
Management of autoimmune and viral hepatitis in immunotherapy: a narrative review※
Introduction
Background
The understanding of immune surveillance, by which innate immune cells eliminate cancer cells, has provided novel therapeutic options for patients with otherwise advanced and devastating cancers. Cancer immunoediting highlights the juxtaposed role of the immune system protecting against tumor growth while also shaping tumor immunogenicity (1). Tumor immunotherapy, modulates the native immune system to attack multiple targets in cancer cells (2). Emerging immunotherapies show promising efficacy in treating not only malignancies but also autoimmune, infectious, and allogenic transplant-related diseases (3).
Alteration of the immune microenvironment can unfortunately result in tissue toxicity, presenting as both acute and chronic immune-related adverse events (irAEs) thereby limiting its clinical use (4).
Rationale and knowledge gap
Liver-related injuries, including immune checkpoint inhibitor (ICI)-mediated hepatitis, are estimated to affect up to 22% of patients receiving immunotherapy (5). Also of concern, is the risk of exacerbating liver injury in patients with autoimmune liver disease or patients infected with chronic hepatitis B virus (HBV) or hepatitis C virus (HCV), as immunotherapy can damage liver function due to the immune response against viral antigens (6,7). With the increasing use of ICI, a more thorough screening and manement of liver disease is imperative to ensure successful outcomes. However, current data and guidelines remain limited.
Objective
In this review article, we highlight the current understanding and management of autoimmune and viral hepatitis in cancer immunotherapy with a strong focus on ICIs. We present this article in accordance with the Narrative Review reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-23-250/rc).
Methods
The authors conducted an independent literature search and review utilizing several databases and search terms (Table 1).
Table 1
| Items | Specifications |
|---|---|
| Date of search | October 5, 2022–February 19, 2023 |
| Databases and other sources searched | PubMed, Scopus, Google Scholar, American Association for the Study of Liver Diseases guidelines, SEER*Stat databases |
| Search terms used | “Immune checkpoint inhibitor”, “autoimmune hepatitis”, “viral hepatitis”, “HBV pathogenesis”, “HCV pathogenesis”, “HBV reactivation”, “HCV reactivation”, “cancer immunotherapy”, “immune related adverse events”, “immune related hepatitis” |
| Timeframe | Studies published prior to 2023 |
| Inclusion criteria | Restricted to English language data, including but not limited to randomized control trials, meta-analysis, systematic reviews, clinical practice guidelines, and case series |
| Selection process | Authors independently performed literature review and selection |
HBV, hepatitis B virus, HCV, hepatitis C virus.
Immunotherapy and autoimmunity
ICIs
Tumor immunotherapy encompasses an expansive group of treatments categorized based on their immune system targets which include immune checkpoints, tumor-infiltrating lymphocyte (TIL) transfer, engineered T cell receptors, chimeric antigen receptor (CAR) T cells, regulatory T cells (Treg), and natural killer (NK) cells (3). Among these immunotherapies, ICI therapy has become an immutable mainstay in the treatment of cancer. Immune checkpoints are molecules that regulate immune responses and are often utilized by tumor cells to evade immunosurveillance (8). Blockade of immune checkpoints augments anti-tumor activity by enhancement of native immune response (9). ICIs including cytotoxic T lymphocyte antigen-4 (CTLA-4), programmed cell death protein 1 (PD-1) and its ligand programmed death-ligand 1 (PD-L1), and lymphocyte-activation gene 3 (LAG-3) activate the immune system, disinhibit T-cell antitumor function, and eliminate tumor cells (10,11) (Figure 1). CTLA-4 inhibits T-cell activation by the downregulation of co-stimulatory ligands CD80 and CD86 (12). Anti-CTLA-4 agents promote T-cell activation and have been shown to induce immune response both in vivo and in vitro to cause tumor regression (13,14). The co-inhibitor receptor PD-1 is activated by PD-L1 and PD-L2, resulting in suppression of T-cell receptor (TCR)-mediated lymphocyte proliferation and cytokine release (15). Increased PD-L1 and PD-L2 gene expression is seen in malignant tissue (9) and is associated with poor disease prognosis in cancer types including renal cell carcinoma (16,17), esophageal cancer (18), urothelial cancer (19) and pancreatic cancer (20). Blockade of this pathway is shown to potentiate the cytotoxic ability of T cells against malignant cells (21). Combination monoclonal antibodies of co-expressed molecules PD-1/LAG-3 are approved for use in advanced melanoma, and have been promising in the management of lung, colorectal, and liver cancer (22). Blockade of this pathway potentiates the cytotoxic ability of T cells against malignant cells (21).

The first ICI agent, Ipilimumab (Bristol-Myers Squibb) was approved in 2011 for the treatment of metastatic melanoma. In an open-label three phase trial of 676 patients with unresectable Stage III or IV metastatic melanoma, Ipilimumab provided greater median overall survival compared to active control [10.0 vs. 6.4 months; hazard ratio (HR) 0.68; P<0.001] (23). Severe irAEs occurred in 10–15% of patients. Pembrolizumab (Merck Sharp & Dohme Corp.) was compared against ipilumumab for the treatment of melanoma and demonstrated improvement in survival hazard ratio of 0.63 (95% CI: 0.47–0.83, P<0.001) thus in 2014 was the first anti-PD-1 granted accelerated approval for treatment of unresectable metastatic melanoma (24). Subsequently, several ICIs have been approved, several of which are approved as first line therapy in solid organ cancers (Figure 2).
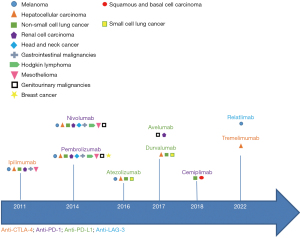
Limitations to ICI therapy include inefficacy due to resistance and intolerance due to adverse effects. Primary resistance can occur in the setting of ICI gene expression on tumor cells whereas secondary (acquired) resistance occurs by loss of function mutations in interferon (IFN) response or inadvertent upregulation of alternative immune checkpoints (4,9). Adverse events may range from mild tissue impairment to fatal toxicities, and have been shown to affect a wide range of organ systems including the liver, colon, lungs, pituitary, thyroid, skin, and less commonly, the heart and nervous system (25).
