
Alleviation of malignant dysphagia in inoperable lung cancer
Highlight box
Key findings
• This paper presents the largest analysis of malignant dysphagia in the course of lung cancer. Esophageal stenting and double stenting (esophagus and bronchi) can improve quality of life and alleviate the symptoms. Furthermore, this treatment approach allows for the use of additional therapies such as chemotherapy, palliative radiotherapy or molecular treatment.
What is known and what is new?
• Lung cancer infiltration into the esophagus is estimated to occur in around 4% of patients, esophagotracheal fistulas occur in approximately 1%. The optimal treatment is not defined, especially in the case of both esophageal and bronchial stenosis and fistulas.
• The article draws attention to the difficult aspect of double stenting (esophagus and bronchial tree) that patients in this group may require. The most common complications after the treatment and the possibility of reintervention are presented.
What is the implication, and what should change now?
• The results are an introduction to in-depth research.
Introduction
Lung cancer and other respiratory cancers are among the most frequent types of malignancy in cancer patients and they cause significant epidemiological and clinical problems. The number of new lung cancer cases worldwide places it in second place behind breast cancer (1). Due to the close anatomical relationship between the bronchial tree and the esophagus, this disease can lead to malignant dysphagia. Indeed lung cancer infiltration into the esophagus is estimated to occur in around 4% of patients (2,3). Meanwhile, infiltration of esophageal cancer into the bronchial tree is even more common and is estimated to occur in around 10% of patients (4). Patients commonly experience a severe general condition with increasing dyspnea, dysphagia and cachexia, which makes them a high-risk group prior to any planned treatment.
This paper presents the largest analysis of malignant dysphagia in the course of lung cancer in the available literature. Its aims are to present our clinical experience, and results of treatment of this rare and difficult medical condition. This article is presented in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1144/rc).
Methods
This study retrospectively analyzed 84 lung cancer patients treated in the Department of Thoracic Surgery between 2008 and 2018. Patients with dysphagia, coexisting bronchial tree and esophageal obstruction, or esophagobronchial fistula, received palliation of the esophagus or esophagobronchial tree through stenting. Patients eligible for treatment suffered either from primary inoperable lung cancer or inoperable relapse after surgery. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The IRB approval was waived by John Paul II Hospital Ethics Board due to retrospective character of the cohort study based exclusively on hospital records. Informed consent was waived due to the retrospective nature of this study. Following standard procedures, patients underwent esophagoscopy, bronchoscopy, endobronchial ultrasound (EBUS), endoscopic ultrasound (EUS), computed tomography (CT), and positron emission tomography-computed tomography (PET-CT), if it was recommended prior to the planned stenting. Cancer classification was based on the Union for International Cancer Control staging (5). The degree of dysphagia was assessed according to a 4-point scale (6):
- 0: no dysphagia;
- 1: able to swallow a semi-liquid diet;
- 2: able to swallow a liquid diet;
- 3: dysphagia to liquids and saliva.
Patients diagnosed with fistula in the course of bronchogenic cancer were stratified into four groups according to fistula location and/or the applied treatment (7):
- Type 1: fistula to the mediastinum;
- Type 2: fistula to the trachea;
- Type 3: fistula to the bronchus;
- Type 4: fistula after stenting.
Dyspnea severity was assessed according to a 4-point scale (7):
- 0: less than 30% stenosis and no dyspnea;
- 1: 30–50% stenosis and dyspnea upon exercise;
- 2: 50–70% stenosis and dyspnea during daily activities;
- 3: more than 70% stenosis and dyspnea while resting.
Intervention
Esophageal stenting
Esophageal stenting was performed under general anesthesia. Location of stenosis was identified endoscopically and in cases with narrow stenosis dilatation of up to 10 mm was performed using Savary-Gilliard dilators. Following dilatation, the neoplastic infiltration length was measured using a small-diameter endoscope. A guidewire was then inserted and a partially covered self-expandable Ultraflex stent (Boston Scientific, Natick, MA, USA) was introduced over the wire under endoscopic control. Deployment of the stent was performed by gradually expanding its proximal end, allowing for the prosthesis to be correctly repositioned before fully expanding. After deployment, the stent location and completeness of expansion were endoscopically assessed.
