
Continuous monitoring of activity and vital signs with load cells under the bed legs in advanced cancer patients: a prospective exploratory observational study—can it represent performance status?
Highlight box
Key findings
• Continuous monitoring of patients on hospital bed may give us useful information in detecting patients’ performance status (PS).
What is known and what is new?
• Long used ECOG-PS is subjective and highly open to bias. A more objective and reliable assessment tool is necessary.
• Activity index, respiratory and heart rate stability, monitored continuously with under the bed non-contact sensors, showed significant change due to worsening of PS.
What is the implication, and what should change now?
• Bed Sensor System, allowing non-contact monitoring of vital signs in hospitalized patients gives us with more detail of patients’ activity. Objective assessment of PS will be useful in decision-making such as treatment options or life-prediction. Some new measurement indexes are proposed.
Introduction
In oncology patients, physical well-being or activity levels determine treatment options, eligibility into clinical trials, and moreover, the prognosis. The activity level is commonly evaluated by Eastern Cooperative Oncology Group performance status (ECOG-PS) (1,2). Since patients spend most of the time outside the hospital, it is often difficult to objectively determine accurate physical function levels. Contrastingly, hospitalized patients spend most of the time on bed even during the daytime, which makes determination of activity levels difficult. A new objective vital sign for diagnosing the physical well-being of patients will be highly usable in oncology determination. Our newly developed contact-free non-restraining continuous bed sensor system (BSS) (3) allows monitoring of not only patient vital signs, but also activities on bed.
The aim of our study was to evaluate continuously monitored BSS parameters to determine patient’s performance in advanced hospitalized cancer patients. We present this article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1235/rc).
Methods
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethics Committee of Graduate School of Medicine, Chiba University approved the conduction of this prospective observational exploratory study (IRB No. #2470). The study was registered at the UMIN Clinical Trial Registry (No. UMIN000042736, Principal investigator: Shiroh Isono, Date of registration: December 13, 2020). Written informed consent was obtained from each consecutive patient admitted to the palliative care unit or the clinical oncology ward during the research period after explaining the risks and purposes of the study.
Study population
Due to the exploratory study purpose, the number of participants was determined by eligibility status of our palliative care ward. Exclusion criteria were (I) those unable to sign informed consents due to impending death or consciousness loss and (II) those with apparent paralysis. Twenty patients, 10 in the palliative care ward (June 2020 to August 2021) and 10 in the clinical oncology ward (December 2020 to August 2021). The reason for the long study period with different duration was due to the corona virus infection pandemic which restricted our bed-use and restriction of our clinical trial in different ward during this period. Some data from palliative care ward were obtained during collaborative research between Chiba University and Minebea Mitsumi Inc. One patient in the clinical oncology group was excluded from the monitoring since emergent hospital discharge was necessary due to exacerbation of delirium exacerbation of on the night of admission. Accordingly, BSS data from 19 patients were included in the final analysis.
Monitoring with BSS
Precise mechanism of the BSS system (Bed Sensor Vital Sign Monitoring System: BSS, Minebea-Mitsumi, Nagano, Japan) is explained in our previous reports (3,4). In brief, four load cell sensors were placed under the wheels of the medical bed and continuous signals were sent to the data logger for data processing and analysis. The four load cells independently measured the total weight on the sensors which detected small movements such as centroid shift due to heart beats, respiration, slight body movements on bed to total bed leave. Magnitude of the weight shift on the sensors was calculated as the acceleration (g/sec2) of the movements and was denoted as activity index (ACI). The primary endpoint of the study was ACI in different PS patients. Secondary endpoints were other BSS monitored activity parameters, such as number of bed-leave during day and night or % time in bed. Other BSS monitored vital signs, which included respiratory rate (RR), respiratory tidal weight (TW) and heart rate (HR), were also included as the secondary endpoint. After calculation of RR and HR, stabilities of RR (RR-S), TW (TW-S) and HR (HR-S) were assessed by measuring the relative changes of RR intervals, TW and HR intervals to those of the preceding breath or heartbeat. Body weight changes, measured in grams (g) were also continuously monitored. When abrupt change of >20% body weight was observed in patient’s in-bed signal, all data including respiratory and circulatory indexes were excluded from data processing. Bed-leave was monitored with distinct change of the full body weight to 0 kg resulting in patient’s out-of-bed signal, during the monitoring period.