Immune-related adverse events
ICI mediated adverse events and toxicity have been extensively reported. Toxicities from ICIs can be divided into irAEs or adverse events of special interest (AEoSI), and infrequently, infusion-related reactions (26). ICIs activate T cells, thus, irAEs are thought of as autoimmune side effects of immunotherapy (27). IrAEs typically occur within the first three months of ICI initiation, but have also been documented to occur up to a year after initiation (13,26,28). Toxicity is graded on the Common Terminology Criteria for Adverse Events (CTCAE) scale with grade 5 representing death (Table 2) (29). The use of anti-CTLA-4 agents is associated with overall and high-grade irAEs, 74% (95% CI: 65–79%), and 24% (95% CI: 18–30%), respectively (30). The incidence of overall and high-grade irAEs with use of anti-PD-1/PD-L1 is 74% (95% CI: 69–79%) and 14% (95% CI: 12–14%), respectively (31). Combination therapy with both agents is associated with the highest incidence of overall and high-grade irAEs, 88% (95% CI: 84–92%) and 41% (95% CI: 35–47%), respectively (31). The presence of irAEs does not adversely affect overall survival.
Table 2
| Grades | Symptoms | Intervention |
|---|---|---|
| Grade 1 | Mild, asymptomatic or mild symptoms | Clinical or diagnostic observations only; intervention not indicated |
| Grade 2 | Moderate; liming age-appropriate instrumental activities of daily living | Minimal, local or noninvasive intervention |
| Grade 3 | Severe or medically significant but not immediately life threatening; disabling; liming | Hospitalization or prolongation of hospitalization indicated |
| Grade 4 | Life threatening consequences | Urgent intervention indicated |
| Grade 5 | Death related to adverse events |
CTCAE, Common Terminology Criteria for Adverse Events.
Various irAEs have been described including pruritis, rash, colitis, liver toxicity, endocrinopathies (e.g., hypothyroidism, new onset type 1 diabetes), hypophysitis, pneumonitis and arthralgias (32). Hypothyroidism and pneumonitis are more commonly seen in patients treated with anti-PD-L1 agents, whereas rash, colitis and hypophysitis are commonly seen in those treated with anti-CTLA-4 agents. Hepatitis, colitis and pancreatitis are among the more clinically severe irAEs (grade 3 or 4) requiring discontinuation of ICIs (33,34). Rarer toxicities include but are not limited to interstitial nephritis, pancreatitis, myocarditis, myositis, arthritis, and ocular toxicities (34).
Hepatic irAEs carry an incidence of 5–10% in single-agent ICI therapy (26). ICI hepatitis, now referred to as immune-mediated liver injury caused by ICI (ILICI) differs based on the type of immunotherapy, dose, and the existence of pre-exisiting liver conditions. Hepatitis, defined as serum elevations of alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST), is graded based on degree of elevation: mild/grade 1, moderate/grade 2, severe/grade 3 and life threatening/grade 4 (Table 3) (35). However, there can be a heterogenous pattern of injury, encompassing hepatocellular, cholestatic, or both (36). Onset of ILICI is typically around 6–12 weeks after initiation of therapy (29). Anti-PD-L1 therapy can lead to a prolonged course of ILICI compared to anti-CTLA-4 agents, 8–9 vs. 3 weeks, respectively (37-39). ILICI typically resolves in 4–6 weeks (40).
Table 3
| Injury | Symptoms | Liver enzymes | Management strategy |
|---|---|---|---|
| Grade 1 | Asymptomatic | AST/ALT >3× ULN and/or Bilirubin >ULN – 1.5× ULN | Continue ICI therapy and recheck enzymes in 1 week |
| Grade 2 | Asymptomatic | AST/ALT >3–5× ULN | Hold ICI and start oral prednisolone 1 mg/kg |
| Grade 3 | Symptomatic liver dysfunction +/− enzymes or compensated cirrhosis | AST/ALT >5–20× ULN and/or bilirubin 3–10× ULN or fibrosis by biopsy | Discontinue ICI; start oral prednisolone 1 mg/kg or IV methylprednisolone 2 mg/kg based on degree of elevation |
| Grade 4 | Decompensated liver function | AST/ALT >20× ULN and/or bilirubin >10× ULN | Permanently discontinue ICI start IV methylprednisolone 2 mg/kg; consider hepatology consult and liver biopsy |
ILICI, immune-mediated liver injury caused by immune checkpoint inhibitor; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ULN, upper limit of normal; ICI, immune checkpoint inhibitor; IV, intravenous.
Occurrence of ILICI was initially reassuring. The phase 2 study of ipilimumab for advanced melanoma reported only 3% ≥ grade 3 liver adverse events and complete resolution of all liver-related AE (41). In a multicenter study of 146 patients treated with ICI, 46.3% developed hepatitis were asymptomatic at presentation, though 45.73% developed hepatitis categorized as severe (42). However, this contrasts with reports of greater burden of hepatic irAEs in real-world settings, including cases of acute liver failure (43-45). In a meta-analysis of fatal toxic effects of ICIs, hepatitis caused 22% of deaths (25). ILICI is higher in patients receiving combination therapy , with a reported incidence of 25–30%, than those on monotherapy, with a reported incidence is 5–10% (5,28,46-48).