Patients with non-resectable bronchial cancer with esophagobronchial fistulas met the criteria for double stenting. The inclusion criteria were the tumor being located ≥2 cm from the upper esophageal sphincter, endoscopically confirmed fistula penetrating the mediastinum or bronchial tree, and airway compression or infiltration that posed a risk of severe airway compromise after the expansion of the esophageal stent.
Airway stenting
Double stenting procedures were performed under general anesthesia using self-expandable Ultraflex stents (Boston Scientific, Natick, MA, USA) for main bronchi, and silicone Y stents (Demed, Mikołów, Poland) in cases were the fistula or obstruction was located in the region of tracheal bifurcation. Stenting with silicone Y stents was performed using Freitag forceps according to the recommended technique (8).
PEG implantation
Esophagoscopy was performed in order to assess the degree of stenosis of the esophagus. If the stenosis was tight (less than 10 mm in diameter), a stent was introduced, following dilation using Savary-Gilliard dilatators. Having restored patency of esophagus, stomach and duodenal bulb were examined, and a proper place for gastrostomy tube was chosen. Gastrostomy tube was pulled transorally using a wire loop passed to the stomach through the needle as described by Gauderer and Ponsky (9).
Follow-up
Chest radiograms were obtained on the day after surgery. Dysphagia and dyspnea scores were also collected at this time, and every 30 days thereafter, along with assessment of the patients’ general condition. If it was not feasible to carry out this follow-up check in person, patients were interviewed over the telephone.
Statistical analysis
All statistical analyses were performed with the Statistica 10 PL software package (StatSoft, USA). The following tests were used: Gehan-Wilcoxon, Kruskal-Wallis, Fisher exact test and chi-square test. The dependent variable regression test was used to analyze data collected before and after surgery. The analyses included survival, respiratory function, dysphagia, weight loss, quality of life (according to the Karnofsky scale), the influence of adjuvant therapy (chemotherapy and/or radiotherapy). Overall survive between analyzed groups of patients was assessed using Kaplan-Meier test. P values <0.05 were considered statistically significant.
Results
Characteristics of patients
Between 2008 and 2018, 2,560 patients were treated for advanced lung cancer, 84 (3.3%) of whom were diagnosed with dysphagia. This cohort included 68 males (81%) and 16 females (19%) with a mean age of 57.8 years, ranging from 28–83 years (Table 1). All patients reported dysphagia as well as weight loss ranging from 4–15 kg, with a mean loss of 7.2 kg. Dyspnea occurred in 10 (11.9%) patients, 5 (6.0%) of whom required emergency restoration and stenting. The length of esophageal stricture ranged from 3–5 cm, with a mean length of 3.4 cm.
