
A review of pharyngocutaneous fistula management in head and neck malignancies
Introduction
Immediate flap reconstruction following tumor resection and concurrent chemoradiotherapy (CCRT) has become the standard treatment protocol for locoregionally advanced head and neck squamous cell carcinoma (HNSCC) (1,2). Despite advancement in microsurgical techniques, the complication rate of developing pharyngocutaneous fistula (PCF) still hovers as high as 16.5% (3) to 21% (4). Numerous studies have identified previous radiotherapy, advanced tumor stages, and positive tumor margins among the risk factors of postoperative fistulae (5-8), yet little is known to avoid such complication effectively.
Postoperative PCF is troublesome as the daunting infection represents high burden of morbidity. Patients suffering from long-term feeding difficulty are prone to malnutrition which further hinders the wound healing process. Persistent infection surrounding the fistula frequently leads to delays in adjuvant treatment that compromises overall oncologic outcomes (9). If left unattended and mistreated, chronic fistulae could eventually erode the overlying soft tissue flap causing subsequent flap necrosis, deep neck infections, or even life-threatening carotid blow-outs (10).
Current practices vary drastically and lack a comprehensive approach in terms of intervention timing, dressing care methods, and surgical procedures to manage post-reconstructive head and neck PCF (11). Extensive surgical interventions in the acute stage could possibly damage the fragile vessel anastomosis site in the transferred free flaps and resulting in total flap loss. However, persistent saliva leakage would lead to deep neck infection and huge dead spaces, rendering primary repair of the fistula almost infeasible. Surgeons must be prudent in selecting the suitable intervention to avoid causing further damage in the extremely fragile tissues after CCRT and infections. In this study, the authors reviewed our own clinical experience and summarized a systematic approach for postoperative PCF accordingly.
Materials and methods
A retrospective review of all hypopharyngeal cancer patients who developed PCF during 2017 to 2021 at E-Da Hospital was performed. Information collected included demographic characteristics, onset of fistula, adopted intervention approaches (conservative or surgical), and resulting outcomes. The acute, subacute, and chronic stages of PCF was defined as persistence for within 2 weeks, 2 weeks to 3 months, and over 3 months respectively.
Results
A total of 17 patients developed PCF in all 321 pharyngeal cancer admissions during this period (Table 1). Fourteen of them received microvascular free flap reconstructions after tumor ablations, two received regional flaps, and one with primary closure. The mean onset time of fistula formation was 2.8 months. Three patients received interventions at acute stage (less than 2 weeks), with two direct repairs and one required regional flap coverage then negative pressure wound therapy (NPWT) for fistula closures. Nine patients received interventions at subacute stages (between 2 weeks to 3 months), with 4 of them resolved after debridement and direct repair, yet another 4 underwent regional flap reconstruction and 1 free flap reconstruction. Five patients with chronic fistula (longer than 3 months) received secondary reconstructions utilizing a double-layered repair of local turn-over flaps for the internal mucosal opening and another flap harvest (four regional flaps and one free flap) to cover the outer skin defect. All patients after the palliative surgery achieved complete remission of fistula at follow-up.
Table 1
| Case | Age/gender | Cancer site | Comorbidities | Initial reconstruction | Onset (after reconstruction) | Fistula stage at intervention | Secondary intervention |
|---|---|---|---|---|---|---|---|
| 1 | 66/M | Hypopharyngeal | HTN | Free ALT | <2 wks | Acute | Debridement, direct repair |
| 2 | 48/M | Hypopharyngeal | DM | Free ALT | <2 wks | Acute | Debridement, direct repair |