All signals and variables were processed at 10 Hz sampling rate within the data logger and stored in a personal computer connected to the data logger for later analyses.
Data collection
Patients in the clinical oncology ward normally received daily fluid infusion and vital signs were assessed by the nurses twice a day, as well as daily body weight check in the morning, while patients in the palliative care ward had once a day vital sign measurement with or without fluid infusion. Daily care, medical check-up, treatments were performed as usual without any intervention or restriction on the patients’ treatments or care. All patients underwent BSS monitoring for the whole admission period. Daily BSS data were divided into 3 periods (AM: 6 a.m. to 2 p.m., PM: 2 p.m. to 10 p.m., night: 10 p.m. to 6 a.m.). The median of each period was calculated for each BSS variable, except for ACI which used averaged data for the hour. BSS data starting on the first night to second PM (total of 6 periods), except for 2 patients in the clinical oncology group who were discharged from the hospital on the second AM period (total of 5 periods), were extracted for the study analysis. Averaged values of 2 days were used for statistical analysis.
Statistical analysis
The data were analyzed using SIGMA Stat version 14.0 (Systat Software Inc., Point Richmond, CA, USA). For normally distributed continuous variables, means and standard deviations (SD) are presented; for non-normally distributed continuous variables, medians with interquartile ranges (IQR) with 25 and 75 percentiles (IQR/25/75) are shown. For continuous data, the univariate analysis between the two groups was conducted using the independent t-test for normally distributed variables and the Ram Sank test for non-normally distributed variables.
Results
Patients’ backgrounds are shown in Table 1. Oncology ward patients whose admission purpose was chemotherapy were assessed as either PS1 or PS2, while palliative care patients were assessed as PS3 or PS4 by respective doctor in charge. Since oncology patients are those with rare cancers, the diagnosis of the cancer in two groups showed wide variability. There was no significant difference in patient background between the groups except for opioid dosing (oral morphine equivalent dose: ram sank test P=0.014); however, actual PS scores were often recorded only once probably due to willful ignorance of patient’s change in physical status during the treatment. Accordingly, comparison of BSS parameters amongst PS was considered accurate when performed within group between better and worse PS (PS1 and PS2, PS3 and PS4).
Table 1
| Characteristic | Oncology group | Palliative care group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All (n=9) | Better PS (PS1) (n=2) | Worse PS (PS2) (n=7) | P value | All (n=10) | Better PS (PS3) (n=5) | Worse PS (PS4) (n=5) | P value | ||
| Age (year) | 63.4±13.0 | 48.5±24.7 | 67.7±5.1 | 0.47 | 65.7±11.7 | 64.4±5.2 | 67±16.6 | 0.75 | |
| Gender | M7:F2 | M1:F1 | M6:F1 | 0.42 | M5:F5 | M4:F1 | M1:F4 | 0.26 | |
| BMI (kg/m2) | 19.9±2.9 | 22.3±6.3 | 19.2±1.4 | 0.61 | 18.6±2.8 | 19.7±2.2 | 17.2±3.1 | 0.22 | |
| Diagnosis | – | – | |||||||
| Digestive organs | 1 | 0 | 1 | 6 | 3 | 3 | |||
| Breast | 0 | 0 | 0 | 2 | 0 | 2 | |||
| Respiratory/intrathoracic | 0 | 0 | 0 | 1 | 1 | 0 | |||
| Urinary tract | 0 | 0 | 0 | 1 | 1 | 0 | |||
| Unknown/other | 6 | 2 | 4 | 0 | 0 | 0 | |||
| Oropharynx/mouth | 2 | 0 | 2 | 0 | 0 | 0 | |||
| Opioid analgesics use (%) | 33 | 0 | 43 | 0.5 | 80 | 80 | 80 | 1 | |
| Oral morphine equivalent dose (mg) | 0 (0, 22.5) | 0 | 0 (0, 30.0) | 0.5 | 48.5 (11.3, 127.5)* | 120 (7.5, 195) | 42 (15, 56.5) | 0.42 | |
Data are shown as n or mean ± SD or medians with interquartile rages (IQR/25/75). P values: better PS versus worse PS within group. *, P<0.05 between groups. PS, performance status; F, female; M, male; BMI, body mass index; SD, standard deviation.
