Noordijk EM, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol 1993;33:583-90. [Crossref] [PubMed]
- Gaspar LE, Mehta MP, Patchell RA, et al. The role of whole brain radiation therapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 2010;96:17-32. [Crossref] [PubMed]
- Kalkanis SN, Kondziolka D, Gaspar LE, et al. The role of surgical resection in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 2010;96:33-43. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 1998;280:1485-9. [Crossref] [PubMed]
- Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 2017;18:1049-60. [Crossref] [PubMed]
- Mahajan A, Ahmed S, McAleer MF, et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol 2017;18:1040-8. [Crossref] [PubMed]
- Palmer JD, Perlow HK, Matsui JK, et al. Fractionated pre-operative stereotactic radiotherapy for patients with brain metastases: a multi-institutional analysis. J Neurooncol 2022;159:389-95. [Crossref] [PubMed]
- Gutschenritter T, Venur VA, Combs SE, et al. The Judicious Use of Stereotactic Radiosurgery and Hypofractionated Stereotactic Radiotherapy in the Management of Large Brain Metastases. Cancers (Basel) 2020;13:70. [Crossref] [PubMed]
- Bindal RK, Sawaya R, Leavens ME, et al. Surgical treatment of multiple brain metastases. J Neurosurg 1993;79:210-6. [Crossref] [PubMed]
- Paek SH, Audu PB, Sperling MR, et al. Reevaluation of surgery for the treatment of brain metastases: review of 208 patients with single or multiple brain metastases treated at one institution with modern neurosurgical techniques. Neurosurgery 2005;56:1021-34; discussion 1021-34. [PubMed]
- Fecci PE, Champion CD, Hoj J, et al. The Evolving Modern Management of Brain Metastasis. Clin Cancer Res 2019;25:6570-80. [Crossref] [PubMed]
- Vogelbaum MA, Brown PD, Messersmith H, et al. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. J Clin Oncol 2022;40:492-516. [Crossref] [PubMed]
- Gondi V, Bauman G, Bradfield L, et al. Radiation Therapy for Brain Metastases: An ASTRO Clinical Practice Guideline. Pract Radiat Oncol 2022;12:265-82. [Crossref] [PubMed]
- Tsai J, Vellayappan B, Venur V, et al. The optimal management of brain metastases from gestational trophoblastic neoplasia. Expert Rev Anticancer Ther 2022;22:307-15. [Crossref] [PubMed]
- Savage P, Kelpanides I, Tuthill M, et al. Brain metastases in gestational trophoblast neoplasia: an update on incidence, management and outcome. Gynecol Oncol 2015;137:73-6. [Crossref] [PubMed]
- Khalifa J, Fléchon A, Chevreau C. Brain metastases from germ cell tumor: time to reconsider radiotherapy? Crit Rev Oncol Hematol 2020;150:102946. [Crossref] [PubMed]
- Gould J. Breaking down the epidemiology of brain cancer. Nature 2018;561:S40-1. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Yamaguchi H, Wakuda K, Fukuda M, et al. A Phase II Study of Osimertinib for Radiotherapy-Naive Central Nervous System Metastasis From NSCLC: Results for the T790M Cohort of the OCEAN Study (LOGIK1603/WJOG9116L). J Thorac Oncol 2021;16:2121-32. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917-29. [Crossref] [PubMed]
- Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol 2018;19:1654-67. [Crossref] [PubMed]
- Drilon A, Siena S, Dziadziuszko R, et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1-2 trials. Lancet Oncol 2020;21:261-70. [Crossref] [PubMed]
- Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol 2013;14:64-71. [Crossref] [PubMed]
- Lin NU, Pegram M, Sahebjam S, et al. Pertuzumab Plus High-Dose Trastuzumab in Patients With Progressive Brain Metastases and HER2-Positive Metastatic Breast Cancer: Primary Analysis of a Phase II Study. J Clin Oncol 2021;39:2667-75. [Crossref] [PubMed]
- Krop IE, Lin NU, Blackwell K, et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol 2015;26:113-9. [Crossref] [PubMed]
- Pérez-García JM, Vaz Batista M, Cortez P, et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: The DEBBRAH trial. Neuro Oncol 2023;25:157-66. [Crossref] [PubMed]
- Bartsch R, Berghoff AS, Furtner J, et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med 2022;28:1840-7. [Crossref] [PubMed]
- Epaillard N, Lusque A, Pistilli B, et al. 260P Antitumor activity of trastuzumab deruxtecan (T-DXd) in patients with metastatic breast cancer (mBC) and brain metastases (BMs) from DAISY trial. Ann Oncol 2022;33:abstr S656.