The incidence of hepatic irAEs is greater in patients treated for primary liver cancers, likely due to the presence of underlying liver disease. In the initial trial, Checkmate 040, evaluating nivolumab (Bristol-Myers Squibb, 2014) in patients with advanced hepatocellular carcinoma (HCC), incidence of ≥ grade 3 ALT elevation was 8% (49) compared to 0% in trials for lung cancer (50-52) and 0–4% in trials for melanoma (5,28,53). A review of clinical trials found that patients with HCC treated with ICI have substantial increases in AST/ALT, though severity did not lead to any interruption of therapy (54). In the KEYNOTE-224 trial evaluating pembrolizumab (Merck, 2016) in patients with HCC, 9% of patients developed ALT elevations of any grade, with 4% of patients with ALT elevation ≥ grade 3 (55). Use of tremelimumab (AstraZeneca, 2022), an anti-CTLA-4 antibody, and tumor ablation for the treatment of HCC was associated with ALT elevations of any grade ≥ grade 3 in 19% and 9% of patients, respectively (56). Nivolumab and ipilimumab combination therapy for the treatment of HCC led to a rise in ALT levels of any grade and ≥ grade 3 in 8–16% and 0–8% of patients, respectively (57). In those receiving the combination of tremelimumab and durvalumab (AstraZeneca, 2017), elevated ALT levels of any grade and ≥ grade 3 were seen in 20% and 5% of patients, respectively (58). The current first line treatment for unresectable HCC is the combination of an anti-PD-L1, atezolizumab (Genentech, 2016), with a vascular endothelial growth factor, bevacizumab (59) based on favorable results from the IMbrave 150 trial which did not demonstrate any increased liver toxicity; ALT elevations of all grades and ≥ grade 3 were seen in 14% and 3.6% of patients, respectively (60). In patients who are undergoing treatment for HCC with ICI agents, it is imperitive that any underlying liver disease is evaluated and adequately managed prior to initiation of ICI treatment given hepatotoxicity concerns (Table 4).
Table 4
| Immunotherapeutic agent (study) | AST or ALT ↑ | Liver failure | Autoimmune hepatitis or immune-mediated hepatitis | HBV or HCV virologic breakthrough |
|---|---|---|---|---|
| Nivolumab (CHECKMATE 459) | + | + | + | Not evaluated |
| Nivolumab + ipilimumab (CHECKMATE 040)* | + | None | + | + |
| Pembrolizumab (KEYNOTE 224) | + | + | + | Not evaluated |
| Tremelimumab + durvalumab (HIMALAYA) | + | + | + | Not evaluated |
| Atezolizumab + bevacizumab (IMbrave 50) | + | + | + | Not evaluated |
| Lenvatinib + pembrolizumab (KEYNOTE 524) | + | + | + | Not evaluated |
↑, increase; +, present; *, study ongoing. ICI, immune checkpoint inhibitor; HCC, hepatocellular carcinoma; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HBV, hepatitis B virus; HCV, hepatitis C virus.
ILICI vs. AIH
While ILICI shares several traits with AIH, they are distinct entities with differing clinicopathological features. Determination of ILICI requires an assessment to exclude other causes such as autoimmune or viral hepatitis. Clinical features of ILICI include systemic symptoms with a rise in aminotransferases. Antinuclear antibodies or IgG elevations are not seen (61). Imaging findings are nonspecific and include hepatomegaly, peri-portal edema, and lymphadenopathy (62) while histological assessment often reveals acute hepatitis with a panlobular distribution of lymphocytic infiltrate with patchy or confluent areas of necrosis, as seen in cases of AIH, viral hepatitis or drug-induced liver injury (63). Pathological changes include presence of histiocytic sinusoidal infiltrates, microgranulomas, and central vein endotheliitis but with the notable absence of a consistent plasma cell predominant infiltrate (64). Histological features may differ between anti-CTLA-4 and anti-PD-1/PD-L1 hepatitis in which granulomatous hepatitis with fibrin-ring granulomas and central vein endotheliitis may be seen in the former and lobular hepatitis can be present in the latter (65). Additionally, immunostaining will reveal increased presence of CD3+ and CD8+ cytotoxic lymphocytes and fewer CD20+ B cells and CD4+ T cells (61,66). A summary of the differences between ILICI and AIH are described in Table 5.
Table 5
| Characteristics | ILICI | AIH |
|---|---|---|
| Clinical features | • Older age | • Younger age |
| • No gender prevalence | • Female predominant | |
| • Symptoms range from asymptomatic to rare cases of acute liver failure | • Symptoms range from asymptomatic to acute liver failure | |
| • Lack of other autoimmune diseases | • Presence of other autoimmune diseases | |
| Laboratory features | • ↑ AST, ALT, ALP/GGT | • ↑ AST, ALT, IgG |
| • Negative ANA (elevated 50%) and normal IgG | • ↑ ALP/GGT, bilirubin (possibly) | |
| • + ANA (high titer), ASMA, anti-LKM1 (possibly) | ||
| Histopathology | • Granulomas | • Plasmacytosis |
| • CD8+ | • CD4+/CD20+ | |
| Treatment | • Steroids may not always be required | • Steroids required |
| • Short courses, high dose | • Other immunosuppressant agents may be required | |
| Recurrence risk | • Rare | • High |
↑, increase; +, present. ILICI, immune mediated liver injury caused by immune check point inhibitors; AIH, autoimmune hepatitis; AST, aspartate aminotransferase; ALT, alanine transaminase; ALP, alkaline phosphatase; GGT, gamma glutamyl transferase; IgG, immunoglobulin gamma; ANA, anti-nuclear antibody; ASMA, anti-smooth muscle antibody; anti-LKM1, anti-liver kidney microsomal antibody-1.
Initial management of ILICI includes pausing immunotherapy. Guidelines for the Society for Immunotherapy of Cancer (SITC), the European Society of Medical Oncology (ESMO) and the American Society of Clinical Oncology (ASCO) recommend treatment with corticosteroids with doses in proportion to grade of hepatitis, up to 2 mg/kg/day (40,51,67). However, liver test findings may dramatically improve with just cessation of immunotherapy alone, without the addition of corticosteroids (36,68). There are several proposed management protocols for patients who develop severe liver toxicity due to ICI use (Table 3) (36,68,69). Challenges in managing ICI toxicity include late recognition, inadequate workup, and delayed treatment (69).