BSS monitored activity
Activity monitoring
ACI (primary end-point) of different PS patients are shown in Figure 1 and data summarized in Table 2. In all PS patients, ACI day was significantly higher than ACI night (rank sum test, P<0.001) with ACI ratio (day/night) >1 (Table 2) suggesting ACI to be a good indicator of activity difference between awake and sleep.

Table 2
| Activity parameters | Oncology group | Palliative care group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All (n=9) | Better PS (PS1: n=2) | Worse PS (PS2: n=7) | P value | All (n=10) | Better PS (PS3: n=5) | Worse PS (PS4: n=5) | P value | ||
| % time on bed | |||||||||
| Daytime | 87.3 (70.3, 92.2) | 57.6 (25.7, 89.6) | 87.3 (70.9, 92.7) | 0.5 | 98.6 (83.3, 99.9) | 88.6 (58.5, 94.8) | 99.9 (99.4, 100) | 0.008 | |
| Nighttime | 96.7 (95.8, 98.1) | 97.0 (95.4, 98.5) | 96.7 (96.3, 97.8) | 1 | 98.6 (92.7, 99.9) | 95.0 (90.9, 98.6) | 99.9 (92.3, 99.9) | 0.151 | |
| No of bed leave/8 hours | |||||||||
| Daytime | 11 (8.3, 15.0) | 12.8 (9.0, 16.7) | 11.0 (7.7, 13.3) | 0.889 | 4.8 (1.6, 6.8)** | 6.8 (4.1, 17) | 1.8 (0.6, 5.3) | 0.056 | |
| Nighttime | 3.5 (2.8, 5.8) | 5 (3.5, 6.5) | 3.5 (2.5, 5.0) | 0.5 | 3.33 (1.38, 4.8) | 4.3 (2.7, 8.5) | 1.5 (0.5, 3.5) | 0.056 | |
| ACI (g/sec2) | |||||||||
| Daytime | 233 [155, 334] | 558 [233, 884] | 220 [141, 326] | 0.333 | 189 [112, 449] | 296 [195, 1,233] | 116 [87, 152] | 0.008 | |
| Nighttime | 83 [72, 139] | 110 [82, 138] | 83 [63, 140] | 1 | 85 [57, 114] | 88 [70, 804] | 72 [47, 103] | 0.31 | |
| ACI ratio (day/night) | 2.8 (1.8, 3.3) | 4.6 (2.9, 6.4) | 2.7(1.6, 2.9) | 0.222 | 1.8 (1.5, 3.4) | 3.4 (1.4, 7.0) | 1.6 (1.3, 2.0) | 0.31 | |
Data are shown as medians with interquartile rages (IQR/25/75). P values: better PS versus worse PS within group. **, P<0.01 between groups. BSS, bed sensor system; ACI, activity index; PS, performance status.
Within the same category of patients, such as those under palliative care or those under cancer treatment, performance state deterioration could be observed with decrease in ACI. In the palliative care group, PS4 patients revealed low ACI throughout the day, while some PS3 patients revealed higher ACI (P=0.008). This appeared to coincide with the oncology group, although the difference between PS1 and PS2 was not statistically significant which may possibly be due to the small number of PS1 patients. It is of note that even amongst hospitalized sedentary patients, ACI of PS4 patients were clearly different from other PS3 patients.
Other activities monitored by BSS, % time on bed, number of bed leave are shown in Table 2. Palliative care group patients were observed on bed 98.6% of the daytime and those with worse PS (PS4) assessment were actually observed 99.9% of the time on or in bed day and night. Those with better PS (PS3) assessment had decreased time on bed during the day, yet above 50% following definition of PS3. In oncology group patients, the observed time on bed during day was as high as 87%. There also was a trend for decrease in better PS (PS1) patients. Number of bed-leave during daytime was observed with more obvious difference with the worsening of PS. Patients in oncology group were observed with a median of 11 bed-leaves in 8 hours during daytime which decreased to 4.8 in palliative care patients (P=0.004, Table 2).
