Correlating symptoms and their changes with survival in patients with brain metastases
Introduction
Approximately 40–60% of advanced cancer patients develop brain metastases, the most common brain neoplasms (1). Certain primary cancer sites are more likely to lead to brain metastases such as breast, colorectal, lung, melanoma, and kidney (1). Fatigue, headaches, and focal weakness are the most prevalent symptoms, although other symptoms can include seizures and visual impairments (2). Symptoms experienced by patients with brain metastases may be due to the cancer itself or as a side effect of treatment (3).
Treatment for brain metastases aims to improve health-related quality of life (HRQOL) by palliating symptoms (4,5). The presence of the blood brain barrier commonly limits the entry of systemic therapies, including chemotherapy; as a result such treatment options are usually not appropriate for these patients (6). For individuals with multiple brain metastases and poor performance status (PS), palliative care with or without whole brain radiotherapy (WBRT) is the standard of care (7,8). However, the full effects of WBRT may take up one month to be experienced, by which point approximately 20–30% of patients will have died (5). Therefore, individuals may never benefit from the prescribed treatment (9). As such, supportive care including symptom management with medication only may be offered for patients with poor PS (7,8). Corticosteroids may palliate symptoms of brain metastases, such as edema and neurological symptoms, in upwards of 70% of patients; however, treatment with corticosteroids alone is indicative of poor survival with a median survival of 2 months (10).
Due to the limited survival of patients with brain metastases, accurate survival prognoses are of the utmost importance to ensure proper prescription of treatment and appropriate care plans. The objective of the study was to determine survival prognosis in patients with multiple brain metastases by using changes in symptoms that patients experienced.
Methods
A retrospective analysis was conducted on prospectively collected databases that were collected from 1999 to 2013. Patients with radiographic evidence of brain metastases verified with CT or MRI were included in the analysis. All patients were treated with WBRT and prescribed varying doses of dexamethasone.
Data collection
Baseline demographic information such as age, gender, Karnofsky performance status (KPS), primary cancer site, number of brain metastases, systemic treatment and dose of dexamethasone (when applicable) were collected for all patients. A total of 14 symptom scores were obtained from six quality of life (QOL) and symptoms questionnaires, which included nausea, pain, anxiety, fatigue, appetite loss, depression, concentration, memory loss, vision changes, weakness, balance, headache, insomnia, concentration. All individuals consented to original data collection and the current study was approved by our institutions Research Ethics Board.
Questionnaires
Edmonton Symptom Assessment Scale (ESAS)
The ESAS is a validated 9-item symptom questionnaire rated on a scale from 0 (no experience of the symptom) to 10 (worse possible degree of the symptom). Six symptoms were evaluated: nausea, pain, fatigue, anxiety, appetite loss and depression.
Spitzer Quality Of Life Index (SQLI)
The SQLI consists of five domains: daily living, health, activity, support, and outlook. An additional symptom questionnaire was also administered rated on a Likert scale from 1 to 4 with 1= none, 2= mild, 3= moderate, and 4= severe. Eight symptoms were evaluated: nausea, concentration, fatigue, memory loss, vision problem, weakness, balance and headache.
European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30)
The EORTC QLQ-C30 is a 30-question QOL assessment for general cancer population. This questionnaire assess symptoms on a scale of 1 to 4, with 1= not at all, 2= a little bit, 3= quite a bit, and 4= very much. Ten symptoms were assessed: nausea, pain, fatigue, insomnia, concentration, memory loss, weakness, anxiety, appetite loss and depression.
European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 15 Palliative (EORTC QLQ-C15-PAL)
The EORTC QLQ-C15-PAL is a QOL assessment for palliative cancer patients consisting of 15 questions. Eight symptoms were assessed: nausea, pain, fatigue, insomnia, anxiety, appetite, weakness and depression. This questionnaire assessed symptoms on a Likert scale of 1 to 4, similar to the C30. Patients who completed the QLQ-C15-PAL also completed the BN20+2. Both questionnaires included the weakness item; as such records from the QLQ-C15-PAL were used if available. If not, the BN20+2 weakness item was used.