In a prospective, multicenter, noninterventional study of patients who developed ≥ grade 3 ILICI, 87% received single agent ICI therapy, among which 75% developed cases of severe irH (39). This cohort of patients were then compared to patients with AIH, who were younger on average (55 vs. 66 years). The AIH group had higher prevalence of cirrhosis (16% vs. 0%, P=0.008) and higher Model For End-Stage Liver Disease (MELD) score (15 vs. 8, P=0.11) than the irH group. An ANA titer >1:80 was seen in 84% of AIH compared to 25% of irH. Patients with AIH had higher median IgG values of 1,706 vs. 916 mg/dL (P<0.001). Also, irH patients required treatment of higher corticosteroid dose (median of 60 vs. 30 mg) initially and for a shorter duration of therapy (2.3 vs. 7 months) compared to the AIH patients. The AIH group also required the use of a second immunosuppressive drug in 97% of patients compared to 42% in the irH group (39).
Autoimmune disease
Patients with underlying autoimmune disease were excluded from trials due to concern for susceptibility for irAEs. Real-world data has supported this theory. In a prospective study including 45 patients with 53 pre-existing autoimmune disease (AD) treated with anti-PD-1 antibody for mainly melanoma and non-small cell lung cancer, irAEs occurred in 44% of patients with preexisting ADs (versus in 29% AD-free) and irAE-free survival time was significantly shorter in preexisting AD patients than AD-free patients (median: 5.4 vs. 13 months, P=0.0002) (70). In this study, the overall survival time and objective response rates, however, did not differ significantly between preexisting AD and AD-free groups (P=0.38 and 0.098, respectively) (70). In a multicenter, retrospective observational study of 751 patients with melanoma and non-small cell lung cancer treated with anti-PD-1 agents, 11% of which had preexisting AD (82% with inactive disease and 18% with active disease), the all-grade incidence of irAEs was 65.9% in patients with preexisting ADs compared to 39.9% in those without (P<0.001) (71). In this study, 47% of patients developed a flare of their underlying autoimmune disease (71).
Exacerbation of underlying autoimmune disease while receiving ICI treatment is a validated concern. In the largest series of 41 patients with preexisting AD and treatment with ipilimumab, 29% experienced flare of their preexisting disease while 29% developed additional irAEs (72). In a systematic review of 49 studies evaluating preexisting autoimmune disease in the setting of ICI use, 92 of 123 patients (75%) had exacerbations of ADs (41%), development of irAEs (25%), or both (11%) (73). Overall, there was no significant difference in frequency of disease flare or irAEs seen between patients with active or inactive disease (67% vs. 75%) (73). A study of 112 patients with preexisting AD reported AD flares and/or irAEs in 71% of patients of which 43% required immunosuppressive therapy and 21% required discontinuation of the agent (74). Patients who were already on immunosuppressive therapy at baseline had worse outcomes with shorter median progression-free survival when compared to those not on baseline immunosuppressive therapy (3.8 vs. 12 months; P=0.006) (74). Exacerbation of AD and development of irAEs were deemed to be manageable in these studies. More recently, a multicenter retrospective study of 123 patients with preexisting ADs treated with ICIs demonstrated exacerbation of underlying AD, development of irAE, or both in 25%, 35% and 10% of patients, respectively (75). Of these, grade 4 exacerbation and fatal toxicity were observed in 9% of patients. There was no no significant difference observed between those receiving anti-CTLA4 agents or anti-PDL1 agents (57.1% vs. 60.3%, respectively; Fisher’s P>0.999) (75). Use of immunotherapy and its expected efficacy must be balanced against potential toxicity issues in patients with underlying autoimmune disease since there is risk of severe flare.
The concern for flare of autoimmune disease also extends to patients with autoimmune liver disease despite the lack of present data. Patients with autoimmune liver disease were not included in studies, but the concern is risk of not only a flare in underlying autoimmune hepatitis (AIH) but additional ICI mediated liver toxicity. Testing and screening for AIH prior to initiating ICI therapy is prudent, especially in patients with other concurrent ADs (67). AIH lacks a signature diagnostic marker thus diagnosis is based on characteristic clinical and laboratory findings along with histological abnormalities (Table 6) (76). First-line treatments including prednisone and azathioprine control hepatic inflammation and achieve biochemical remission with an ideal laboratory response as normalization of serum ALT, AST and IgG levels (77). Presence of AIH itself should not be a contraindication to treatment with ICI, although close monitoring and follow-up are essential to monitor for AD flare and de novo irAEs. More longitudinal studies are needed to assess definitive effects of ICIs in patients with autoimmune liver disease.
Table 6
| Features | Criteria |
|---|---|
| Clinical | • Exclusion of viral, hereditary, metabolic, cholestatic, and drug-induced diseases |
| Laboratory | • ↑ AST, ALT |
| • ↑ Serum IgG levels | |
| Autoantibodies | • + ANA |
| • + ASMA | |
| • + Anti-LKM1 | |
| Histopathology* | • Interface hepatitis |
| • Plasma cell infiltration | |
| • Lobular hepatitis |
↑, increase; +, positive; *, histopathological diagnosis must be present along with one of the other (clinical, laboratory or serological markers) features as supporting evidence to make a diagnosis of autoimmune hepatitis. AST, aspartate aminotransferase; ALT, alanine transaminase; ANA, anti-nuclear antibody; ASMA, anti-smooth muscle antibody; Anti-LKM1, anti-liver kidney microsomal antibody-1; IgG, immunoglobulin gamma.
Immunotherapy and viral hepatitis
HBV infection is a global health concern affecting approximately two billion people worldwide (78). An estimated 71 million people worldwide are living with chronic hepatitis C (79). Active and persistent viral infection is associated with hepatic disease progression and risk of development of HCC. Management of hepatitis B and C infection in the setting of immunotherapy hinges upon understanding differences in pathogenesis.
