
Total parenteral nutrition for patients with gastrointestinal cancers: a clinical practice review
Introduction
Background
The use of artificial nutrition through enteral and parenteral routes remains a controversial topic in the management of cancer cachexia, mechanical obstruction, and/or malfunctioning absorption secondary to gastrointestinal malignancies. Anorexia and cachexia are striking manifestations of advancing cancer, impacting a patient’s functional status, mood, and appearance. These changes often invoke emotional responses from the patient, caregivers, and clinicians.
Rationale and knowledge gap
The use of parenteral nutrition to address cancer-related cachexia varies widely across healthcare systems, countries, cultures, and disease states. Although society guidelines exist about the proper implementation of nutritional support for cancer patients, they are often vague. The decision to initiate nutritional support must be made carefully, considering patient-specific factors such as functional status and life expectancy (1,2). This practice review is designed to highlight factors that should be considered prior to offering parenteral nutrition.
Objective
In this article, we discuss the use of total parenteral nutrition (TPN) in patients with gastrointestinal malignancies receiving palliative-intent systemic cancer treatment or comfort-focused care. This includes an exploration of the indications for TPN, of patient-specific factors that should be considered when contemplating its use, and of the impact it has on outcomes relevant to the practice of palliative care.
Case review and discussion
Case description
Ms. W is a 63-year-old female with esophageal adenocarcinoma. She was initially treated with definitive chemotherapy and radiation and had a complete response to treatment. On routine follow-up imaging at 18 months, she was found to have pulmonary lesions. She underwent a biopsy to confirm diagnosis. Fifteen days after the procedure, she presented for an acute visit to her oncologist with early satiety and abdominal pain. She had not been feeling well since the biopsy, and oral intake had progressively decreased due to nausea and discomfort. For 5 days prior to presentation, she could only tolerate consuming broth. It had been a week since her last bowel movement. Physical examination revealed abdominal distension and a computed tomography (CT) scan showed ascites, carcinomatosis, and small bowel obstruction with a transition point in the first part of the duodenum and a massively distended stomach. She was admitted to the hospital for further management, including nasogastric decompression of the stomach and surgical consultation.
The biopsy results confirmed disease recurrence with poorly differentiated adenocarcinoma consistent with esophageal primary. Her tumor was Her-2 positive (3+) by immunohistochemistry. Surgical intervention of the obstruction was considered, but due to her poor nutritional status, surgery was delayed for a week to maximize nutritional support and monitor for improvement. The patient was not a candidate for percutaneous endoscopic gastrostomy (PEG) feeding tube due to distal obstruction of the gastrointestinal tract, so TPN was initiated. She tolerated the intervention well and her nutritional status improved. She had no further vomiting. She then underwent exploratory laparotomy, which revealed a two centimeter serosal implant on the outer aspect of the duodenum causing obstruction. After surgery, she was continued on TPN for nutritional support during the healing process. Three weeks after surgery, the patient started palliative-intent chemotherapy. She was successfully weaned off TPN 5 weeks after surgery. She had an excellent radiographic response to treatment on a follow-up CT scan after three cycles of treatment and had no further obstructive symptoms.
Discussion
What is TPN?
The term “total parenteral nutrition” is used when all or almost all nutrients are delivered intravenously (3). TPN is considered when all other avenues for nutritional support, such as supplements, appetite stimulants, and enteral tube feedings have been exhausted and providing parenteral nutrition is within a patient’s goals. A nutrition support team determines the required nutritional components and adjusts the nutritional solution based on laboratory and physical exam findings. Teams usually consist of a physician, a pharmacist, and a nutritionist, although larger interdisciplinary teams exist (2). Physicians in this role are most commonly gastroenterologists, surgeons, or internists who have developed a strong interest in alternative ways of feeding. TPN is often initiated and administered in the hospital to allow for close monitoring. However, it can also be administered at home for patients who are equipped with the necessary education, team support and supplies that enable safe use outside of an institutionalized setting.