Table 1
| Demographic | Results |
|---|---|
| Age, years | |
| Median | 57.8 |
| Range | 28–83 |
| Male:female, n (%) | 68:16 (81:19) |
| Primary tumor site, n (%) | |
| Left lung | 69 (82.14) |
| Right lung | 15 (17.85) |
| Type of cancer, n (%) | |
| NSCLC | 74 (88.1) |
| SCC | 33 (39.3) |
| ADC | 37 (44.0) |
| Mixed type | 7 (8.3) |
| SCLC | 5 (6.0) |
| Other | 2 (2.4) |
| Recurrence after surgery, n (%) | |
| Lobectomy | 3 (3.6) |
| Pneumonectomy (left: right) | 3:1 |
| Inoperable | 2 (2.4) |
| Mode of presentation obstruction and treatment (stenting), n (%) | |
| Esophagus | 64 (76.2) (X) |
| Esophagus and bronchial tree without fistula | 12 (14.3) |
| Esophagus and bronchial tree with fistula | 8 (9.5) (X+Y) |
| Other additional treatment, n (%) | |
| Chemo and or radiotherapy after stenting | 57 (67.85) |
| PEG | 12 (14.28) |
| Type of fistula, n (%) | |
| Type 1 | 1 (1.2) |
| Type 2 | 1 (1.2) |
| Type 3 | 5 (6.0) |
| Type 4 | 1 (1.2) |
| Reintervention, n (%) | |
| Esophageal stent migration (re-stenting) | 3 (3.6) |
| Re-stenting due tissue esophageal stent overgrowth | 5 (6.0) |
| Re-stenting due bronchial stent obstruction | 1 (1.2) |
| PEG | 5 (6.0) |
| Survival, days | |
| Median | 93.4 |
| Range | 28–296 |
| Median survival after esophageal stenting [range] | 133 [68–296] |
| Median survival after double stenting without fistula [range] | 110 [80–287] |
| Median survival after double stenting with fistula [range] | 79 [5–225] |
| Mechanical ventilation, n (%) | 3 (3.6) |
| Mortality, n (%) | 1 (1.2) |
X, esophageal stenting; X+Y, double stenting (esophageal and bronchial tree stenting). NSCLC, non-small-cell lung cancer; SCC, squamous-cell carcinoma; ADC, adenocarcinoma; SCLC, small-cell lung cancer; PEG, percutaneous endoscopic gastrostomy.
Localization of stenosis
Esophageal stenosis was found 20–22 cm from the incisors in 2 (2.4%) patients, 22–25 cm from the incisors in 80 (95.2%), and 26–28 cm from the incisors in 2 (2.4%) patients.
Patients with esophageal compression without the obstruction of the bronchial tree (Figure 1)
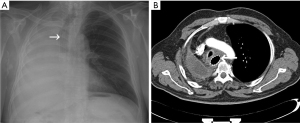
Among 64 patients treated for dysphagia without obstruction of the bronchial tree, all achieved resolution after stenting, with scores of 2.68 [2–3] before and 1.2 [0–1] after stenting (P=0.0001). Quality of life was assessed according to the Karnofsky scale, and was found to be 59 [50–70] before and 72 [60–80] after stenting (P=0.0001). In this group, 3 (3.6%) patients required PEG implantation. Bronchial tree stenosis in the range of 10–20% occurred in 5 (6.0%) patients post-stenting with no intervention needed. Another 2 (2.4%) patients developed clinically important bronchial tree stenosis, presenting dyspnea after esophageal stenting and required additional airways stenting. Patients survived for 68–296 days, with a mean of 133 days.
Patients with esophageal compression and bronchial tree obstruction without fistula features
Of the patients studied, 12 (14.3%) with esophageal and bronchial tree obstruction required double stenting (Figure 2). Bronchial tree stenosis was found to range between 40–90%, with a mean of 67%. Three (3.6%) patients with tracheal bifurcation stenosis above 70% required emergency treatment. Three (3.6%) patients required left bronchus stenting and 3 (3.6%) right bronchus. This group of patients received self-expandable bronchial covered stents. Six (7.1%) patients received stenting using a silicone Y stent. In this group, Four (4.8%) patients required PEG implantation. All patients experienced resolution of dysphagia, with a mean score of 2.76 [2–3] before stenting, and 1.3 [0–1] after stenting (P=0.0001). Dyspnea was also resolved, with a mean score of 2.86 [2–3] before stenting, which was reduced to 0.4 [0–1] after stenting (P=0.001). Furthermore, quality of life also improved with a mean score of 56 [50–70] before stenting and 70 [60–80] after stenting (P=0.0001). Patients survived for 80–187 days with a mean survival of 110 days.