HBV pathogenesis
The HBV is an enveloped circular and partially double-stranded Hepadnaviridae DNA virus that infects hepatocytes (80). The stages of HBV infection are hallmarked by various genes corresponding with infection activity: S gene encoding hepatitis B surface antigen (HBsAg), C gene encoding pre-genomic RNA which forms the hepatitis B core antigen (HBcAg), and a precore protein derivative that encodes the hepatitis B e antigen (HBeAg) (Figure 3) (81). In contrast to other hepatic viruses, HBV DNA embeds into the host hepatocyte genome and converts into a covalently closed circular DNA (cccDNA) that is stabilized in the hepatocyte allowing it to persist in a latent state (82). A complete cure of HBV infection is defined by HBV cccDNA eradication, rendering viral replication impossible (83).

In an acute infection, HBV spreads quickly and effectively throughout hepatic parenchyma due to the highly vascular nature of hepatic tissue and ability to evade detection by innate immunity (84). Clearance of HBV infection is via the adaptive immune system and thought to be dependent on CD8+ T cell response, which can be negated by poor CD4 T cell function via weak IFN-γ activation (85). The majority of immunocompetent adults who develop HBV infection by horizontal transmission are able to successfully clear infection, with less than 10% of these cases becoming chronic HBV. Alternatively, vertical transmission of HBV becomes chronic in more than 90% of cases due to HBV precore protein (HBeAg) that crosses the placenta and facilitates viral persistence by inhibiting induction of the T cell response and creating immune tolerance (86).
Chronic HBV infection is differentiated into 4 phases based on HBV DNA level, ALT level, HBeAg positivity and liver histology (Table 7) (81,87). The prolonged, continuous exposure to high levels of viral antigen results in exhaustion of HBV-specific CD8+ T cell activity causing impairment of multiple immune processes (84,88). Active liver injury is only avoided if HBV replication remains inhibited by antiviral therapy (89). In the chronic inactive state, a reservoir of stable circular HBV DNA and its viral proteins exist in hepatocytes while serum HBV DNA remains undetectable (90).
Table 7
| Phase of chronic infection | HBV load | HBeAg | ALT level | Histological inflammation or fibrosis |
|---|---|---|---|---|
| Immune tolerant | +++ | + | Normal | None to minimal |
| Immune active | ++ | + | Elevated | Moderate to severe |
| Chronic inactive | − | − | Normal | Absent with variable fibrosis |
| Chronic immune-reactivation HBV | + | − | Variable | Variably present |
+, positive; −, negative. HBV, hepatitis B virus; HBeAg, hepatitis B e antigen; ALT, alanine transaminase.
Hepatitis B reactivation
Complete eradication of cccDNA has not been achieved with either the host immune response or antiviral treatment with nucleos(t)ide analogues. The presence of cccDNA allows for HBV reactivation (HBVr) when there is significant disturbance in the balanced state between viral replication and the host immune system. This can occur sponataneously or in response to therapeutic agents that affect the host immune system and immune microenvironment (91). Various definitions have been proposed, but the updated guideline by the American Association for the Study of Liver Diseases (AASLD) defines HBVr when any of the following criteria are fulfilled: (I) a ≥2 log (100-fold) increase in HBV DNA compared to the baseline level; (II) HBV DNA ≥3 log(Jeny1,000) IU/mL in a patient with previously undetectable level; or (III) HBV DNA ≥4 log(Jeny10,000) IU/mL if the baseline level was not available (87).
Various factors affect reactivation of HBV including HBsAg positivity, HBeAg positivity and higher HBV DNA levels (>10,000 IU/mL) (92,93). Clinical presentation of HBVr ranges from silent without overt hepatitis to fulminant liver failure (93). HBVr is associated with multiple different treatments including ICIs, tumor necrosis factor-a inhibitors, immunosuppression by corticosteroids, systemic chemotherapy, biologic antibodies such as anti-CD20 like rituximab or anti-tumor necrosis factor-α like infliximab, and even locoregional hepatic interventions such as transarterial chemoembolization (TACE) to treat HCC (94).
HBV seropositivity was evaluated for predisposition for HBVr in a historical cohort study which found reactivation occurred in 1% of HBsAg positive patients (5 of 511) compared with 0% of HBsAg negative patients (0 of 2,954) (95). Patients receiving antiviral prophylaxis had 0.4% reactivation rate compared to 6.4% in those without prophylaxis (57).
HCV pathogenesis
HCV is an enveloped RNA Hepacivirus with significant heterogeneity, thus its pathogenesis is not completely understood (96). Initial response to infection is via innate immunity (96). Over time, HCV has mutated and evolved to evade this detection, further contributing to the high rate of conversion to chronic HCV infection (96). Adaptive immunity-mediated elimination of HCV relies on helper T cell response. Sustained immune response is often insufficient in clearing HCV; most patients develop chronic HCV infection, with 74–86% of patients developing persistent viremia (97).
Persistent HCV replication in chronic infection promotes an immunosuppressive microenvironment by exhausting HCV-specific CD8+ T cells and increasing FoxP3+ T regulatory cells (Treg) (98). This increased Treg activity dampens other immune cells including lymphocytes, NK cells, and antigen presenting cells (99). Chronically HCV infected cells are characterized by decreased glutathione levels which promote increased oxidative stress and liver injury (100). Prolonged inflammatory state causes hepatocytes to secrete pro-fibrogenic cytokines and to activate myofibroblasts that drive formation of hepatic fibrosis (100).
Patients with chronic HCV who undergo immunomodulatory interventions are described as developing enhanced HCV replication, and while there is no widely accepted definition of HCV reactivation (HCVr) one proposed definition includes an increase in HCV-RNA level of ≥1 log IU/mL from baseline HCV-RNA (101). HCVr is less common and results in less severe consequences than HBVr, but is more likely to occur in patients with hematologic malignancies (78% vs. 42%, P=0.002), particularly lymphoma (50% vs. 22%, P=0.05) (102). This is especially true following implementation of highly effective direct-acting antiviral (DAA) therapy for HCV treatment (103). Retrospective immunotherapy studies that included patients with HCV-RNA monitoring pre- and post-intervention demonstrated that HCVr occurred in 23–36% of patients (101,102,104).