Composition of the nutritional solutions can vary based on the caloric and amino acid requirements of the patient. Patients with cancer may have higher metabolic needs given the activity of the cancer itself. On the other hand, functional decline in patients with cancer can lower caloric needs due to decreased overall energy expenditure. If energy needs are unknown, as is often the case, it is recommended to supplement nutrition based on the needs of healthy patients, ranging from 25–30 kcal/kg/day (1). Solutions are now made to be more lipid-dense, as opposed to glucose-rich, which decreases the risk of hyperglycemia classically associated with the use of TPN. Newer emulsions include olive and fish oils with anti-inflammatory properties (4). If oral intake has been poor for an extended period, parenteral nutrition should be gradually increased, while patients are closely monitored for refeeding syndrome, which can occur when malnourished patients have drastic and potentially fatal shifts in electrolytes when parenteral nutrition is initiated (1).
“Supplemental parenteral nutrition” (SPN) is the peripheral administration of nutritional elements to patients whose complete nutritional needs cannot be met by enteral nutrition alone. SPN may be utilized if there is concern that malnutrition is interfering with or delaying cancer treatment (2). Because the risks associated with SPN are similar to those with TPN, it is incumbent upon the medical team to continually reassess and justify its use.
Prior to the initiation of any parenteral nutrition, attempts should be made to seek dietary counseling, maximize vitamin supplementation, engage in physical exercise (as tolerated), and employ pharmacological agents such as steroids. A comprehensive nutritional assessment is a critical component of developing a stepwise approach to supplementing and if necessary, replacing oral nutrition. This includes understanding pre-morbid nutritional status and lifestyle changes since the diagnosis of cancer, exploring how socioeconomic factors may be affecting diet, and making recommendations that are practical and evidence-based. Most physicians lack the training needed to provide expert guidance around nutritional support, thus necessitating an interdisciplinary approach.
Patients receiving TPN are closely monitored for electrolyte imbalances, liver dysfunction, and hyperglycemia, as well as for signs of nutritional gains, which include increased energy, weight gain, and increased body mass. Patients and families may believe that patients are benefiting from nutrition even when objective data is lacking, but the effect of this belief on patients and families in terms of ameliorating quality of life and coping is unclear (5,6). As with mechanical ventilation or renal-replacement therapies, harms and benefits of TPN must be continuously reassessed by the medical team and patient in the context of disease-burden and prognosis.
Indications for the use of TPN in patients with gastrointestinal cancers
Identifying the patient-specific principal indication for the use of TPN before it is offered as part of a treatment plan is essential. Common indications for TPN initiation in adult patients with cancer include malignant bowel obstruction (MBO), severe mucositis due to cancer treatments, malabsorptive syndromes, intractable nausea and vomiting, and cancer cachexia (7,8). American and international guidelines for the use of TPN exist, yet decisions about offering nutritional support are often based on institutional culture and provider experience and comfort level. Differentiating TPN as a short-term intervention to bridge to therapy with specific, measurable goals from indefinite TPN with no clear endpoint is vital when approaching conversations about risks and benefits; this differentiation is important for both the physician and the patient and both parties need to be clear about the purpose for using TPN. Palliative care providers can support clear communication to help patients and caregivers grasp the nuances of TPN use. If there is uncertainty around whether parenteral nutrition will be temporary or permanent, it is critical to identify markers of success that are independent of time. Patients should be empowered to have an active voice in how and when TPN is tapered or discontinued.
The most common indication for TPN in patients with gastrointestinal malignancies is MBO (8,9). Bowel obstructions include malfunctioning bowel secondary to functional obstruction related to tumor infiltration of adjacent tissues or mechanical intestinal obstruction. Bowel obstructions can be categorized as operable or inoperable. TPN is primarily, although not exclusively, used when an obstruction is considered inoperable. MBOs affect an estimated 3% to 15% of patients with all types of cancer and occur in 10% to 40% of patients who have primary colorectal or gastric tumors (10-12).