| 3 | 61/M | Hypopharyngeal | Nil | Free ALT | 2 wks | Acute | Direct repair, then IMA flap coverage but partial flap failed and healed under NPWT |
| 4 | 55/M | Hypopharyngeal | Nil | Free ALT | 4 wks | Subacute | Debridement, direct repair |
| 5 | 63/M | Hypopharyngeal | Nil | Free ALT | 4 wks | Subacute | Debridement, direct repair |
| 6 | 52/M | Hypopharyngeal | Nil | Free ALT | 4 wks | Subacute | Debridement, direct repair |
| 7 | 46/M | Hypopharyngeal | Nil | Free ALT | 4 wks | Subacute | Debridement, DP flap coverage |
| 8 | 52/M | Hypopharyngeal | Liver cirrhosis | Primary closure | 4 wks | Subacute | Free ALT reconstruction |
| 9 | 57/M | Hypopharyngeal | Nil | Free ALT | 4 wks | Subacute | Debridement, eventually DP flap coverage |
| 10 | 57/M | Oropharyngeal | CVA | PMMC flap | 2 ms | Subacute | Debridement, then DP flap coverage |
| 11 | 59/M | Hypopharyngeal | Nil | Free ALT | 2 ms | Subacute | Debridement, direct repair |
| 12 | 42/M | Hypopharyngeal | Nil | Free ALT | 2 ms | Subacute | Debridement, NPWT |
| 13 | 54/M | Hypopharyngeal | HTN | PMMC + SCM muscle flap | 5 ms | Chronic | Free ALT |
| 14 | 55/M | Hypopharyngeal | DM, HTN | Free ALT | 7 ms | Chronic | IMA flap coverage |
| 15 | 49/M | Hypopharyngeal | Nil | Free ALT | 8 ms | Chronic | IMA flap coverage |
| 16 | 64/M | Hypopharyngeal | HTN | Free ALT | 9 ms | Chronic | IMA flap coverage |
| 17 | 54/M | Hypopharyngeal | CAD, DM | Free ALT | 12 ms | Chronic | IMA flap coverage |
M, male; HTN, hypertension; ALT, anterolateral thigh flap; wks, weeks; DM, diabetes mellitus; IMA, internal mammary artery; NPWT, negative pressure wound therapy; DP, deltopectoral; CVA, cerebrovascular accident; PMMC, pectoralis major myocutaneous; ms, months; SCM, sternocleidomastoid; CAD, coronary artery disease.
Case presentations
Direct repair in acute stage (case 1)
The 66-year-old patient reconstructed with a tubular anterolateral thigh (ALT) flap suffered from acute onset of fistula due to dehiscence at the flap edge sutured to the remaining hypopharynx. Direct repair was performed at the fifth day postoperatively. No leakage was noted after the surgical intervention and retaining enteral feeding bypass for 2 weeks.
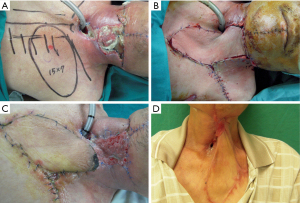
Regional flap reconstruction and NPWT-assisted closure in acute stage (case 3)
The 61-year-old pharyngeal cancer patient reconstructed with a free ALT tubular flap after cancer excision. Patient suffered from PCF and neck skin necrosis due to severe infection. A regional internal mammary artery perforator flap was designed to cover the neck wound at 10 days after the first surgery. However, poor healing and suspected fistula recurrence was noted at the submental neck area. After debridement, we closed the inner side with watertight sutures and applied NPWT for 2 weeks. Good granulation tissues were then found after the treatment and the wound eventually achieved complete resolution (Figure 1).
NPWT-assisted closure in subacute stage (case 12)
The 42-year-old hypopharyngeal cancer patient received initial free ALT flap reconstruction after tumor ablation yet PCF was noted 2 months postoperatively. After serial debridement, the mucosal side of the fistula was sutured to form a watertight seal. NPWT was applied at 4 weeks postoperatively after the transferred free flap stabilizes and was kept for a total of 2 weeks. Enteral bypass with nasogastric tube was also kept during the period. The fistula wound was then completely healed after the treatment.
Double-layer flap closure of PCF in chronic stage (case 15)
The 49-year-old man receive initially free ALT flap reconstruction for the circumferential defect after pharyngeal cancer ablations and received further CCRT. However, PCF was noted at 8 months postoperatively. Secondary reconstruction was performed at 6 months after CCRT, utilizing a double-layered coverage with local turn-over flaps to repair of the inner opening of the fistula and a regional internal mammary artery perforator flap rotated for second layer coverage. Patient achieved complete remission after the procedure (Figure 2).