Viral hepatitis in ICI
Universal screening of patients with newly diagnosed cancer for HBV and HCV is not routine in oncology practice, although most guidelines recommend it. Thus the prevalence of HBV and HCV in those with malignancies is unknown and underreported. In a recent multicenter prospective cohort study of 3,092 patients with newly diagnosed cancers, the observed infection prevalence for previous HBV infection was 6.5% (95% CI: 5.6–7.4%), chronic HBV was 0.6% (95% CI: 0.4–1.0%) and chronic HCV was 2.4% (95% CI: 1.9–3.0%) (105).
PD-1 and CTLA-4 are potent regulators in T-cell mediated pathways and are known to alter activity in chronic HBV and HCV infections (103). The risk of HBVr and HCVr can be explained by the immune activation of hepatocytes which have been chronically infected in an immunosuppressed environment; it is also possible that inhibition of CTLA-4 may result in the activation of Treg, therefore impairing the ability of T cells to further suppress HBV and HCV (106).
Overall, data regarding incidence, prevalence, morbidity, and mortality of HBV reactivation and enhanced HCV replication after initiation of ICI therapy is limited. ICI clinical trials exclude patients with underlying chronic infections due to concerns for reactivation, cellular toxicity, and presumed lack of efficacy (103). The theoretical risk of inefficacy is attributed to chronic viral infections suppressing T cell function (4,32).
Chronic viral hepatitis is characterized by chronic hepatic inflammation, promoting fibrin formation and eventually, cirrhosis (107). This chronically inflamed state promotes hepatocarcinogenesis via mechanisms that inhibit antitumor activity (e.g., impaired NK cell and CD8+ T cell activity) and lead to the development of hepatocellular carcinoma (107). In contrast to many other hematologic and solid-organ tumors, HCC development and maintenance relies heavily on its immune microenvironment, providing a compelling basis for utilizing ICI therapy for treatment, particularly as advanced HCC is difficult to treat with immunosuppressive chemotherapy (107).
In the IMbrave150 trial, patients with unresectable HCC were treated with either combination atezolizumab and bevacizumab (multikinase inhibitor) or sorafenib with 12-month survival outcomes of 67.2% (95% CI: 61.3–73.1%) and 54.6% (95% CI: 45.2–64%), respectively (60). The study excluded history of autoimmune disease and HBV/HCV coinfection, but the most reported adverse event in atezolizumab/bevacizumab therapy was immune-mediated hepatitis (53% of patients) (60), while viral reactivation was not a reported adverse event (108). This combination immunotherapy has been shown to be more effective in patients with underlying viral liver disease (HBV hazard ratio 0.58, 95% CI: 0.40–0.83; HCV hazard ratio 0.43, 95% CI: 0.25–0.73) compared to non-viral etiology (hazard ratio for death 1.05, 95% CI: 0.68–1.63) (108,109). In the Checkmate 040 trial, 9% of HBV-infected patients (7/82) and 10% of HCV-infected patients (4/39) had virologic breakthrough, defined by the study as 1-log increase in HBV DNA or HCV RNA from baseline (57). In KEYNOTE-224, a non-randomized, open-label trial studying pembrolizumab in sorafenib-refractory patients with HCC, response rate was 16% (95% CI: 7–29%) and did not result in any viral-induced hepatitis flares in the 104 patients included (110). Pembrolizumab monotherapy however, did lead to ILICI in 2.9% of patiens (111).
In the HIMALAYA trial, combination regimen tremelimumab plus durvalumab was compared against sorafenib and results showed an increased median overall survival for treatment of unresectable HCC, 16.43 months (95% CI: 14.06–19.12) versus 13.77 months (95% CI: 12.25–16.13), respectively, with hazard ratio 0.78 (96% CI: 0.65–0.93, P=0.0035) (112). Durvalumab monotherapy was noninferior to sorafenib for the treatment of unresectable HCC, with median overall survival of 16.56 months (95% CI: 14.06–19.12) with hazard ratio of 0.86 (95.67% CI: 0.73–1.03). HBV and HCV accounted for 31% and 27% of the etiologies for chronic liver disease. Although reactivation events were not reported, the most commonly reported immune-mediated adverse event was immune-mediated hepatitis, with 7.5% of patients requiring steroid treatment and 2.3% of patients requiring discontinuation of combination tremelimumab plus durvalumab, comparable to durvalumab monotherapy with 6.4% and 1.3% respectively (112).
HBV and HCV infected patients with HCC who were treated with ICI therapy were compared to non-infected patients and both groups responded similarly to ICI therapy with no significant differences in pre- and post-immune microenvironments (113). Additionally, hepatic viruses were found to be integrated in both malignant and normal hepatocytes, suggesting that the resultant HCC is likely driven more by chronic inflammatory process than by viral infection itself. This meta-analysis confirms that viral status in HCC should not disqualify patients from receiving ICI treatments as outcomes were not significantly different.
Conceptually, via inhibition of these CTLA-4 and PD-1 pathways, activation of T cell response should reverse the T cell exhaustion seen in chronic HBV and HCV thus, promoting viral clearance (106). This immune effect was observed in an ex vivo study in which HBV-specific T cell proliferation and increased IFN production was observed after PD-1/PD-L1 blockade (88). The theoretical antiviral effect of ICI therapy is supported by limited data from trials and several cases. In a small retrospective case series, 7 of 9 patients receiving ipilimumab for advanced melanoma with underlying viral hepatitis experienced viral stability, or in 2 cases, HCV regression attributable to ipilimumab alone (114). In an open-label Phase II clinical trial of tremelimumab for the treatment of HCC with underlying chronic HCV, anti-CTLA-4 agent tremelimumab was found to decrease HCV load, from 378×103 IU/mL on day 0, to 30.2×103 IU/mL on day 120 (n=11, P=0.011), and 1.69×103 IU/mL on day 210 (n=6, P=0.017) (115). Viral response, defined as >1 log decrease in HCV load, was seen in 75% (9 of 12) patients (115). In a study of 133 patients, 1.5% (2 of 133) of patients with underlying HBV or HCV developed viral reactivation (98). There is promising evidence to demonstrate an antiviral effect of ICIs, with a small percentage of patients developing paradoxical viral reactivation (98).