Patients who develop a MBO can become highly symptomatic quickly and often experience pain, nausea, vomiting, and abdominal distension. A MBO is considered a poor prognostic indicator and without a rapid and definitive treatment, death usually follows within weeks. If a patient is unable to take anything by mouth, including water, death occurs within days to short weeks; if a patient is able to take water or clear liquids but not food, prognosis may stretch to weeks or short months. Patients with a MBO from a gastrointestinal malignancy have typically lived longer than patients with a MBO from a gynecologic malignancy due to the widespread peritoneal carcinomatosis more commonly associated with gynecologic cancer (9). While complete obstructions are more life-threatening than partial obstructions, patients who are not fully obstructed may have anorexia from fear of developing symptoms when they do eat that can make the partial obstruction as serious as a complete obstruction. Given the morbidity associated with MBO, aggressively managing symptoms and addressing the underlying cause of obstruction are essential. When the underlying cause of obstruction cannot be resolved, TPN is used in an attempt to prolong survival. In a 2015 systematic review of survival, quality of life, and cost-effectiveness of home TPN in patients with inoperable MBOs, Naghibi et al. found a mean survival rate of 116 days for these patients (9).
Systemic cancer treatments and radiation may cause anorexia, nausea, vomiting, and diarrhea. TPN has been used for patients undergoing systemic treatment in an effort to maximize nutrition with the rationale that malnourishment could decrease the efficacy of cancer therapies or preclude survival benefit. In these cases, TPN is viewed as a bridge therapy. It may be difficult to elucidate whether treatment of cancer leads to improvements in symptoms and quality of life or if better nutrition is directly causing these benefits (2). In the case presented at the start of the article, Ms. W had a clear indication for TPN as bridge to treatment. For this patient, TPN allowed her to prepare for and heal from a life-altering surgery, which afforded her the opportunity to continue cancer-directed therapy with an effective therapeutic drug. In the perioperative setting, TPN supports patients while they regain the ability to take nutrition by mouth. The hallmark of using TPN as an effective bridge is to allow patients to survive and heal sufficiently to undergo treatments that have high response rates after surgical intervention.
Enhanced recovery after surgery (ERAS) protocols exist for cancer patients undergoing surgical intervention and provide guidance around advancing diet when appropriate (13). Disease of adjacent tissues such as metastatic disease to the mesentery and malignant ascites can cause bowel dysfunction that limits oral intake. Mucositis, damage to gastrointestinal innervation, and sloughing of the intestinal lining can alter absorption. Liver and pancreatic dysfunction can affect the metabolic process leading to functional “intestinal failure”. When this occurs as a side effect of systemic treatments or as the result of surgical interventions, TPN may be considered. However, “intestinal failure” usually leads to a permanent dependence on parenteral nutrition and puts patients at increased risk of complications. These cases can become particularly challenging as patients become sicker or develop new medical problems. Many patients and clinicians struggle with the decision to discontinue parenteral nutrition. This necessitates an interdisciplinary approach with an emphasis on the psychosocial aspects of the palliative cessation of TPN.
TPN has also been offered to patients who have cancer cachexia that is refractory to medical management. Cancer cachexia is a “multifactorial syndrome defined by ongoing loss of skeletal muscle mass that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment” (14). Unbalanced metabolic states lead to energy and protein deficits that are incompatible with life (14). The use of TPN to address cancer cachexia varies widely in practice. Cancer cachexia is perhaps one of the most controversial indications for the use of TPN. In the case of Ms. W, TPN was used to enhance nutritional status to enable her to continue life-prolonging cancer-targeted therapies, but in cancer cachexia the main driver of mortality is the cachexia so there are different considerations (2). If the cachexia is caused by cancer and is a poor prognostic indicator, treating the cachexia with TPN may be considered to be prolonging the dying process.