Discussion
PCF remains one of the most challenging and serious post-reconstructive complications in head and neck malignancies. In our study, 5.3% (17/321) of hypopharyngeal cancers developed postoperative PCF. Preoperative radiation therapy was found with an 10% absolute risk increase to developing PCF in a systemic review with meta-analysis (8); yet adjuvant radiotherapy is mostly inevitable for disease control and no practical measures could be found to alleviate the effect of such major risk factor. Nevertheless, premeasurements in morbidity control are worth considering to prevent fistula formation from early on. Nutrition status is one of the main issues, as these patients tend to suffer from long-term cachexia owing to feeding difficulty. Preoperative survey of body mass index, hemoglobin, and albumin test should be performed routinely. We suggest providing nasogastric tube feeding or parenteral feeding during admission prior to the definite surgery to improve the overall nutrition status. This facilitates postoperative wound healing and endurance against CCRT side effects. Surgical principles and techniques also affect the complication rates as perioperative measures could prevent serious salivary leakage and subsequent fistula formation. In head and neck tumor resections, the resultant defects often cause digestive tract exposures and contamination of the surrounding tissues. Thus, watertight sutures should always be applied on the flap edges to conceal digestive juice properly. Despite recent development of collagen patches or tissue glues (12-14), the outcomes of such barrier materials remain controversial and the authors do not recommend surgeons to completely replace the basic surgical techniques with these biochemical assistances. Moreover, a double-layered flap reconstruction is recommended for through-and-through oral defects (15) and circumferential hypopharyngeal defects (16) as it provides robust barriers against salivary submergence and reduces subsequent leakage rates. Standardized postoperative care protocol is also crucial in avoiding complications. Nasogastric tube decompressions should be kept for at least 2 weeks postoperatively to alleviate the pressure within the oropharyngeal tract and to avoid flap dehiscence predisposing fistula formation (17).
If unfortunately, fistula does occur, the approaching management should be contemplated according to its timing of post-reconstructive stages and wound healing phases (Figure 3). Typically, acute wounds are defined as either within 72 hours or 2–3 weeks before entering the remodeling stage (18). However, for patients who undergone free flap reconstructions, we define the acute phase as 2 weeks postoperatively as the microcirculation in the transferred flap requires this minimal period to fully stabilize (19). During this stage, surgeons should be careful in performing surgical debridement and repair on such patients as the fragile microsurgical anastomosis site could be severed and resulted in flap necrosis. Conventional dressing care is recommended since any NPWT could possibly compromise pedicle patency and risk flap failures. Enteral bypass from either nasogastric or gastrostomy tube feedings or parenteral nutrition are advised in such circumstances to alleviate the intra-luminal pressures. The fistula is usually minor at this point and could sometimes heal spontaneously after proper compressive dressings and oral feeding avoidance (11,20-22).
The fistula then enters the subacute phase between 2 weeks to 3 months postoperatively, which frequently coincides with the postoperative CCRT in head and neck cancers. Most patients receiving a standard radiotherapy went through a period of receiving two Gray (Gy) fractions per day, 5 days per week for a total dose of 60 to 70 Gy (23). Tissues surrounding the fistula are often extremely fragile and inflamed while persistent leakage of saliva can lead to deep neck infections with huge dead spaces, both hindering direct fistula repair and rendering definite reconstruction infeasible and prone to recurrence. NPWT could eliminate dead spaces and salivary leaks around the fistula (24). It is known, however, that NPWT should be avoided in untreated sepsis and wound infections of evident necrotic tissues, osteomyelitis, or purulent discharge (25,26); thereby, the authors suggest performing thorough debridement to remove all infected tissues before application. In order to maintain adequate negative pressure drainage instead of drawing excessive salivary secretion from the digestive tract itself, a vacuum tight seal must be achieved before NPWT application so that the negative pressure could seal those dead spaces in the neck to facilitate wound healing. The authors prefer performing watertight suturing on the mucosal side of the fistula (24) as it provides a much durable barrier and could be managed right after the debridement in a single-staged operation. Other measures as applying hydrogum dental paste (25) or inserting Penrose drain into the fistula (27) have also been reported to achieve similar seal. NPWT has demonstrated comparable fistula closure rates to patients treated with conventional wound care, yet achieved so in a significantly shorter amount of time (11). Even if spontaneous closure is not achieved after NPWT, such treatment could optimize wound bed condition for further reconstructions (28) and allow time for multi-disciplinary teams to focus on morbidity control and nutrition support (29).
The fistula eventually enters the chronic wound stage if failed to heal after 3 months postoperatively (30). At this time, most patient have completed their adjuvant radiotherapy and the wound inflammation begins to subside. In our experience, we preferred to perform definite surgical reconstructions to cover the fistula 6 months after the radiotherapy. The choice of soft tissue flaps differed among studies found in the literature. However, we do not recommend using local flaps for coverage as the surrounding tissues within the irradiation field were often fragile and inflamed as well. Regional pedicled flaps such as pectoralis major myocutaneous flaps, deltopectoral flaps or sternocleidomastoid flaps away from the radiation field are all sensible choices; or surgeons should opt for distant free flap reconstructions instead. Regardless of the flap choice, it is best to achieve a double-layered closure utilizing the viable tissues surrounding the fistula for turn-over closure and an additional flap for second layer coverage to avoid placing the breakpoints of tensions on the same plane (29). The authors were able to achieve low recurrence rate and aesthetically pleasant results with this reconstruction method.