Prevention and management of viral hepatitis in ICI therapy
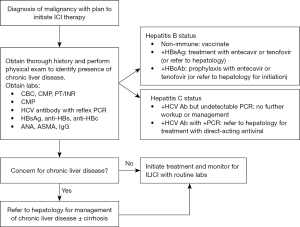
The guideline for screening for chronic HBV infection is in those who are at risk, including persons needing immunosuppressive therapy as they are more likely to develop chronic HBV infection after acute infection (116). Screening is performed using both HBsAg and anti-HBs. The presence of HBsAg establishes the diagnosis of hepatitis B infection. In those who do not have immunity, vaccination against HBV infection is recommended.
In a single-center, retrospective study, 1% of HBsAg negative patients developed acute hepatitis on chemotherapy compared to 33% of HBsAg positive patients (117). Later reports suggest HBV reactivation occurs in 41–53% (118) of HBsAg-positive, anti-HBc-positive patients and 8–18% (119) of HBsAg-negative, anti-HBc-positive patients receiving anticancer treatments. Thus, screening with anti-HBc to determine prior exposure is recommended in those who will receive immunosuppressive therapies (120,121). Interpretation of screening tests for HBV is summarized in Table 8.
Table 8
| HBsAg | Anti-HBs | Anti-HBc | Interpretation | Action |
|---|---|---|---|---|
| + | − | + | Acute or chronic infection | Evaluation and further testing |
| − | +/− | + | Exposure to HBV; at risk for reactivation | Follow-up as appropriate |
| − | + | − | Immune due to vaccination | No further action required |
| − | − | − | At risk for HBV infection | Vaccinate |
+, positive; −, negative. HBsAg, hepatitis B surface antigen; Anti-HBs, anti-hepatitis B surface antibodies; Anti-HBc, anti-hepatitis B core antigen; HBV, hepatitis B virus.
There are currently six therapeutic agents approved for the treatment of chronic HBV infection IFN and 5 nucleos(t)ide analogues, which are competitive inhibitors of HBV polymerases and work by inhibiting further HBV DNA synthesis and replication. The 5 available nucleos(t)ide analogues are lamivudine, telbivudine, entecavir, adefovir, and tenofovir but preferred initial therapy is with with Peg-IFN, entecavir or tenofovir (87). Therapy success is determined by biochemical, serological, virological and histological endpoints. Hepatitis B treatment aims to suppress viral replication and can be monitored for efficacy by surrogate markers including the normalization of ALT and loss of HBeAg. Treatment duration with NAs is driven by HBeAg presence, HBV DNA suppression, and complications of liver disease and cirrhosis.
There has been emerging data on antiviral prophylaxis in those undergoing immunosuppressive and immune-modulating drugs. HBsAg-positive patients are at high risk of HBVr thus should receive anti-HBV prophylaxis before the initiation of immunosuppressive therapy (122,123). Patients who receive lamivudine prophylaxis have significantly lower incidence of hepatitis (relative risk =0.40, 95% CI: 0.26–0.63, P<0.0001), and have reduced rate in overall mortality and mortality attributed to HBVr (RR 0.45, 95% CI: 0.29–0.70, P=0.0004 and RR 0.41, 95% CI: 0.20–0.84, P=0.01) compared to those who did not receive prophylactic treatment (124). HBsAg-negative, anti-HBc-positive patients are at lower risk of HBVr and depending on clinical situation can be initiated on anti-HBV prophylaxis or monitored with intention of on-demand therapy at the first signs of reactivation (87). Although antiviral prophylaxis has not been studied in randomized controlled trials, most guidance for anti-HBV therapy is to treat for 12 months following immunosuppressive therapy, especially in the case of B cell deleting therapies (e.g., rituximab) (90,94,121). The prolonged duration of treatment is to account for possible delayed reactivation.
In contrast to the limited effects of successful HBV therapy, achieving complete cure is possible with HCV antiviral treatment. Cure is defined as undetectable HCV RNA 12 weeks after completion of antiviral therapy. Since HCV infection is a curable disease, a one-time, routine, opt-out HCV testing is recommended for all individuals aged 18 years or older (125). Initial screening should be performed with HCV-antibody testing with reflex HCV RNA polymerase chain reaction testing (126). Eradication of HCV infection has numerous health benefits including reduced rates of all-cause mortality, cirrhosis, hepatic decompensation and HCC (127).
The advent of direct acting antiviral (DAA) therapy has revolutionized treatment of HCV and provided therapeutic tools required to strive for elimination (128). Given the highly efficacious nature of treatment, current guidelines recommend antiviral therapy in all adults with acute or chronic HCV infection (125). There are several currently available DAA regimens (Figure 4) that provide high sustained virologic response (SVR) rates of >95% (129).

In HCV patients without cirrhosis or those who are treatment-naïve, simplified antiviral regimen with either 8 weeks of glecaprevir/pibrentasvir or 12 weeks of sofosbuvir/velpatasvir is recommended (125). Antiviral treatment recommendations for HCV patients with more decompensated liver disease are more complex. As found in the ASTRAL-4 trial, HCV patients with Child-Pugh-Turcotte class B cirrhosis demonstrated lower sustained virologic response 12 weeks after treatment (SVR12), with 83% (95% CI: 74–90%) on sofosbuvir-velpatasvir therapy, and 94% (95% CI: 87–98%) on sofosbuvir-velpatasvir-ribavirin (130). Given the efficacy and tolerability of DAA treatment, HCV treatment can be approached in several ways for patients who undergo immunotherapy. Though there is a paucity of data to support a consensus recommendation for treatment, DAA therapy can be initiated prior to or in combination with immunosuppressive therapy, or initiated at the onset of HCVr in patients who receive HCV-RNA monitoring (102).