Complications of TPN use
Complications from the use of parenteral nutrition are rare but can have a profound impact on morbidity and mortality. Some complications, such as infection and thrombosis are tangible, while others, such as decrease in quality of life, are harder to identify or quantify. The requirements of TPN therapy—frequent blood draws, ongoing interaction with the medical system, and the need to adhere to strict protocols around administration—can be burdensome and worsen a patient’s quality of life.
While improvements in hygiene and TPN catheter maintenance protocols have improved outcomes related to catheter-associated infections, the risk is not insignificant, especially given the immunocompromised state of cancer patients undergoing chemotherapy. Rates of infection have been reported to be 0.05 to 3.08 per 1,000 catheter days. Five studies calculated central venous catheter sepsis rates ranging from 0.4 to 2.89 per 1,000 days (9,15). A separate study found rates of catheter-related bloodstream infection to be 0.18 episodes/catheter years (8). Education about line hygiene and handling, as well as daily inspection of line sites, are tools that have led to the reduction of infection rates (16,17). International guidelines exist to guide site selection, sterile technique for insertion, and the development of line maintenance protocols.
Indwelling lines are also associated with thrombosis. In a systematic review by Naghibi et al., thrombotic complications were reported in two studies, one indicating a rate of 0.19 per 1,000 catheter-days and the other reporting a rate of 4.34 per 1,000 catheter-days (9). Blood clots not only are at risk of propagation and infection, but also preclude the administration of parenteral nutrition for extended periods of time.
Patients on home TPN are at high risk of rehospitalization. A study from 1997, though dated, found that of 164 patients with advanced cancer on home TPN, 95 patients underwent 155 readmissions to the hospital. Time in the hospital consisted of 15–23% of their survival time (18). Little data exists about the causes of rehospitalization in patients receiving TPN. Given that patients on TPN are seriously ill, it is reasonable to consider that there are multiple potential causes of hospitalization that are not direct complications of TPN; however, it is likely that some of these hospitalizations are related to the use of parenteral nutrition. There is little data on whether patient location influences the rate of complications associated with the use of parenteral nutrition. Similarly, it is unclear how the use of TPN impacts hospital length-of-stay.
Although the rate of metabolic complications from TPN is challenging to measure, changes in the composition of TPN solutions have had a favorable impact. With the addition of fatty acids to solutions, glucose load is reduced, which in turn decreases the number of metabolic complications (4,16). Most nutritional solutions now contain all amino acids in sufficient amounts. Studies in critically ill patients have demonstrated that high protein nutrition has been associated with decreased mortality (19). Soy bean oils, olive oil, and fish oil have been introduced to increase patients’ antioxidant levels and decrease omega-6 fatty acids, which may contribute to inflammation and immune system activation (16). Nutritional solutions are now tailored to meet patients’ needs, and nutritionists consider insulin-resistance and other comorbidities when guiding treatment. One systematic review found metabolic complications were reported in 3 studies with a range of 0.32 to 1.37 per 1,000 days (9). Newer formulations are “all-in-one” admixtures instead of separate components administered separately. This reduces likelihood of contamination, line infections, and cost (16).
Hepatobiliary dysfunction in patients receiving long-term TPN has been well-documented. Associated complications include hepatic steatosis, fibrosis, cholelithiasis, and acalculous cholecystitis (20). The reported incidence of liver-related complications in patients receiving TPN ranges widely and it is often unclear if alterations in liver function are clinically significant. The incidence of abnormal liver function tests following TPN initiation, ranges from 25–100% of patients across early studies (20). Many of these studies included heterogenous groups of patients with varying degrees of liver dysfunction and disease burden at baseline (20). The abnormal liver function tests in these patients are predominately correlated with steatosis of the liver. Of note, malnutrition itself can predispose patients to the development of liver steatosis. The steatosis associated with the use of peripheral nutrition is thought to be reversible and mild, especially as nutritional formulations have been made to be more protein-balanced and less glucose-rich. The consequences of biliary stasis, a byproduct of parenteral nutrition, include the development of cholelithiasis and cholecystitis (20). The duration of TPN is associated with the degree of biliary stasis that results, with patients receiving TPN over a longer period of time experiencing the development of more biliary sludge. For many patients, especially those with limited-life expectancy, the risk of likely minor hepatobiliary complications may be outweighed by the benefits of receiving nutrition.