Daily body weight changes
Continuous body weight monitoring allows us to share subtle changes of weight in different PS groups. Body weight increase/decrease per day from 1st night to 2nd night was +1.7 (1.4, 2.0) kg in PS1, +0.2 (−0.01, 1.2) kg in PS2 patients showing slight increase of body weight. On the contrary, PS3 and PS4 patients showed decrease in body weight −0.1 (−0.8, 0.4) kg and −0.85 (−2.1, 1.6) kg in PS3 and PS4, respectively).
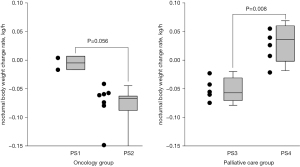
Nocturnal changes in body weight
A new vital measurement denoting changes in nocturnal body weight, which physiologically declines during the night. The slope of the weight decrease per night was estimated as the slope of the linear regression line, of which average of 2 nights were compared. As shown in Figure 2, PS1 and PS2 patients showed a minus slope denoting physiological decrease in spite of continued hydration. However, in poor PS patients, namely PS4 patients, showed a positive slope, suggesting increase in body weight at night (P=0.008).
BSS vital sign monitors
Respiratory and circulatory indexes
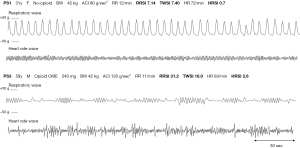
Figure 3 shows the representative wave observed in PS1 (above) and PS3 (below) patients. PS3 patient is observed with irregular small breathing and heart rate as is calculated as high RRSI, TWSI and HRSI.
Respiratory and circulatory BSS monitored indexes
Average respiratory rate during the day and night in both groups were similar and showed slight but insignificant decrease at night (Table 3). Respiratory rate stability index is defined as % change in respiratory intervals which is calculated for every breath and averaged per hour. Tidal weight stability index is the % change of weight changes due to respiration, assumed to parallel the tidal volume changes. Consequently, the higher the irregularity, the more unstable or ataxic the respiration becomes. Reliable data can be obtained only when body activities are low, and accordingly only night data are compared.
Table 3
| Vital sign parameters | Oncology group | Palliative care group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All (n=9) | Better PS (PS1: n=2) | Worse PS (PS2: n=7) | P value | All (n=10) | Better PS (PS3: n=5) | Worse PS (PS4: n=5) | P value | ||
| Respiratory variables | |||||||||
| Respiratory rate (/min) | |||||||||
| Daytime | 15.6 (13.0, 17.3) | 13.0 (12.7, 13.2) | 16.0 (14.7, 17.6) | 0.04 | 14.3 (12.5, 17.3) | 16.1 (13.1, 17.3) | 14.0 (10.2, 17.1) | 0.47 | |
| Nighttime | 14.7 (11.0, 18.6) | 10.9 (9.2, 12.5) | 15.4 (13.9, 21.5) | 0.11 | 13.4 (11.8, 16.0) | 12.1 (11.8, 16.4) | 13.8 (10.2, 17.1) | 0.87 | |
| RR stability index (%) | |||||||||
| Nighttime | 7.3 (6.5, 9.9) | 6.8 (6.1, 7.5) | 7.3 (6.9, 10.6) | 0.2 | 14.6 (10.1, 26.0)* | 11.3 (7.8, 26.2) | 21.8 (11.1, 31.7) | 0.47 | |
| TW stability index (%) | |||||||||
| Nighttime | 9.0 (8.0, 10.3) | 7.6 (7.2, 7.9) | 9.4 (8.5, 10.4) | 0.08 | 16.5 (9.2, 23.2) | 11.1 (9.1, 19.0) | 18.2 (11.2, 32.0) | 0.22 | |
| % tachypnea | |||||||||
| Nighttime | 0.4 (0.2, 26.1) | 0.1 (0, 1.0) | 0.9 (0.4, 51.2) | 0.06 | 2.2 (0.2, 6.2) | 1.3 (0.2, 4.0) | 5.8 (0.3, 7.4) | 0.22 | |
| % bradypnea | |||||||||
| Nighttime | 0.9 (0.4, 6.2) | 4.0 (0.6, 7.5) | 0.9 (0.2, 4.8) | 0.67 | 3.1 (0.6, 8.7) | 5.3 (0.6, 6.4) | 1.0 (0.5, 25.5) | 0.84 | |
| Circulatory indexes | |||||||||
| Heart rate (/min) | |||||||||
| Daytime | 64.6 (61.2, 67.6) | 63.4 (62.2, 64.6) | 67.5 (60.2, 67.7) | 0.67 | 57.9 (51.0, 63.0)* | 55.4 (48.2, 63.6) | 58.5 (52.4, 63.4) | 0.69 | |
| Nighttime | 64.6 (60.8, 67.4) | 68.4 (64.6, 72.1) | 63.1 (60.2, 67.1) | 0.3 | 56.5 (49.4, 61.5)* | 56.2 (46.6, 62.1) | 56.9 (50.0, 63.4) | 0.69 | |
| HR stability index (%) | |||||||||
| Nighttime | 0.8 (0.6, 1.2) | 0.9 (0.8, 1.0) | 0.8 (0.6, 1.3) | 0.89 | 1.5 (0.8, 1.9)* | 1.4 (0.7, 2.1) | 1.7 (0.8, 1.9) | 0.92 | |