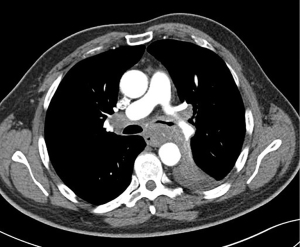
Patients with dysphagia and esophagotracheal fistula
Fistula of the bronchial tree was detected in 8 (9.5%) of the treated patients (in 1 of Type 1, in 1 of Type 2, in 5 of Type 3, and in 1 of Type 4). Six (7.14%) patients received double stenting using the silicone Y and esophageal stents, whilst 2 (2.4%) patients received the self-expanding bronchial and esophageal stents (Figure 3). In this group, 5 (6.0%) patients underwent Percutaneous Endoscopic Gastrostomy (PEG). Resolution of dysphagia was observed in all patients, with a mean score of 2.74 [2–3] before stenting and a mean score of 1.4 [0–1] after stenting (P=0.0001). Dyspnea was also reduced, from 2.89 [2–3] before stenting, to 0.5 [0–1] post-stenting (P=0.0001). Furthermore, quality of life scores increased from 53 [50–70] before stenting to 67 [60–80] after stenting (P=0.0001). Two (2.4%) patients required emergency treatment. Patients survived for 28–154 days with a mean of 79 days. On the fifth postoperative day, 1 (1.19%) patient died due to circulatory failure.
Complications after stenting (Table 2) (10)
Table 2
| Grade | Number (%) | Definition |
|---|---|---|
| I | 29 (36.2) | 14 (17.5%) chest pain, 15 (17.9%) cough. Any deviation from the normal postoperative course without the need for pharmacological treatment or surgical, endoscopic and radiological interventions. Allowed therapeutic regimens are: drugs as antiemetics, antipyretics, analgesics, diuretics and electrolytes and physiotherapy. This grade also includes wound infections opened at the bedside |
| II | 0 | Requiring pharmacological treatment with drugs other than such allowed for grade I complications. Blood transfusions and total parenteral nutrition are also included |
| III | 12 (14.3) | 12 (14.3%) retention of mucus secretions patients required bronchoscopy Requiring surgical, endoscopic or radiological intervention |
| IIIa | 0 | Intervention not under general anesthesia |
| IIIb | 2 (2.4) | 2 (2.4%) bronchial tree stenting. Intervention under general anesthesia |
| IV | 3 (3.6) | Respiratory insufficiency, life-threatening complication (including CNS complications)* requiring IC/ICU-management |
| IVa | 0 | Single organ dysfunction (including dialysis) |
| IVb | 0 | Multi organ dysfunction |
| V | 1 (1.2) | Death of a patient |
*, brain hemorrhage, ischemic stroke, subarachnoid bleeding, but excluding transient ischemic attacks. CNS, central nervous system; IC, intermediate care; ICU, intensive care unit.
There were a number of complications recorded after stenting, including both minor and major events. In terms of minor complications, 14 (17.5%) patients developed a sensation of chest distension and pain that required oral and intravenous analgesics. Meanwhile, 15 (17.9%) patients developed a cough that was relieved by inhalation and oral agents.
Major complications were found in 3 (3.6%) patients who developed respiratory failure that required artificial ventilation. Meanwhile, respiratory fistula occurred in 2 (2.4%) patients on 39th and 63th postoperative day, and 1 (1.2%) developed bronchial tree and esophageal stenosis. These patients were decannulated on postoperative days two and three, and no further complications occurred on subsequent days. Symptoms of retained mucus secretion in the bronchial tree that required bronchoaspiration and clearing occurred in 12 (14.3%) patients. Bronchial tree stenosis requiring stenting occurred in 2 (2.4%) patients after esophageal stenting. During the postoperative course, 1 (1.2%) patient with esophagotracheal fistula died due to circulatory failure on the fifth day.
Re-intervention
Five (6.0%) patients suffered from recurrence of dysphagia due to granuloma formation on the upper pole of the prosthesis between the 36th and 78th day, which led to removal and replacement of the prosthesis. In 3 (3.6%) patients with esophageal compression, stents migrated after follow-up chemo-radiotherapy, leading to stent removal and the introduction of PEG. Obstruction of a tracheal stent occurred in one patient, which was removed and replaced with a silicone Y stent.