The expense of TPN is a barrier to use. Line placement, supplies including nutritional solutions, funding for a nutritional support team, monitoring of patients on therapy, and the management of complications contribute to a high cost. The economic impact of supporting TPN across all settings is an important consideration. Given its high cost, there is a pressing need for more research to determine the utility of TPN for patients with gastrointestinal malignancies and at the end of life. In order to ensure the equitable allocation of resources, evidence-based guidelines must be developed to determine the proper use of TPN in patients receiving cancer treatments with palliative intent. Naghibi et al. estimated a cost of around £107,000/QALY (quality-adjusted life year) in 2013 for the administration of parenteral nutrition, noting that £20,000–£30,000/QALY is the threshold for coverage under the National Institute of Clinical Excellence Guidelines in England (9).
Recommendations for the use of TPN
A review of the current literature yields several recommendations to aid in decision-making around whether a patient with a gastrointestinal malignancy will likely benefit from the initiation of TPN.
Quality of life
Adding parenteral nutrition to the treatment plan for medically-complex patients introduces significant logistical burdens to patients and caregivers. TPN should not be initiated in cancer patients receiving palliative cancer-directed therapies if they have uncontrolled symptoms or an untenable quality of life by their definition. In this population, the goal of parenteral nutrition is to prolong life and it is imperative to fully assess whether extending life will lead to a meaningful and dignified existence for the patient. In one study, parenteral nutrition was found to transiently improve health-related quality of life after 2 months, but effects diminished by 6 months (4). Moreover, the clinical significance of this finding is questionable given the small gains in quality of life scores of <20% (4). Perhaps more useful to clinical decision-making is the finding that quality of life wanes in the final 2–3 months of life even with parenteral nutrition, highlighting the critical importance of prognostication in the decision-making process around TPN initiation (21). Palliative care providers are adept at discussing goals and values and synthesizing information to make sound evidence-based recommendations. This essential skill, coupled with knowledge around prognostication, makes palliative care providers an excellent resource for teams considering whether it is appropriate to offer TPN to a patient.
The role of prognostication
Patients can benefit from artificial nutrition if it is believed that their life expectancy is limited by malnutrition rather than other complications from cancer or treatment, such as pleural effusions or pathologic fractures. Generally, parenteral nutrition should only be initiated in patients who are expected to survive for at least 2 months and who will otherwise suffer from malnutrition (2). Amano et al. demonstrated that in a group of 1,453 cancer patients admitted to palliative care units, those receiving parenteral nutrition had a longer median survival time than those with poor oral intake who did not receive enteral or parenteral nutrition (22). In another prospective study of 414 palliative cancer patients receiving TPN at home and with a life-expectancy of >6 weeks at time of initiation, 50% of patients survived for 3 months while 22.9% of patients survived to 6 months (8). These patients had incurable solid-tumor cancer of variable types and were malnourished at time of enrollment (21).
Prognostic tools are used to predict survival time and to estimate the likelihood that patients will benefit from the initiation of parenteral nutrition. Bozzetti et al. found that the Glasgow Prognostic Score (GPS), the Karnofsky Performance Status (KPS), tumor spread, in combination with tumor type, could be used to predict survival in patients with incurable cancer on TPN at 3 and 6 months (Figure 1) (23). Both the KPS and GPS were independently and significantly associated with survival over these time periods. They found that 33% of patients with a KPS ≤50 and GPS =2 were alive at 3 months whereas 79% of patients with KPS ≥50 and GPS =0 survived after the same period of time (2). Patients with higher functional status scores were more likely to survive longer and to see survival benefit while receiving parenteral nutrition (8). Naghibi et al. found that patients with a KPS >50% had longer survival time than those <50% (median survival 183 vs. 91 days) (9). Patients who make gains in KPS after the initiation of parenteral nutrition, compared to patients who do not make gains or experience loss in functional status, tend to live longer as well (9).