Data are shown as medians with interquartile rages (IQR/25/75). P values: better PS versus worse PS within group. *, P<0.05 between groups. BSS, bed sensor system; RR, respiratory rate; TW, tidal weight; HR, heart rate.
Palliative care patients were observed with significantly higher RR stability index (P=0.03, Table 3), and higher TW stability index compared to those of oncology patients (P=0.06, Table 3). % tachypnea and % bradypnea are % time spent with RR >22 or RR <8, respectively. These data showed no difference between the two groups statistically, though trend for increased % was observed in palliative care patients. Average heart rate during the day and night in both groups were similar during daytime and night; however, intergroup difference was significant between palliative care and oncology patients in all periods, with higher HR in the oncology patients (P=0.03, Table 3). HR stability index, % change in the heart rate intervals calculated for every beat and averaged for one hour showed small but significant difference between the groups with instabilities higher in palliative care patients (P=0.04, Table 3).
Discussion
In this prospective observational exploratory study in advanced cancer patients, palliative care patients were observed with (I) low number of bed leave during the day, (II) high respiratory rate instability, (III) low heart rate with high heart rate instabilities, compared with oncology patients with better PS under curative treatment. Activities compared within groups showed worse PS patients (PS4) to be detected with (I) high % of time on bed, (II) low ACI and (III) increase of nocturnal body weight.
PS or the ability to move physically needs to be accurately assessed in cancer patients for the purpose of informing patients as to their eligibility of oncological treatment and prognosis prediction (5). ECOG-PS, though simple and prevalent, is subjective and thus open to bias (6); therefore, an objective measurement system to help physicians’ assessment is recommended (7). Objective evaluation of PS has been attempted by use of wearable monitors in patients with ECOG-PS between 0 to 3 (8), and may have potential for clinical use. These data used intensity levels of physical activity, such as step counts, travel distance and number of stairs climbed during the day with wearable monitors. ECOG-PS and wearable monitors both have difficulty in assessing patients already bedridden, such as patients with bone metastasis, paralysis, or patients suffering from chemotherapy side-effects. Our findings advocate the use continuous under the bed legs monitoring system for detection of physical state worsening in already moderately deteriorated patients.
ACI
ACI was first chosen for possible indicator for PS (primary hypothesis), which decreased significantly at night, reliably showing activity decrease in bed at night. Within the same group of patients, ACI was lower in worse PS patients, although intergroup difference was not clear. The fact that PS determination itself was open to bias due to treatment purpose and the physician’s intent may be reasons for the ACI indifference. However, patients in the oncology group, though physically fit, whose purpose of admission being chemotherapy, were likely to have stayed comfortably in bed resting during the treatment, which is likely the major reason for low ACI in good PS oncology patients and the major limitation to diagnose PS in hospitalized patients. Obviously, %time in bed will not be a useful index for hospitalized-patients’ activity evaluation since most patients spend time on bed, even if they may not be asleep in bed. Number of bed-leave during the day was observed with significant difference between the two groups, a candidate measure to reflect physical fitness.