European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Brain Module (EORTC QLQ-BN20 or BN20+2)
The BN20 is a 20-item questionnaire to supplement the QLQ-C30, while the BN20+2 is a 22-item in-development tool to accompany the QLQ-C15-PAL. Both questionnaires assess symptoms on a Likert scale from 1 to 4, similar to the QLQ-C30 or QLQ-C15-PAL. Four symptoms were assessed by both questionnaires: fatigue, vision problem, coordination, and headache, while the BN20+2 assess two additional symptoms: concentration, memory loss.
Functional Assessment of Cancer Therapy-Brain Scale (FACT-Br)
The FACT-Br assesses QOL in five domains: physical well-being, social/family well-being, emotional well-being, functional well-being, and additional concerns related to the brain. It is rated on a Likert scale from 0 to 4, with 0= not at all, 1= a little bit, 2= somewhat, 3= quite a bit, and 4= very much. Twelve symptoms were assessed: nausea, pain, fatigue, vision problems, weakness, coordination, headache, anxiety, depression, insomnia, concentration and memory loss. Insomnia, concentration and memory loss were reversed due to wording of the question.
Statistical analysis
Overall survival (OS) was calculated from consultation date to the death/last follow-up in months. Survival was defined from date of consult to date of death as baseline assessments were conducted at initial clinic consultation. All patients included in this study were assessed in a rapid referral radiotherapy clinic where the majority of patients receive treatment within 1 week of initial consultation. Patients who were still alive at time of analysis were censored at their last follow-up date.
Baseline symptoms
Univariate Cox proportional hazard (PH) model of OS was conducted in patients with symptom items. The time (months) to death or last follow-up was considered as the outcome variable. Analysis was separated into two groups: group 1 included all patients who completed the ESAS on a scale of 0 to 10; group 2 included all patients who completed one or more of the following questionnaires the EORTC QLQ-C30, SQLI, EORTC QLQ-BN20 or BN20+2, EORTC QLQ-C15 and Fact-Br. To search for the parameters most predictive of time to death, all variables with P<0.10 obtained from the univariate analysis were selected to the backward stepwise selection procedure in the multivariate analysis. Kaplan-Meier OS curve with 95% confidence interval (CI) was conducted in group 1 or group 2 patients. For those significant symptoms obtained from the multivariate analysis, Kaplan-Meier OS curves and Log-rank test were performed.
Symptom change
Only subjects who completed baseline assessment and at least one follow-up QOL questionnaire were included in the analysis. For the symptom change analysis, all patients from both group 1 and group 2 were analyzed together. Symptom changes were calculated between baseline and each follow-up record (month 1, 2, or 3). Kaplan-Meier OS curves with Log-rank test were performed in patients with month 1, 2, or 3 measurements. Symptom changes were sorted into three categories: increase (baseline score was less than follow-up score; severity of symptoms increased or worse QOL), no change (baseline score was equal to follow-up score; same severity), and decrease (baseline score was greater than follow-up score; severity of symptoms decreased or better QOL).
Univariate Cox PH model of OS was also conducted in all patients at month 1, 2, and 3, respectively, with demographic parameters and symptom changes (increase, no change, or decrease). Backward stepwise selection procedure in the multivariate analysis was also performed, using all variables with P<0.10 obtained from univariate analysis. In the final multivariate Cox PH model, we only kept the most significant predictors (P<0.05). All analyses were performed using Statistical Analysis Software (SAS version 9.4 for Windows). P value <0.05 was considered statistically significant.