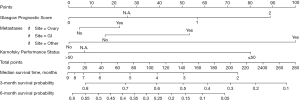
A systematic review of the use of TPN in patients with advanced cancer demonstrated that parenteral nutrition improves physical functioning but may not be superior to dietetic counseling for malnourished patients. The same review found that for patients receiving antineoplastic treatment, TPN may be useful in improving functional status but TPN is not associated with functional improvement in patients who are no longer candidates for cancer-directed therapies. For this latter subset of patients, TPN may improve nutritional status but not functional status (4). The degree of cachexia at time of parenteral nutrition initiation has a significant impact on prognosis. Cancer cachexia is defined as weight loss >5%, body mass index (BMI) of <20 coupled with a weight loss >2%, or sarcopenia coupled with a weight loss of >2%. It is also associated with reduced survival compared to patients with pre-cachexia. Refractory cachexia is defined as having a life expectancy ≤3 months with a cancer that is pro-catabolic and not responding to treatments (8). In patients receiving home parenteral or enteral artificial nutrition, survival rates are shorter for patients with refractory cachexia (11.9±13.8 weeks) than for those with cachexia (17.1±20.3 weeks) and pre-cachexia (28.7±35.9 weeks) (8). Although no firm cut-offs exist regarding initiation of TPN based on degree of cachexia, data suggests that patients with refractory cachexia are the least likely to gain survival and functional benefit from TPN.
Type of cancer likely influences survival after the initiation of parenteral nutrition. Patients with gastrointestinal malignancy have longer survival times than those with gynecologic malignancy (9). However, TPN is generally recommended for patients with a reasonably long life expectancy (≥2 months) regardless of their cancer etiology, who would suffer from nutritional deprivation due to starvation without intervention (2).
Education and support
Educating patients and family members about the goals of treatment and expected benefits is paramount to providing comprehensive, goal-concordant care. Patients and families often have pre-existing beliefs about parenteral nutrition and hydration. In one Japanese study, 80–90% of bereaved family members responding to a survey expressed a need for parenteral nutrition for cancer patients who had poor oral intake. Despite this, 70% of the respondents in this study said they had insufficient information about TPN and half did not feel they were given sufficient information about parenteral nutrition or hydration (5). Families understandably wish to nourish loved ones who appear to be dying, but the realities of providing this type of care are often overlooked.
Open and empathetic discussions with patients and families regarding the feelings of helplessness that often arise in caring for a seriously ill loved one can be a first step. These conversations can include candidly addressing the disconcerting physical manifestations of cancer, acknowledging the link between feeding and the expression of love, and a reframing of malnourishment as a symptom of an underlying, progressive disease instead of the cause of death. Cultural factors, religious beliefs, healthcare systems, socioeconomic considerations, and individual preferences interact to affect decision-making around TPN (2,9). Healthcare providers must consider how these factors influence if and when parenteral nutrition will be offered, and their recommendations must be evidence-based and goal-concordant. The support of an interdisciplinary team can help patients and their families grapple with the emotional and spiritual dimensions of such a consequential decision.
TPN is discontinued in patients for a variety of reasons: when there is evidence that cancer is progressing (often close to the time of death), when patients or families refuse to continue, and when complications arise (2). We recommend including anticipatory guidance about TPN discontinuation—e.g., discussing with patients and families at what point TPN is no longer a helpful therapy, and thus should be stopped—in the initial conversations about beginning parenteral nutrition. Sensitivity to the emotional significance of the discontinuation is an important part of supportive care for both patient and family.