Nocturnal body weight changes
Accuracy of BSS monitored body weight changes and its usefulness of long-term measurement on bed-ridden patients has been reported in a previous report (9). In healthy individuals, without diet at night, perspiration, and morning urination result in lowest body weight in the morning before breakfast. Our BSS system allows precise monitoring of the body weight changes. Thus, this is the first to report daily measured weight body decrease during sleep. Our data showed constant decrease in nocturnal body weight even in those with continuous 3,000–4,000 mL/day hydration in the oncology patients. On the contrary, patients in the palliative care unit were observed with positive slope or increase in weight at night even with no or limited hydration and this was significant in PS4 patients in spite of body weight decrease after 24 hours. Though not the scope of this study, it is noteworthy that within the palliative care patients, 4 positive slope patients were observed with short prognosis of 6.5 days(median) while 6 negative slope patients showed a median prognosis of 18.5 days (P=0.25, t-test). Peripheral edema, ascites or pleural effusion known as overhydration-related symptoms which we experience in terminal-ill cancer patients, may parallel this non physiological increase of the body weight at night. A similar phenomenon is reported in intensive care unit patients where non-survivors were observed to have significant increase in daily body weight compared with survivors (10). Comparatively, body weight loss in chemotherapy-planned patients is predicted to have poor prognosis (11). Not only daily body weight measurement, but nocturnal body weight changes or body-weight changes during hydration, may also act as reliable tools in predicting the patients’ wellness.
Respiratory rate and instability
BSS monitoring system has been previously reported to be reliable for rate (3) and also for a new measurement, TW to represent tidal volume (12). Our study showed RR instability to be significantly increased in palliative care patients, although the reason for this instability is yet unclear. We speculate this to be either due to worsening of PS or higher opioid use in palliative care patients. We have previously reported opioid dose dependent increase in respiratory rate instability in terminal cancer patients and a predominant risk factor for ataxic breathing pattern, especially in female patients (5). As opioid dose increase is usually accompanied with worsening of PS, these two phenomena are difficult to discriminate nor probably clinically not required.
Heart rate and instability
To our surprise, palliative care patients were found with low heart rate and high heart rate instability. Previous reports have proven increase in resting heart rate to be closely related to all-cause mortality (13). On the contrary, heart rate variability or RR-interval variability in ECG has been known to be a measure of sympathetic activity and our data follows previous reports showing increased variability to be an important clinical indicator for mortality in myocardial infarction patients (14). Autonomic dysfunction (AD) in advanced cancer patients have recently been reported and have been focused as a mortality risk (15). Cancer itself, certain cancer drugs and radiotherapy to certain area and other combined cancer-associated lifestyle disturbances have been reported to contribute to autonomic disturbances in advanced cancer patients; however, this AD is characterized by elevated heart rate and reduced heart rate variability which coincides with our study. The reason for low heart rate and high heart rate instability in palliative care patients is unclear and needs to be confirmed in future research.
Limitations of the study
Clearly, the small number of participants in this study and the rare cancer population in the oncology department are the major limitation for generalization of the data. As this was an exploratory prospective study using a new monitoring system, further clinical trials with a larger number of participants are mandatory. Another limitation of the study is that the BSS system, at present, can only be utilized in hospital beds. Whether respiratory and heart rate stabilities can be monitored for even short times, as few hours on out-patients’ chemotherapy beds, need to be confirmed in future studies. Home based BSS system and tele-monitoring may be another choice in the future.
Conclusions
Objective determination of the PS of patients under cancer treatment is important, for the patients as well as for the clinicians in order to understand what to expect and decide on the choices given in their remaining lives. Our result showed that “activity index” in bed, was useful in detecting PS4, low activity patients. Heart rate instability and respiratory rate instability were observed to be a candidate index for performance deterioration. BSS system, allowing non-contact monitoring of vital signs may give us the opportunity to observe patients in detail for further clinical decision-making without burden on the patients.
Acknowledgments
Authors would like to thank Dr Yuichi Takiguchi (Chair of the Department of Clinical Oncology, Chiba University Hospital, Chiba, Japan) for his generous permission for conducting clinical trial on his patients. Special thanks to Sara Shimizu, MD (Shimizu Orthopedic Plastic Surgery Clinic, Tokyo, Japan) for her assistance with improving the manuscript.