- Tolaney SM, Sahebjam S, Le Rhun E, et al. A Phase II Study of Abemaciclib in Patients with Brain Metastases Secondary to Hormone Receptor-Positive Breast Cancer. Clin Cancer Res 2020;26:5310-9. [Crossref] [PubMed]
- Kumthekar P, Tang SC, Brenner AJ, et al. ANG1005, a Brain-Penetrating Peptide-Drug Conjugate, Shows Activity in Patients with Breast Cancer with Leptomeningeal Carcinomatosis and Recurrent Brain Metastases. Clin Cancer Res 2020;26:2789-99. [Crossref] [PubMed]
- Tawbi HA, Forsyth PA, Algazi A, et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N Engl J Med 2018;379:722-30. [Crossref] [PubMed]
- Kartolo A, Deluce J, Holstead R, et al. Impact of Baseline Corticosteroids on Immunotherapy Efficacy in Patients With Advanced Melanoma. J Immunother 2021;44:167-74. [Crossref] [PubMed]
- Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAF(V600)-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol 2017;18:863-73. [Crossref] [PubMed]
- Marquez-Rodas I, Arance Fernandez AM, Berciano Guerrero MA, et al. 826P Encorafenib and binimetinib followed by radiotherapy for patients with symptomatic BRAF mutated melanoma brain metastases: GEM1802/E-BRAIN clinical trial. Ann Oncol 2022;33:abstr S926.
- Li B, Yu J, Suntharalingam M, et al. Comparison of three treatment options for single brain metastasis from lung cancer. Int J Cancer 2000;90:37-45. [Crossref] [PubMed]
- Rades D, Pluemer A, Veninga T, et al. Whole-brain radiotherapy versus stereotactic radiosurgery for patients in recursive partitioning analysis classes 1 and 2 with 1 to 3 brain metastases. Cancer 2007;110:2285-92. [Crossref] [PubMed]
- Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009;10:1037-44. [Crossref] [PubMed]
- Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol 2014;32:3810-6. [Crossref] [PubMed]
- Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol 2013;15:1429-37. [Crossref] [PubMed]
- Borgelt B, Gelber R, Kramer S, et al. The palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 1980;6:1-9. [Crossref] [PubMed]
- Murray KJ, Scott C, Greenberg HM, et al. A randomized phase III study of accelerated hyperfractionation versus standard in patients with unresected brain metastases: a report of the Radiation Therapy Oncology Group (RTOG) 9104. Int J Radiat Oncol Biol Phys 1997;39:571-4. [Crossref] [PubMed]
- Trifiletti DM, Ballman KV, Brown PD, et al. Optimizing Whole Brain Radiation Therapy Dose and Fractionation: Results From a Prospective Phase 3 Trial (NCCTG N107C [Alliance]/CEC.3). Int J Radiat Oncol Biol Phys 2020;106:255-60. Erratum in: Int J Radiat Oncol Biol Phys 2020;106:1111. [Crossref] [PubMed]
- Tsao MN, Xu W, Wong RK, et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev 2018;1:CD003869. [PubMed]
- Borgelt B, Gelber R, Larson M, et al. Ultra-rapid high dose irradiation schedules for the palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 1981;7:1633-8. [Crossref] [PubMed]
- Komarnicky LT, Phillips TL, Martz K, et al. A randomized phase III protocol for the evaluation of misonidazole combined with radiation in the treatment of patients with brain metastases (RTOG-7916). Int J Radiat Oncol Biol Phys 1991;20:53-8. [Crossref] [PubMed]
- Phillips TL, Scott CB, Leibel SA, et al. Results of a randomized comparison of radiotherapy and bromodeoxyuridine with radiotherapy alone for brain metastases: report of RTOG trial 89-05. Int J Radiat Oncol Biol Phys 1995;33:339-48. [Crossref] [PubMed]
- Sundström JT, Minn H, Lertola KK, et al. Prognosis of patients treated for intracranial metastases with whole-brain irradiation. Ann Med 1998;30:296-9. [Crossref] [PubMed]
- Williams GR, Butala AA, Manjunath SH, et al. Radiation Therapy for Plasma Cell Disease of the Brain and Skull: Poor Palliation and Survival After Treatment for Central Nervous System Involvement. Adv Radiat Oncol 2021;6:100720. [Crossref] [PubMed]