Strengths and limitations
There are limitations to this practice review. First and foremost, the literature search, though comprehensive, was performed based on key words thought to produce the most relevant literature. This review was not done systematically, though attempts were made to compile the most up-to-date and relevant information on this topic. The use of TPN in patients with particular cancer types was not addressed here and was deemed beyond the scope of this paper. It is important to note that work has been done looking at the benefits and risks of TPN in patients with specific malignancies, such as gastric and esophageal. Finally, this serves as summary of important findings that have come from original research studies. We encourage readers to review original publications cited here when applying these principles to their work. This paper was written with the support of an interdisciplinary team including palliative care physicians and an oncologist. We attempted to provide a thoughtful review and encourage further studies on this complicated topic.
Conclusions
In the management of cachexia, mechanical obstruction, or malfunctioning absorption secondary to gastrointestinal malignancies, the use of TPN remains a controversial issue. Although guidelines for the proper use of nutritional support in oncologic care exist, the guidelines are often vague and decisions about initiating treatment are frequently based on institutional culture and provider comfort rather than a careful evaluation of patient-specific factors such as functional status and life-expectancy (1). It is important to identify the principal indications for TPN, which include: adult cancer patients with MBO, severe mucositis due to cancer treatments, malabsorptive syndromes, intractable nausea and vomiting, and with a life-expectancy of >2 months and who would benefit from further cancer-directed therapy. Differentiating TPN as a short-term intervention with specific measurable goals versus planning for indefinite TPN is an essential step in discussing risks and benefits with patients.
Patients who are no longer candidates for cancer-directed therapies are unlikely to benefit from the initiation of TPN. Improvements in functional status, quality of life, and life expectancy have been demonstrated in some studies; however, these gains are short-term and are limited to only the healthiest patients receiving parenteral nutrition. Offering intensive therapies instead of withholding them in the face of serious and progressive illness may seem more compassionate to the provider, this can instead lead to increased suffering and a loss of opportunity to prioritize what matters most at the end of life for patients and families. As with any major medical or surgical intervention, medical teams must take a comprehensive approach to weighing the risks and benefits of offering parenteral nutrition. This involves understanding the severity of disease and the goals of the patient, anticipating the disease trajectory, and considering prognosis. A cohesive interdisciplinary team is essential for cases involving complex medical decision-making, especially when patients are approaching death. Palliative care providers, with expertise in serious illness communication, prognostication, and cancer care, can play a critical role in determining who will likely benefit from TPN. Medical teams continue to support patients with cancer even when interventions are not offered or discontinued. Through close contact and consistent communication, providers can honor the wishes of their patients, even when that means pivoting away from intensive treatments such as TPN.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Palliative Medicine, for the series “Palliative Care in GI Malignancies”. The article has undergone external peer review.
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1380/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1380/coif). The series “Palliative Care in GI Malignancies” was commissioned by the editorial office without any funding or sponsorship. KA served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Muscaritoli M, Arends J, Bachmann P, et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin Nutr 2021;40:2898-913. [Crossref] [PubMed]