Funding: This work was supported by research fund from Daiichi-Sankyo (No. A22-1073 to primary investigator: Prof. Shiroh Isono), which will be used as an article processing charge.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1235/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1235/dss
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1235/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1235/coif). The authors report that this study used some data obtained during collaborative research between Chiba University and Minebeamitsumi Inc. No funding from Minebeamitsumi Inc. was used for this particular study. SI, as a head of the Department of Anesthesiology, Chiba University, is one of the inventors of the vital sign monitoring system used in this study and received royalties and grants for the industry-Academia Joint Research from Minebeamitsumi Inc. SI is also one of the inventors for the issued or pending patents of the devise used in this study and received research funding from Daiichi-Sankyo, A22-1073 which will be used as an article processing charge. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This prospective observational study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and approved by the Ethics Committee of the Graduate School of Medicine, Chiba University (IRB No. #2470). Written consent was obtained from each consecutive patient admitted to the palliative care unit or the clinical oncology ward during the research period after explaining the risks and purposes of the study. The study was registered at the UMIN Clinical Trial Registry (No. UMIN000042736, principal investigator: Shiroh Isono; date of registration: December 13, 2020).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. [Crossref] [PubMed]
- Karnofksy DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM, editor. Evaluation of chemotherapeutic agents. New York: Columbia University Press; 1949.
- Isono S, Nozaki-Taguchi N, Hasegawa M, et al. Contact-free unconstraint respiratory measurements with load cells under the bed in awake healthy volunteers: breath-by-breath comparison with pneumotachography. J Appl Physiol (1985) 2019;126:1432-41. [PubMed]
- Hasegawa M, Nozaki-Taguchi N, Shono K, et al. Effects of opioids on respiration assessed by a contact-free unconstraint respiratory monitor with load cells under the bed in patients with advanced cancer. J Appl Physiol (1985) 2021;130:1743-53. [PubMed]
- Allende-Pérez S, Rodríguez-Mayoral O, Peña-Nieves A, et al. Performance status and survival in cancer patients undergoing palliative care: retrospective study. BMJ Support Palliat Care 2022;bmjspcare-2022-003562.
- Chow R, Bruera E, Temel JS, et al. Inter-rater reliability in performance status assessment among healthcare professionals: an updated systematic review and meta-analysis. Support Care Cancer 2020;28:2071-8. [Crossref] [PubMed]
- Simcock R, Wright J. Beyond Performance Status. Clin Oncol (R Coll Radiol) 2020;32:553-61. [Crossref] [PubMed]
- Gresham G, Hendifar AE, Spiegel B, et al. Wearable activity monitors to assess performance status and predict clinical outcomes in advanced cancer patients. NPJ Digit Med 2018;1:27. [Crossref] [PubMed]
- Ishikawa T, Sakai I, Amemiya A, et al. Long-term body weight change assessed by non-contact load cells under the bed in older people with and without eating assistance: a preliminary study. Sci Rep 2022;12:8107. [Crossref] [PubMed]
- Antonio ACP, Fernandes VR, Azzolin KO. The Correspondence Between Fluid Balance and Body Weight Change Measurements in Critically Ill Adult Patients. J Crit Care Med (Targu Mures) 2021;7:46-53. [Crossref] [PubMed]
- Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 1980;69:491-7. [Crossref] [PubMed]
- Inada A, Inaba S, Matsumura Y, et al. Contact-free assessments of respiratory rate and volume with load cells under the bed legs in ventilated patients: a prospective exploratory observational study. J Appl Physiol (1985) 2023;134:1341-8. [PubMed]
- Zhang D, Shen X, Qi X. Resting heart rate and all-cause and cardiovascular mortality in the general population: a meta-analysis. CMAJ 2016;188:E53-63. [Crossref] [PubMed]
- Johnston BW, Barrett-Jolley R, Krige A, et al. Heart rate variability: Measurement and emerging use in critical care medicine. J Intensive Care Soc 2020;21:148-57. [Crossref] [PubMed]
- Coumbe BGT, Groarke JD. Cardiovascular Autonomic Dysfunction in Patients with Cancer. Curr Cardiol Rep 2018;20:69. [Crossref] [PubMed]