Results
Baseline symptoms
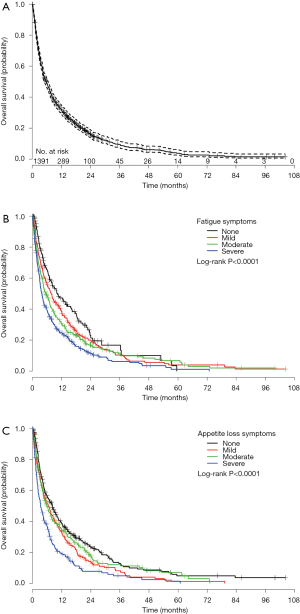
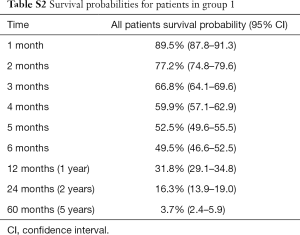
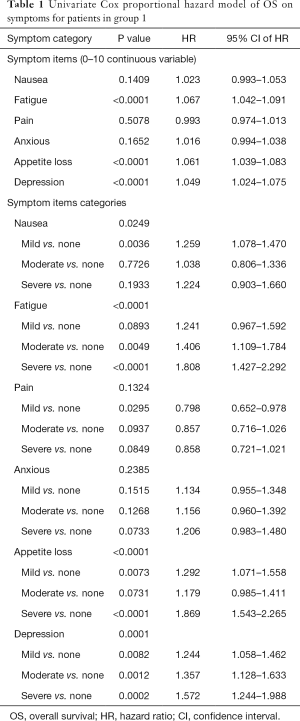
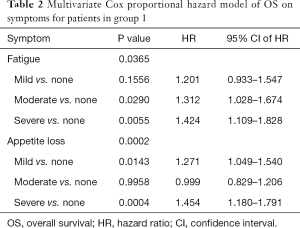
Group 1 consisted of 1,391 patients who completed the ESAS symptom questionnaire at baseline rated on an 11 point Likert scale from 0 to 10. The mean age was 68 years, with 53% being male. The most common primary cancer sites were lung (36%), genitourinary (26%), and breast (20%). Median KPS was 60, ranging from 10 to 100 (Table S1). Among these 1391 patients, 925 (66%) patients were dead and 466 (34%) patients were alive and censored. Duration of follow-up was ranged between 0.1 and 104 months and the actuarial median survival time was 5.8 months (95% CI of 4.9–6.5 months). Figure 1A shows the Kaplan-Meier OS curve with 95% CI in all patients (group 1). In Table S2, the survival probabilities at 1-, 2-, and 3-month were 89.5%, 77.2%, and 66.8% respectively. Fatigue, nausea (only categorical), appetite loss, and depression were significantly related to OS (Table 1). Through multivariate analysis, patients with moderate (HR =1.31) or severe (HR =1.42) fatigue were more likely to have shorter duration of survivals comparing to those without any fatigue symptoms. As well, patients with mild (HR =1.27) or severe (HR =1.45) appetite loss were more likely to have higher risk of death or shorter survival, as compared to those without such symptoms (Table 2, Figure 1B,C).
Full table
Full table
Full table
Full table
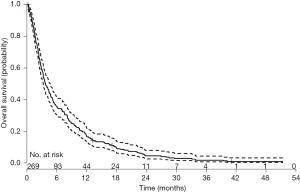
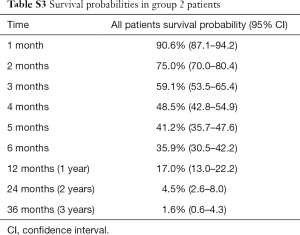
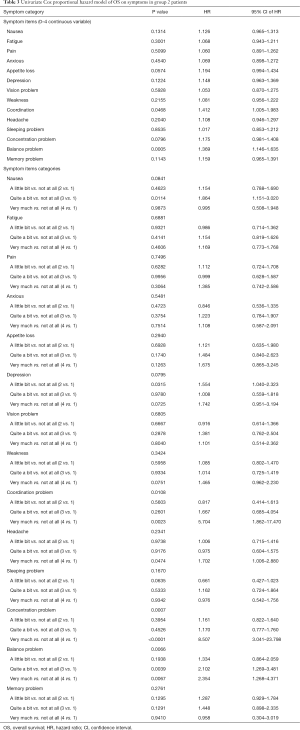
In group 2, 269 patients completed a symptom questionnaire (EORTC QLQ-C30, SQLI, EORTC QLQ-BN20 or BN20+2, EORTC QLQ-C15 and Fact-Br) at baseline. The mean age was 63 years, with 69% male. The most common primary cancer sites were lung (54%) and breast (25%). Median KPS was 70 ranging from 30 to 100 (Table S1). Among 269 patients, 259 (96%) patients died and 10 (4%) patients alive. Duration of follow-up was ranged from 0.2 to 51 months. Figure S1 shows the Kaplan-Meier OS curve with 95% CI in patients from group 2. The actuarial median survival time was 3.8 months with 95% CI of 3.2–4.6 months. In Table S3, the survival probabilities at 1-, 2-, and 3-month were 90.6%, 75.0%, and 59.1%, respectively. In the univariate analysis, coordination, balance, and concentration (only categorical) were significantly related to OS (Table 3). All symptoms items were not significant in the multivariate analysis for group 2 patients.