- Bozzetti F. Parenteral nutrition. Nutrition 2019;66:101-7. [Crossref] [PubMed]
- Scolapio JS, Picco MF, Tarrosa VB. Enteral versus parenteral nutrition: the patient’s preference. JPEN J Parenter Enteral Nutr 2002;26:248-50. [Crossref] [PubMed]
- Tobberup R, Thoresen L, Falkmer UG, et al. Effects of current parenteral nutrition treatment on health-related quality of life, physical function, nutritional status, survival and adverse events exclusively in patients with advanced cancer: A systematic literature review. Crit Rev Oncol Hematol 2019;139:96-107. [Crossref] [PubMed]
- Amano K, Maeda I, Morita T, et al. Beliefs and Perceptions About Parenteral Nutrition and Hydration by Family Members of Patients With Advanced Cancer Admitted to Palliative Care Units: A Nationwide Survey of Bereaved Family Members in Japan. J Pain Symptom Manage 2020;60:355-61. [Crossref] [PubMed]
- Abe A, Amano K, Morita T, et al. Beliefs and Perceptions About Parenteral Nutrition and Hydration by Advanced Cancer Patients. Palliat Med Rep 2022;3:132-9. [Crossref] [PubMed]
- Feng YL, Lee CS, Chiu CC, et al. Appropriateness of Parenteral Nutrition Usage in Cancer Patients. Nutr Cancer 2015;67:1014-7. [Crossref] [PubMed]
- Ruggeri E, Giannantonio M, Agostini F, et al. Home artificial nutrition in palliative care cancer patients: Impact on survival and performance status. Clin Nutr 2020;39:3346-53. [Crossref] [PubMed]
- Naghibi M, Smith TR, Elia M. A systematic review with meta-analysis of survival, quality of life and cost-effectiveness of home parenteral nutrition in patients with inoperable malignant bowel obstruction. Clin Nutr 2015;34:825-37. [Crossref] [PubMed]
- Gwilliam B, Bailey C. The nature of terminal malignant bowel obstruction and its impact on patients with advanced cancer. Int J Palliat Nurs 2001;7:474-81. [Crossref] [PubMed]
- Dolan EA. Malignant bowel obstruction: a review of current treatment strategies. Am J Hosp Palliat Care 2011;28:576-82. [Crossref] [PubMed]
- Ripamonti C, De Conno F, Ventafridda V, et al. Management of bowel obstruction in advanced and terminal cancer patients. Ann Oncol 1993;4:15-21. [Crossref] [PubMed]
- Shida D, Tagawa K, Inada K, et al. Modified enhanced recovery after surgery (ERAS) protocols for patients with obstructive colorectal cancer. BMC Surg 2017;17:18. [Crossref] [PubMed]
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489-95. [Crossref] [PubMed]
- Ozcelik H, Gozum S, Ozer Z. Is home parenteral nutrition safe for cancer patients? Positive effects and potential catheter-related complications: A systematic review. Eur J Cancer Care (Engl) 2019;28:e13003. [Crossref] [PubMed]
- Hellerman Itzhaki M, Singer P. Advances in Medical Nutrition Therapy: Parenteral Nutrition. Nutrients 2020;12:717. [Crossref] [PubMed]
- Inchingolo R, Pasciuto G, Magnini D, et al. Educational interventions alone and combined with port protector reduce the rate of central venous catheter infection and colonization in respiratory semi-intensive care unit. BMC Infect Dis 2019;19:215. [Crossref] [PubMed]
- Pironi L, Ruggeri E, Tanneberger S, et al. Home artificial nutrition in advanced cancer. J R Soc Med 1997;90:597-603. [Crossref] [PubMed]
- Zusman O, Theilla M, Cohen J, et al. Resting energy expenditure, calorie and protein consumption in critically ill patients: a retrospective cohort study. Crit Care 2016;20:367. [Crossref] [PubMed]
- Quigley EM, Marsh MN, Shaffer JL, et al. Hepatobiliary complications of total parenteral nutrition. Gastroenterology 1993;104:286-301. [Crossref] [PubMed]
- Bozzetti F, Santarpia L, Pironi L, et al. The prognosis of incurable cachectic cancer patients on home parenteral nutrition: a multi-centre observational study with prospective follow-up of 414 patients. Ann Oncol 2014;25:487-93. [Crossref] [PubMed]
- Amano K, Maeda I, Ishiki H, et al. Effects of enteral nutrition and parenteral nutrition on survival in patients with advanced cancer cachexia: Analysis of a multicenter prospective cohort study. Clin Nutr 2021;40:1168-75. [Crossref] [PubMed]
- Bozzetti F, Cotogni P, Lo Vullo S, et al. Development and validation of a nomogram to predict survival in incurable cachectic cancer patients on home parenteral nutrition. Ann Oncol 2015;26:2335-40. [Crossref] [PubMed]