- Grade M, Koenig J, Qian Y, et al. Outcomes and Characteristics of Patients Treated with Emergent Palliative Radiation Therapy. Pract Radiat Oncol 2019;9:e203-9. [Crossref] [PubMed]
- Verduin M, Zindler JD, Martinussen HM, et al. Use of Systemic Therapy Concurrent With Cranial Radiotherapy for Cerebral Metastases of Solid Tumors. Oncologist 2017;22:222-35. [Crossref] [PubMed]
- Son CH, Jimenez R, Niemierko A, et al. Outcomes after whole brain reirradiation in patients with brain metastases. Int J Radiat Oncol Biol Phys 2012;82:e167-72. [Crossref] [PubMed]
- Sadikov E, Bezjak A, Yi QL, et al. Value of whole brain re-irradiation for brain metastases--single centre experience. Clin Oncol (R Coll Radiol) 2007;19:532-8. [Crossref] [PubMed]
- Logie N, Jimenez RB, Pulenzas N, et al. Estimating prognosis at the time of repeat whole brain radiation therapy for multiple brain metastases: The reirradiation score. Adv Radiat Oncol 2017;2:381-90. [Crossref] [PubMed]
- Cooper JS, Steinfeld AD, Lerch IA. Cerebral metastases: value of reirradiation in selected patients. Radiology 1990;174:883-5. [Crossref] [PubMed]
- Wong WW, Schild SE, Sawyer TE, et al. Analysis of outcome in patients reirradiated for brain metastases. Int J Radiat Oncol Biol Phys 1996;34:585-90. [Crossref] [PubMed]
- Chidambaram S, Pannullo SC, Schwartz TH, et al. Reirradiation of Recurrent Brain Metastases: Where Do We Stand? World Neurosurg 2019;125:156-63. [Crossref] [PubMed]
- Hyun JW, Jeong IH, Joung A, et al. Leptomeningeal metastasis: Clinical experience of 519 cases. Eur J Cancer 2016;56:107-14. [Crossref] [PubMed]
- Buszek SM, Chung C. Radiotherapy in Leptomeningeal Disease: A Systematic Review of Randomized and Non-randomized Trials. Front Oncol 2019;9:1224. [Crossref] [PubMed]
- Nguyen TK, Sahgal A, Detsky J, et al. Predictors of leptomeningeal disease following hypofractionated stereotactic radiotherapy for intact and resected brain metastases. Neuro Oncol 2020;22:84-93. [Crossref] [PubMed]
- NCCN. National Comprehensive Cancer Network: Central Nervous System Cancers (Version 2.2022). 2022.
- Park JH, Kim YJ, Lee JO, et al. Clinical outcomes of leptomeningeal metastasis in patients with non-small cell lung cancer in the modern chemotherapy era. Lung Cancer 2012;76:387-92. [Crossref] [PubMed]
- Kingston B, Kayhanian H, Brooks C, et al. Treatment and prognosis of leptomeningeal disease secondary to metastatic breast cancer: A single-centre experience. Breast 2017;36:54-9. [Crossref] [PubMed]
- Kim DW, Barcena E, Mehta UN, et al. Prolonged survival of a patient with metastatic leptomeningeal melanoma treated with BRAF inhibition-based therapy: a case report. BMC Cancer 2015;15:400. [Crossref] [PubMed]
- Yan F, Rinn KJ, Kullnat JA, et al. Response of Leptomeningeal Metastasis of Breast Cancer With a HER2/neu Activating Variant to Tucatinib: A Case Report. J Natl Compr Canc Netw 2022;20:745-52. [Crossref] [PubMed]
- Ahn MJ, Chiu CH, Cheng Y, et al. Osimertinib for Patients With Leptomeningeal Metastases Associated With EGFR T790M-Positive Advanced NSCLC: The AURA Leptomeningeal Metastases Analysis. J Thorac Oncol 2020;15:637-48. [Crossref] [PubMed]
- Alder L, Trapani D, Van Swearingen A, et al. Abstract 5257: Durable clinical and radiographic responses in a series of patients with HER2+ Breast Cancer (BC) Leptomeningeal Disease (LMD) treated with trastuzumab deruxtecan (T-DXd). Cancer Res 2022;82:5257. [Crossref]
- Glantz MJ, Hall WA, Cole BF, et al. Diagnosis, management, and survival of patients with leptomeningeal cancer based on cerebrospinal fluid-flow status. Cancer 1995;75:2919-31. [Crossref] [PubMed]
- Yang JT, Wijetunga NA, Pentsova E, et al. Randomized Phase II Trial of Proton Craniospinal Irradiation Versus Photon Involved-Field Radiotherapy for Patients With Solid Tumor Leptomeningeal Metastasis. J Clin Oncol 2022;40:3858-67. [Crossref] [PubMed]
- Yang TJ, Wijetunga NA, Yamada J, et al. Clinical trial of proton craniospinal irradiation for leptomeningeal metastases. Neuro Oncol 2021;23:134-43. [Crossref] [PubMed]