Full table
Full table
Symptom change
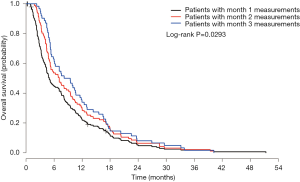
Two hundred and one patients were included in the symptom change analysis if they had at least one follow-up. The mean age was 63 years, with 68% of the patients being male. The most common primary cancer sites were lung (58%) and breast (23%). Median KPS was 70 ranging from 30 to 100 (Table S1). Among 190 patients with month 1 follow-up, 183 died and 7 alive with a censored rate of 3.7%; among 97 patients with month 2 follow-up, 96 died and 1 alive with a censored rate of 1.0%; among 62 patients with month 3 symptoms records, all of them died and 0% censored. The actuarial median survival time was 5.0 months (95% CI: 4.3–7.0), 7.1 (95% CI: 5.2–9.5), and 8.8 (95% CI: 5.8–11.5) in patients who had month 1, 2, or 3 measurements. Log-rank test shows significant difference among three curves (P=0.029; Figure S2).
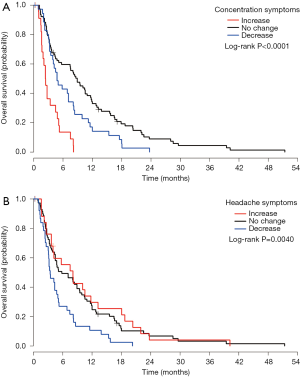
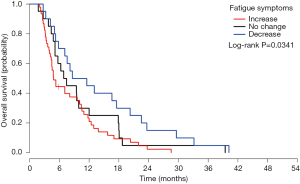
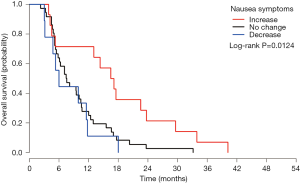
At month 1, the symptom changes of appetite loss, headache, concentration and balance were significantly related to OS (Table 4). Symptom changes of concentration and headache remained significant in the multivariate analysis. Patients with increased concentration difficulty (Figure 2A) had higher risk of impending death compared to patients with no change (HR =4.74) or with decreased scores (HR =3.19). While for headache (Figure 2B), patients with decreased symptom experience were more likely to have shorter survival, compared to patients with no change (HR =1.85) or with increased symptoms experience (HR =1.79) (Table S4). For month 2, symptom change of fatigue was significantly related to OS (Table 4). Patients with increased experience of fatigue (Figure 3) had shorter survival compared to patients with decreased score (HR =2.06) (Table S4). For month 3, three symptom changes were significantly related to OS: nausea, appetite loss, and concentration difficulty (Table 4). Patients with increased nausea (Figure 4) had higher risk of impending death comparing to patients with decreased score (HR =2.06) (Table S4).
Full table

Full table
Discussion
Brain metastases are a common neurological consequence of advanced cancer and are associated with significant morbidity and mortality. Clinical presentation of certain symptoms can act as prognostic factors to help physicians formulate survival estimates. Presenting symptoms and symptom changes can help inform the physician of the patient’s PS and necessary action, such as referral to supportive care or further case management.
Several studies have shown that global reductions in QOL and increased severity of other symptom scores are associated with shorter survival (8-10). In one study, higher symptom burden in dyspnea, drowsiness, appetite, and nausea predicated death; however, upon multivariate analysis only dyspnea and drowsiness were significantly correlated with shorter survival (11). One study examined total symptom burden in palliative cancer patients and found it was significantly associated with time to death (12). Interestingly, this study saw no association between psychological symptom burden, such as depression and anxiety, and time of death (12). Another study found that all ESAS items significantly deteriorated in the last month of life (13). It appears that certain symptoms intensify at the end of life with the most symptom burden contributed by worsening fatigue, appetite, and wellbeing (11,13).