Survival
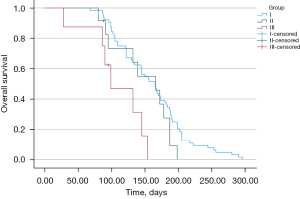
There was a statistically significant difference in survival time between patients from analysed groups (P=0.003). Post hoc analyses showed that patients from groups I and II had longer survival time than patients from group III (Figure 4). Mean time of survival for patients from group I equaled M =160.8 (SD =6.68) days. For patients from group II: M =148.3 (SD =12.73) days, and for patients from group III: M =108.3 (SD =15.35) days. The median (range) survival after stenting was 94.3 [5–296] days. Survival time was not affected by chemotherapy or radiotherapy (P=0.54).
Discussion
Esophageal stenting is the gold standard of palliative treatment in inoperable esophageal cancer. While the results of treatment in this group of patients are well documented, patients with dysphagia in the setting of lung cancer are sparse. Therefore, studies usually involve small groups of patients, and only a few reports have presented an analysis of 40–50 patients (11,12).
It is estimated that dysphagia in the course of lung cancer occurs in approximately 3–4% of patients, which has been confirmed in this report. It effects older patients in particular, with 1 in 25 presenting with symptoms prior to a diagnosis being made. It is an indicator of poor prognosis, with survival significantly shorter, around 12 months, when compared to those without dysphagia. Usually, this group requires a longer stay in hospital, has an increased likelihood of complications and are at greater risk of dying in hospital. Furthermore, treatment of this condition is difficult, and the studies conducted on small groups of retrospectively analyzed patients have not been able to pinpoint the optimal treatment strategy (2,3).
The stenting procedure in dysphagia due to lung cancer should generally not differ from the standard for patients with inoperable esophageal cancer. The causes of dysphagia may include external compression or infiltration of a lung cancer mass into the esophagus, compression or infiltration of mediastinal lymph nodes, or esophageal stenosis secondary to antecedent mediastinal radiotherapy (13). Such an advanced stage of cancer can make it very difficult to maintain patency of the esophagus and the airways. In 80% of our patients with left lung cancer, esophageal stenosis was short and located adjacent to the bronchial tree. In 75% of patients, stenosis was the result of external compression without features of endoscopic tumor infiltration and thus was not verifiable. Therefore, ultrasound techniques such as EBUS-guided transbronchial needle aspiration and EUS should be applied to obtain verification. Stenting as a treatment of neoplastic esophageal stenosis is a simple method with a low-rate of complications and perioperative mortality (14).
Esophageal stenosis in the course of lung cancer is most commonly located in the middle part of the esophagus, approximately 23 cm from the incisors. Usually, the stenosis is short, which allows for the stent to be implanted without difficulties. However, this location of esophageal stenosis and the inserted stent may result in compression and narrowing of the airway which may then require stenting. Such patients require bronchoscopic evaluation before and after esophageal stenting. Patients with primary inoperable esophageal cancer also require bronchoscopic evaluation. However, they are predisposed to tumor infiltration of the left main bronchus and would therefore require stenting of this location if it were to become obstructed.
In our report, we would like to draw attention to patients requiring double stenting. Patients with esophago-respiratory fistula are qualified for double stenting in accordance with the recommendations. Patients without fistula, with esophageal compression and bronchial tree obstruction however constitute separate group. In these cases, to stent esophagus alone may lead to critical bronchial tree obstruction which restoration could be technically challenging. In such patients, we suggest considering double stenting, which requires the experience of the treating team. In our study, all patients from group II underwent double stenting. Altogether it was performed in 23 (27.4%) cases and 12 (14.3%) patients required alternative route of nutrition.
In the course of lung cancer, esophagotracheal fistulas occur in approximately 1% of patients (15). Patients with such complicated cancer are most often in a severe general condition with increasing dyspnea and cachexia, and represent a group of high-risk patients prior to any further planned treatment. As our results show, in this group of patients the survival is statistically shorter than in groups I and II.