- Walker GV, Shihadeh F, Kantarjian H, et al. Comprehensive craniospinal radiation for controlling central nervous system leukemia. Int J Radiat Oncol Biol Phys 2014;90:1119-25. [Crossref] [PubMed]
- Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol 2006;7:813-20. [Crossref] [PubMed]
- Calaminus G, Kortmann R, Worch J, et al. SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol 2013;15:788-96. [Crossref] [PubMed]
- Pinnix CC, Yahalom J, Specht L, et al. Radiation in Central Nervous System Leukemia: Guidelines From the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys 2018;102:53-8. [Crossref] [PubMed]
- Yan W, Liu Y, Li J, et al. Whole brain radiation therapy does not improve the overall survival of EGFR-mutant NSCLC patients with leptomeningeal metastasis. Radiat Oncol 2019;14:168. [Crossref] [PubMed]
- Morris PG, Reiner AS, Szenberg OR, et al. Leptomeningeal metastasis from non-small cell lung cancer: survival and the impact of whole brain radiotherapy. J Thorac Oncol 2012;7:382-5. [Crossref] [PubMed]
- Hirano Y, Konishi K, Ejima Y. Utility of whole brain radiation therapy for leptomeningeal carcinomatosis. Int J Clin Oncol 2020;25:1432-9. [Crossref] [PubMed]
- Lee JM, Mehta UN, Dsouza LH, et al. Long-term stabilization of leptomeningeal disease with whole-brain radiation therapy in a patient with metastatic melanoma treated with vemurafenib: a case report. Melanoma Res 2013;23:175-8. [Crossref] [PubMed]
- Figura NB, Rizk VT, Mohammadi H, et al. Clinical outcomes of breast leptomeningeal disease treated with intrathecal trastuzumab, intrathecal chemotherapy, or whole brain radiation therapy. Breast Cancer Res Treat 2019;175:781-8. [Crossref] [PubMed]
- El Shafie RA, Böhm K, Weber D, et al. Palliative Radiotherapy for Leptomeningeal Carcinomatosis-Analysis of Outcome, Prognostic Factors, and Symptom Response. Front Oncol 2019;8:641. [Crossref] [PubMed]
- Ajithkumar T, Horan G, Padovani L, et al. SIOPE - Brain tumor group consensus guideline on craniospinal target volume delineation for high-precision radiotherapy. Radiother Oncol 2018;128:192-7. [Crossref] [PubMed]
- Chamberlain MC. Radioisotope CSF flow studies in leptomeningeal metastases. J Neurooncol 1998;38:135-40. [Crossref] [PubMed]
- Chamberlain MC, Kormanik P, Jaeckle KA, et al. 111Indium-diethylenetriamine pentaacetic acid CSF flow studies predict distribution of intrathecally administered chemotherapy and outcome in patients with leptomeningeal metastases. Neurology 1999;52:216-7. [Crossref] [PubMed]
- Ozdemir Y, Yildirim BA, Topkan E. Whole brain radiotherapy in management of non-small-cell lung carcinoma associated leptomeningeal carcinomatosis: evaluation of prognostic factors. J Neurooncol 2016;129:329-35. [Crossref] [PubMed]
- Prabhu RS, Turner BE, Asher AL, et al. A multi-institutional analysis of presentation and outcomes for leptomeningeal disease recurrence after surgical resection and radiosurgery for brain metastases. Neuro Oncol 2019;21:1049-59. [Crossref] [PubMed]
- Boogerd W, van den Bent MJ, Koehler PJ, et al. The relevance of intraventricular chemotherapy for leptomeningeal metastasis in breast cancer: a randomised study. Eur J Cancer 2004;40:2726-33. [Crossref] [PubMed]
- Kim JY, Kim ST, Nam DH, et al. Leukoencephalopathy and disseminated necrotizing leukoencephalopathy following intrathecal methotrexate chemotherapy and radiation therapy for central nerve system lymphoma or leukemia. J Korean Neurosurg Soc 2011;50:304-10. [Crossref] [PubMed]
- DeAngelis LM, Seiferheld W, Schold SC, et al. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol 2002;20:4643-8. [Crossref] [PubMed]
- Pan Z, Yang G, He H, et al. Concurrent radiotherapy and intrathecal methotrexate for treating leptomeningeal metastasis from solid tumors with adverse prognostic factors: A prospective and single-arm study. Int J Cancer 2016;139:1864-72. [Crossref] [PubMed]