Our study found that baseline symptoms of fatigue, nausea, and appetite loss, as measured by the ESAS, were significantly related to OS. In particular, individuals scoring with moderate to severe fatigue and appetite loss had shorter survival than those presenting with mild or no symptoms. These findings were similar to previous studies (9,10). However, in contrast to Cheung et al., our study found that depression, as measured by the ESAS, was also significantly related to OS. For individuals assessed by the QLQ-C30, QLQ-C15-PAL, BN20, BN20+22, SQLI and Fact-Br, co-ordination, balance and concentration difficulty were significantly related to OS. Greater burden of appetite loss, headache, and difficulty concentrating were also correlated with shorter OS. As time progressed, increased concentration difficulties, issues with balance, and fatigue intensity were associated with higher risk of death. A previous study found that neurocognitive function (NCF) and QOL were correlated and that declines in NCF predicated deteriorations of QOL (14). Our findings regarding concentration difficulties further corroborate this study suggesting that NCF may be an important component of the prognosis estimate.
Several other studies have examined the prognostic significance of symptoms amongst other entities such as characteristics of the brain metastases and patients themselves, and the prescribed treatment. Caballero et al. examined prognostic factors for patients with recurrent brain metastases who underwent SRS after previous WBRT (15) and concluded that prognostic factors were dependent on primary site. Another study examined patients with brain metastases where the majority of patients were treated with WBRT (84%) (16). Survival differed depending on prescribed treatment, with corticosteroids alone having the lowest median survival (1.3 months). Other prognostic factors were identified, which included PS, corticosteroid response, systemic tumor activity, and serum lactate dehydrogenase levels. Lesser prognostic factors included age, primary tumour site, and number of BM. Lagerwaard et al. also found variable prognostic factors between primary cancer sites (16). For instance in lung cancer patients, sex had a significant impact on survival and for breast cancer patient length of the period between primary tumour occurrence and development of BM were of prognostic significance. Future studies should be conducted to address the lack of consistency in the literature to determine what symptoms are of prognostic significance.
In addition to symptoms caused by the disease itself, WBRT is associated with certain side effects that may also contribute to QOL debilitations, such as increased fatigue. One study found that patients experienced no difference in fatigue following WBRT if prescribed dexamethasone (2). As fatigue is associated with worsened QOL, it is imperative to monitor and address such debilitating symptoms to prevent further functional declines. In patients presenting with multiple lesions and poor PS, adequate symptom control can be managed through corticosteroid prescription alone without added side effects from WBRT, which may be more appropriate in palliative circumstances (7). For patients where benefits from WBRT and other radiotherapy techniques are indicated, concurrent corticosteroid prescription should be considered to manage side effects of radiation, such as fatigue to prevent functional debilitations and preserve QOL at the end of life.
Recently, Jones et al. provided commentary on the interim results of the Quality of Life after Treatment for Brain Metastases (QUARTZ) study (17). The non-inferiority study aimed to elucidate the impact of WBRT on OS and QOL between individuals who received dexamethasone and optimal supportive care with or without WBRT. The interim results of the study showed no differences between the two groups in regards to survival, average QOL, and symptom scores. Interestingly, the QUARTZ cohort had shorter survival than previous studies had observed which highlights the appropriateness of WBRT in certain circumstances. As previously set forth by Tsao et al., patients with poor prognosis with single or multiple fractions can be managed solely by palliative care with optional WBRT (8). The utility of WBRT is particularly important in certain circumstances when the intent of treatment is improved brain control, multiple brain metastases are present, or the chance of brain recurrence in other areas is high (8).