According to National Comprehensive Cancer Network and European Society of Gastrointestinal Endoscopy recommendations, double stenting is the treatment of choice (16,17). However, Altemur Karamustafaoglu and Yoruk analyzed a group of patients treated for esophageal obstruction in the course of lung cancer and treated 8 patients with esophagobronchial fistula, and only 1 patient received double stenting (18). In contrast, Yanık et al. treated 12 patients with esophageal fistula and achieved complete coverage of the fistula and stabilization of their general condition using esophageal stenting (12). Likewise, Kim et al. also treated patients with fistula by unilateral stenting of the esophagus (19). Of course, this procedure can be controversial since it is not always possible to achieve full tightness and control of the fistula. Patients with fistula are clinically challenging and may require repeated procedures as well as an additional route of nutrition, such as PEG. Kim et al. analyzed patients after esophageal stenting, along with PEG implantation or conventional gastrostomy, and found that the two methods were comparable, though better nutritional control was achieved in the group with PEG implantation.
An important consideration for electing patients for stenting is the assessment of respiratory capacity. Both lung cancer with obstructions of the airways and previous lung resection restricting the respiratory area can lead to an increase in respiratory failure. Indeed, LeRoux reported that 1–2% of lung cancer patients presented with wheeze and stridor, with advanced bronchial cancer (20). In this study, dyspnea occurred in 10 (11.9%) patients, and they required double stenting. Of course, this treatment is avoided as much as possible because it is technically difficult, and due to the pressure of the implanted stents on the bronchial tree and esophagus it may lead to necrosis and respiratory fistula. In our analysis, the group of patients who required double stenting accounted for 20% of those treated.
Although double stenting is safe, it still has high complication rates during the peri- and postoperative period, which has been confirmed by reports from other authors (21,22).
It is important to monitor and report on patients following surgical intervention. In this analysis, 7 (8.3%) recurrent cancer patients with esophageal infiltration were treated. Such obstruction of the esophagus increases the risk of aspiration pneumonia and due to the limited respiratory reserves of these individuals this can lead to respiratory failure and death. This group of patients required esophageal stenosis restoration and an alternative route of nutrition. In our experience, the maintenance of proper nutrition is only possible if an alternative route of nutrition is employed (23). All patients received esophageal stents, though this group may also require bronchial tree stenting.
Patients require close monitoring after stent implantation for possible life-threatening complications. Bronchial fistula occurred in 1 (1.2%) patient and this required stent removal and re-stenting, which can be technically difficult. Other serious complications included stent migration and granuloma formation. Such complications usually require stent removal and, in some patients, re-stenting. Furthermore, the outcome of such treatment is often uncertain, and in our opinion an alternative simultaneous route of nutrition is necessary.
Among the limitations of herby presented work are relatively small sample size and its retrospective character. Another limitation that may be discussed is the lack of impact of chemo and/or radiotherapy on patient survival. We undertake the analysis in a heterogeneous group of patients after prior treatment (surgical treatment, chemotherapy) with a relatively short survival time. The results are an introduction to in-depth research.
Conclusions
Treatment of malignant dysphagia in the setting of inoperable lung cancer requires diversified endoscopic management, including unilateral esophageal stenting, double stenting of both the esophagus and the bronchial tree, and the creation of an alternative nutrition route. The proposed treatment can improve quality of life and alleviate the symptoms of cancer. Furthermore, this approach allows for the use of additional therapies such as chemotherapy, palliative radiotherapy or molecular treatment, that will allow for a longer and better survival.