In palliative care for head and neck malignancies, fistula formation can be a serious psychosocial issue as the purulent discharge and daunting erosions sever patient’s appearance and dignity. The connection between digestive tract and cutaneous opening may also result in feeding difficulty, exacerbating cachexia and depriving the basic satisfaction from oral intake. Unfortunately, laryngopharyngeal cancer patients are usually too fragile to undergo series of reconstructions or revision procedures; thereby preventing fistula from deteriorating to severe complications is pivotal in post-reconstructive care. Prevention is still the most effective in terms of complications and surgeons should always optimize morbidity control and nutrition status before the definite surgical resection and reconstruction. Rigorous practice of surgical technique and standardized postoperative care protocols also helps in minimizing the change of salivary leakage. NPWT has been proven to be a promising and beneficial tool in facilitating fistula closures, yet with very low risk of subsequent complications (31). In the authors perspective, NPWT is only beneficial when done properly with premeasures of debridement and vacuum tight seal techniques so that no further damage or uncontrollable leakage would result from the persistent low-pressure suction. Nevertheless, if chronic fistula ensues, surgeons may consider intervene early with regional flap coverage before the infection worsens to mutilating organ exposures or carotid blow-outs. Identifying the exact stage and timing of the fistula formation and escalate the option ladder accordingly could greatly improve our clinical practice in managing postoperative PCF in palliative surgery.
Conclusions
PCF should be managed with a series of different conservative dressing care and surgical interventions according to their stages of acute, subacute, or chronic phases in palliative care.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Marios Papadakis) for the series “Palliative Reconstructive Surgery” published in Annals of Palliative Medicine. The article has undergone external peer review.
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1475/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1475/coif). The series “Palliative Reconstructive Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Consent was provided for use of all images and case-related information.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Anderson G, Ebadi M, Vo K, et al. An Updated Review on Head and Neck Cancer Treatment with Radiation Therapy. Cancers (Basel) 2021;13:4912. [Crossref] [PubMed]
- Cheraghlou S, Schettino A, Zogg CK, et al. Changing prognosis of oral cancer: An analysis of survival and treatment between 1973 and 2014. Laryngoscope 2018;128:2762-9. [Crossref] [PubMed]
- Michael RC, Das S, Mani S, et al. Pharyngocutaneous Fistula Following Primary and Salvage Laryngectomy: Aetiology and Predictive Factors. Indian J Otolaryngol Head Neck Surg 2022;74:2139-48. [Crossref] [PubMed]
- Wang M, Xun Y, Wang K, et al. Risk factors of pharyngocutaneous fistula after total laryngectomy: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol 2020;277:585-99. [Crossref] [PubMed]
- Paydarfar JA, Birkmeyer NJ. Complications in head and neck surgery: a meta-analysis of postlaryngectomy pharyngocutaneous fistula. Arch Otolaryngol Head Neck Surg 2006;132:67-72. [Crossref] [PubMed]
- Lau V, Chen LM, Farwell DG, et al. Postoperative radiation therapy for head and neck cancer in the setting of orocutaneous and pharyngocutaneous fistula. Am J Clin Oncol 2011;34:276-80. [Crossref] [PubMed]
- Liang JW, Li ZD, Li SC, et al. Pharyngocutaneous fistula after total laryngectomy: A systematic review and meta-analysis of risk factors. Auris Nasus Larynx 2015;42:353-9. [Crossref] [PubMed]
- Dedivitis RA, Aires FT, Cernea CR, et al. Pharyngocutaneous fistula after total laryngectomy: systematic review of risk factors. Head Neck 2015;37:1691-7. [Crossref] [PubMed]
- Murphy CT, Galloway TJ, Handorf EA, et al. Survival Impact of Increasing Time to Treatment Initiation for Patients With Head and Neck Cancer in the United States. J Clin Oncol 2016;34:169-78. [Crossref] [PubMed]