Treatment for brain metastases may not be curative in nature, but survival estimates are essential in providing the patient with information regarding their condition allowing them to prepare for end of life circumstances. Utilizing both presenting symptoms and symptom changes can allow accurate survival predictions to be made. Such predictions can inform proper treatment prescription, ensure that appropriate care goals are formed by the patient and physician, and allow patients to deal with end-of-life matters. Updating prognosis information throughout the treatment process is additionally important so that treatment and goals of care can be adapted accordingly. Furthermore, symptoms and symptom changes associated with poorer survival may act as a powerful indicator for the involvement of palliative care. Palliative care should be incorporated into a patient’s care plan, if not at initial diagnosis, then at least at the first presentation of symptoms that are correlated with poorer survival. However, it remains a challenge for healthcare professionals to provide accurate predictions to their patients; therefore, it is necessary to determine what symptoms should be assessed and monitored to help form accurate prognosis estimates. The current literature on this topic is limited and inconsistent, and future studies should be conducted on the prognostic significance of symptoms experienced by the patients themselves.
Acknowledgements
We thank the generous support of Bratty Family Fund, Michael and Karyn Goldstein Cancer Research Fund, Joey and Mary Furfari Cancer Research Fund, Pulenzas Cancer Research Fund, Joseph and Silvana Melara Cancer Research Fund, and Ofelia Cancer Research Fund.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by our institutions Research Ethics Board and written informed consent was obtained from all patients.
References
- Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol 2005;75:5-14. [Crossref] [PubMed]
- Pulenzas N, Khan L, Tsao M, et al. Fatigue scores in patients with brain metastases receiving whole brain radiotherapy. Support Care Cancer 2014;22:1757-63. [Crossref] [PubMed]
- Portenoy RK, Thaler HT, Kornblith AB, et al. Symptom prevalence, characteristics and distress in a cancer population. Qual Life Res 1994;3:183-9. [Crossref] [PubMed]
- Wong J, Hird A, Kirou-Mauro A, et al. Quality of life in brain metastases radiation trials: a literature review. Curr Oncol 2008;15:25-45. [PubMed]
- Hall WA, Djalilian HR, Nussbaum ES, et al. Long-term survival with metastatic cancer to the brain. Med Oncol 2000;17:279-86. [Crossref] [PubMed]
- Mehta MP, Paleologos NA, Mikkelsen T, et al. The role of chemotherapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol 2010;96:71-83. [Crossref] [PubMed]
- Bezjak A, Adam J, Barton R, et al. Symptom response after palliative radiotherapy for patients with brain metastases. Eur J Cancer 2002;38:487-96. [Crossref] [PubMed]
- Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol 2012;2:210-25. [Crossref] [PubMed]
- Edwards A, Gerrard G. Clinical management. The management of cerebral metastases. Eur J Palliat Care 1998;5:7-11.
- Borgelt B, Gelber R, Kramer S, et al. The palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 1980;6:1-9. [Crossref] [PubMed]
- Palmer JL, Fisch MJ. Association between symptom distress and survival in outpatients seen in a palliative care cancer center. J Pain Symptom Manage 2005;29:565-71. [Crossref] [PubMed]
- Cheung WY, Barmala N, Zarinehbaf S, et al. The association of physical and psychological symptom burden with time to death among palliative cancer outpatients. J Pain Symptom Manage 2009;37:297-304. [Crossref] [PubMed]
- Zeng L, Zhang L, Culleton S, et al. Edmonton symptom assessment scale as a prognosticative indicator in patients with advanced cancer. J Palliat Med 2011;14:337-42. [Crossref] [PubMed]
- Li J, Bentzen SM, Li J, et al. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys 2008;71:64-70. [Crossref] [PubMed]
- Caballero JA, Sneed PK, Lamborn KR, et al. Prognostic factors for survival in patients treated with stereotactic radiosurgery for recurrent brain metastases after prior whole brain radiotherapy. Int J Radiat Oncol Biol Phys 2012;83:303-9. [Crossref] [PubMed]
- Lagerwaard FJ, Levendag PC, Nowak PJ, et al. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys 1999;43:795-803. [Crossref] [PubMed]
- Jones JA, Simone CB 2nd. Whole brain radiotherapy for patients with poor prognosis: possibilities for the impact of the QUARTZ trial. Ann Palliat Med 2015;4:58-60. [PubMed]