Acknowledgments
Funding: This article was supported by the science fund of the John Paul II Hospital, Cracow, Poland (No. FN/1/2023 to PO).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1144/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1144/dss
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1144/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1144/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The IRB approval was waived by John Paul II Hospital Ethics Board due to retrospective character of the cohort study based exclusively on hospital records. Informed consent was waived due to the retrospective nature of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global Cancer Observatory, WHO. Available online: https://gco.iarc.fr/, accessed on 22.12.2022
- Marmor S, Cohen S, Fujioka N, et al. Dysphagia prevalence and associated survival differences in older patients with lung cancer: A SEER-Medicare population-based study. J Geriatr Oncol 2020;11:1115-7. [Crossref] [PubMed]
- Patel DA, Krishnaswami S, Steger E, et al. Economic and survival burden of dysphagia among inpatients in the United States. Dis Esophagus 2018;31:1-7. [Crossref] [PubMed]
- Madeya S, Borsch G. Upper intestinal endoscopy in 188 bronchial cancer patients and 118 breast cancer patients with abdominal symptoms. Med Klin (Munich) 1992;87:631-36. [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Blazeby JM, Williams MH, Brookes ST, et al. Quality of life measurement in patients with oesophageal cancer. Gut 1995;37:505-8. [Crossref] [PubMed]
- Włodarczyk J, Gil T, Warmus J, et al. Double stenting for malignant airway and esophageal obstructions. Disease of the Esophagus. 2017;8:1-9. [Crossref]
- Freitag L, Tekolf E, Steveling H, et al. Management of malignant esophagotracheal fistulas with airway stenting and double stenting. Chest 1996;110:1155-60. [Crossref] [PubMed]
- Gauderer MW, Ponsky JL, Izant RJ Jr. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg 1980;15:872-5. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Markos P, Sisko Markos I, Ivekovic H, et al. Self-expandable metal stent for dysphagia caused by mediastinal masses in patients with lung cancer. Arab J Gastroenterol 2019;20:28-31. [Crossref] [PubMed]
- Yanık F, Karamustafaoğlu YA, Yörük Y. Esophageal self-expandable metal stent placement for the palliation of dysphagia due to lung cancer. Turk Gogus Kalp Damar Cerrahisi Derg 2019;27:88-92. [Crossref] [PubMed]
- Camidge DR. The causes of dysphagia in carcinoma of the lung. J R Soc Med 2001;94:567-72. [Crossref] [PubMed]
- van der Bogt RD, Vermeulen BD, Reijm AN, et al. Palliation of dysphagia. Best Pract Res Clin Gastroenterol 2018;36-37:97-103. [Crossref] [PubMed]
- Hyde L, Hyde CI. Clinical manifestations of lung cancer. Chest 1974;65:299-306. [Crossref] [PubMed]
- Spaander MCW, van der Bogt RD, Baron TH, et al. Esophageal stenting for benign and malignant disease: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2021. Endoscopy 2021;53:751-62. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Bentrem DJ, et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2019;17:855-83. [Crossref] [PubMed]
- Altemur Karamustafaoglu Y, Yoruk Y. Self-expandable esophageal stents placement for the palliation of dysphagia as a result of lung cancer. Dis Esophagus 2010;23:561-4. [Crossref] [PubMed]
- Kim J, Min YW, Lee H, et al. Comparative Study of Esophageal Self-expandable Metallic Stent Insertion and Gastrostomy Feeding for Dysphagia Caused by Lung Cancer. Korean J Gastroenterol 2018;71:124-31. [Crossref] [PubMed]
- Le Roux BT. The presentation of bronchial carcinoma. Scott Med J 1968;13:31-7. [Crossref] [PubMed]
- Lecleire S, Antonietti M, Di Fiore F, et al. Double stenting of oesophagus and airways in palliative treatment of patients with oesophageal cancer is efficient but associated with a high morbidity. Aliment Pharmacol Ther 2007;25:955-63. [Crossref] [PubMed]
- Herth FJ, Peter S, Baty F, et al. Combined airway and oesophageal stenting in malignant airway-oesophageal fistulas: a prospective study. Eur Respir J 2010;36:1370-4. [Crossref] [PubMed]
- Włodarczyk JR, Kużdżał J. Safety and efficacy of oesophageal stenting with simultaneous percutaneous endoscopic gastrostomy as a supplementary feeding route in unresectable proximal oesophageal cancer. Wideochir Inne Tech Maloinwazyjne 2018;13:176-83. [Crossref] [PubMed]