- Chiesa Estomba CM, Betances Reinoso FA, Osorio Velasquez A, et al. Carotid blowout syndrome in patients treated by larynx cancer. Braz J Otorhinolaryngol 2017;83:653-8. [Crossref] [PubMed]
- Khoo MJW, Ooi ASH. Management of postreconstructive head and neck salivary fistulae: A review of current practices. J Plast Reconstr Aesthet Surg 2021;74:2120-32. [Crossref] [PubMed]
- Lee DW, Chung S, Lee WJ, et al. Use of a collagen patch for management of pharyngocutaneous fistula after hypopharyngeal reconstruction. J Craniofac Surg 2010;21:1674-6. [Crossref] [PubMed]
- Choi HJ. Repair of the complicated orocutaneous fistula using atelocollagen sponge. J Craniofac Surg 2015;26:978-9. [Crossref] [PubMed]
- Brennan PA, Kiwanuka T, Aldridge T, et al. The use of Tisseal™ fibrin glue in the management of chronic oro-cutaneous fistula in the radiotherapy treated neck - a technical note. Br J Oral Maxillofac Surg 2016;54:828-9. [Crossref] [PubMed]
- Boyd JB, Morris S, Rosen IB, et al. The through-and-through oromandibular defect: rationale for aggressive reconstruction. Plast Reconstr Surg 1994;93:44-53. [Crossref] [PubMed]
- Spyropoulou GC, Lin PY, Chien CY, et al. Reconstruction of the hypopharynx with the anterolateral thigh flap: defect classification, method, tips, and outcomes. Plast Reconstr Surg 2011;127:161-72. [Crossref] [PubMed]
- Dong YB, Yuan LN, Luo JK, et al. Delayed oral feeding reduces pharyngocutaneous fistula formation after open surgical treatment of primary hypopharyngeal cancer: A case-control study. Ear Nose Throat J 2022; Epub ahead of print. [Crossref] [PubMed]
- Gonzalez AC, Costa TF, Andrade ZA, et al. Wound healing - A literature review. An Bras Dermatol 2016;91:614-20. [Crossref] [PubMed]
- Hamilton K, Wolfswinkel EM, Weathers WM, et al. The Delay Phenomenon: A Compilation of Knowledge across Specialties. Craniomaxillofac Trauma Reconstr 2014;7:112-8. [Crossref] [PubMed]
- Mäkitie AA, Niemensivu R, Hero M, et al. Pharyngocutaneous fistula following total laryngectomy: a single institution's 10-year experience. Eur Arch Otorhinolaryngol 2006;263:1127-30. [Crossref] [PubMed]
- Virtaniemi JA, Kumpulainen EJ, Hirvikoski PP, et al. The incidence and etiology of postlaryngectomy pharyngocutaneous fistulae. Head Neck 2001;23:29-33. [Crossref]
- McLean JN, Nicholas C, Duggal P, et al. Surgical management of pharyngocutaneous fistula after total laryngectomy. Ann Plast Surg 2012;68:442-5. Erratum in: Ann Plast Surg 2016;76:474. [Crossref] [PubMed]
- Pignon JP, Bourhis J, Domenge C, et al. Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet 2000;355:949-55. [Crossref] [PubMed]
- Yang YH, Jeng SF, Hsieh CH, et al. Vacuum-assisted closure for complicated wounds in head and neck region after reconstruction. J Plast Reconstr Aesthet Surg 2013;66:e209-16. [Crossref] [PubMed]
- Tian B, Khoo D, Tay AC, et al. Management of orocutaneous fistulas using a vacuum-assisted closure system. Head Neck 2014;36:873-81. [Crossref] [PubMed]
- Al Deek NF, Wei FC, Tsao CK. Fistulae After Successful Free Tissue Transfer to Head and Neck: Its Prevention and Treatment. Clin Plast Surg 2016;43:739-45. [Crossref] [PubMed]
- Umezawa H, Matsutani T, Yokoshima K, et al. A Novel Tube-Drainage Technique of Negative Pressure Wound Therapy for Fistulae after Reconstructive Surgery. Plast Reconstr Surg Glob Open 2018;6:e1885. [Crossref] [PubMed]
- Dhir K, Reino AJ, Lipana J. Vacuum-assisted closure therapy in the management of head and neck wounds. Laryngoscope 2009;119:54-61. [Crossref] [PubMed]
- Sadigh PL, Wu CJ, Feng WJ, et al. New double-layer design for 1-stage repair of orocutaneous and pharyngocutaneous fistulae in patients with postoperative irradiated head and neck cancer. Head Neck 2016;38:E353-9. [Crossref] [PubMed]
- Kyaw BM, Järbrink K, Martinengo L, et al. Need for Improved Definition of "Chronic Wounds" in Clinical Studies. Acta Derm Venereol 2018;98:157-8. [Crossref] [PubMed]
- Lin FY, Huang PY, Cheng HT. Systematic review of negative pressure wound therapy for head and neck wounds with fistulas: Outcomes and complications. Int Wound J 2020;17:251-8. [Crossref] [PubMed]