This evidence of benefit for screening for HBV and HCV has yet to translate to all guideline-directed practice when implementing ICI therapy. Though there exist some viral screening recommendations for hematologic malignancies and hematopoietic stem cell transplants, guidance for viral screening is limited in other non-hepatic solid organ malignancies utilizing ICI therapy. At a single-institution study at MD Anderson Cancer Center, only 14% of cancer patients starting ICI were screened for HCV (103), despite United States Preventive Services Taskforce (USPSTF) recommendations for once-in-lifetime HCV screening of all adults.
Discussion
ICI therapy has become the mainstay therapy for a large number of cancers including advanced and unresectable HCC. Although ICI therapy has changed the landscape of cancer management, irAEs has been a limitation for overall patient outcomes. ILICI contributes to roughly 5–10% of these irAEs and greater incidence is observed in patients with underlying liver disease, including in the treatment of HCC.
With evolving advancements in the treatment of viral and AIH, it imperative to screen for HBV, HCV, and AIH when initiating ICI to ensure better patient outcomes. Unfortunately, efficacy and safety data in patients with these diseases are lacking, as they have been excluded from major ICI clinical trials due to concern for viral reactivation or AIH flare on initiation of therapy.
We propose a screening algorithm (Figure 5) to identify and treat patients with hepatic comorbidities prior to ICI initiation. The authors also encourage early involvement of a hepatology in patients with HCC (Figure 5A) and select patients with non-HCC malignancies (Figure 5B). This algorithm provides a multidisciplinary approach to ICI therapy and subsequent ILICI management. By utilizing this proposed algorithm and emphasizing routine screening for viral and AIH in patients prior to initiating ICI treatment, substantial improvement in morbidity and mortality can be achieved, allowing for more patients with underlying liver diseases to be safely managed with ICI therapy.
Conclusions
With the growing landscape of ICI therapy, it is crucial to identify and understand the associated risks of therapy to allow for appropriate management. The high morbidity and mortality associated with hepatic toxicity especially highlights the need for careful screening of underlying diseases including autoimmune and viral hepatitis prior to initiation of ICI therapy. As treatment options for advanced and unresectable HCC expand to include more ICI therapy, the prevalence of patients with pre-treatment AIH and chronic HBV and HCV infections will increase. These patients will require close monitoring during treatment and diligent surveillance following its completion.
Use of immunotherapy must be coupled with standard practices of thorough liver evaluation and monitoring. Screening for HBV and HCV infections is paramount in patients undergoing immunomodulatory therapy in order to avoid severe liver injury, viral reactivation, and even fulminant liver failure.
Currently, literature providing evidence for long-term adverse events and survival benefits is scarce. More longitudinal studies that include patients with underlying autoimmune and viral hepatitis are required to definitively guide management.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Sukeshi Patel Arora and Sherri Rauenzahn Cervantez) for the series “Comprehensive Care for Patients with Hepatocellular Carcinoma: Insights from the 2022 San Antonio Liver Cancer Symposium” published in Annals of Palliative Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-23-250/rc
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-23-250/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-23-250/coif). The series “Comprehensive Care for Patients with Hepatocellular Carcinoma: Insights from the 2022 San Antonio Liver Cancer Symposium” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
※Special series on Comprehensive Care for Patients with Hepatocellular Carcinoma: Insights from the 2022 San Antonio Liver Cancer Symposium.
References
- Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin 2020;70:86-104. [Crossref] [PubMed]
- Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013;342:1432-3. [Crossref] [PubMed]
- Weber EW, Maus MV, Mackall CL. The Emerging Landscape of Immune Cell Therapies. Cell 2020;181:46-62. [Crossref] [PubMed]
- Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer 2017;123:1904-11. [Crossref] [PubMed]
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373:23-34. [Crossref] [PubMed]
- Wong GL, Wong VW, Hui VW, et al. Hepatitis Flare During Immunotherapy in Patients With Current or Past Hepatitis B Virus Infection. Am J Gastroenterol 2021;116:1274-83. [Crossref] [PubMed]
- Ding ZN, Meng GX, Xue JS, et al. Hepatitis B virus reactivation in patients undergoing immune checkpoint inhibition: systematic review with meta-analysis. J Cancer Res Clin Oncol 2023;149:1993-2008. [Crossref] [PubMed]
- Pardoll D. Cancer and the Immune System: Basic Concepts and Targets for Intervention. Semin Oncol 2015;42:523-38. [Crossref] [PubMed]
- Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol 2021;16:223-49. [Crossref] [PubMed]
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015;348:56-61. [Crossref] [PubMed]
- Sharma P, Hu-Lieskovan S, Wargo JA, et al. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017;168:707-23. [Crossref] [PubMed]
- Wong YC, Tay SS, McCaughan GW, et al. Immune outcomes in the liver: Is CD8 T cell fate determined by the environment? J Hepatol 2015;63:1005-14. [Crossref] [PubMed]
- Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol 2020;17:807-21. [Crossref] [PubMed]
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996;271:1734-6. [Crossref] [PubMed]
- Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027-34. [Crossref] [PubMed]
- Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A 2004;101:17174-9. [Crossref] [PubMed]
- Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res 2006;66:3381-5. [Crossref] [PubMed]
- Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 2005;11:2947-53. [Crossref] [PubMed]
- Nakanishi J, Wada Y, Matsumoto K, et al. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother 2007;56:1173-82. [Crossref] [PubMed]
- Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res 2007;13:2151-7. [Crossref] [PubMed]
- Hirano F, Kaneko K, Tamura H, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res 2005;65:1089-96. [Crossref] [PubMed]
- Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N Engl J Med 2022;386:24-34. [Crossref] [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Barone A, Hazarika M, Theoret MR, et al. FDA Approval Summary: Pembrolizumab for the Treatment of Patients with Unresectable or Metastatic Melanoma. Clin Cancer Res 2017;23:5661-5. [Crossref] [PubMed]
- Wang DY, Salem JE, Cohen JV, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol 2018;4:1721-8. [Crossref] [PubMed]
- Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv119-42. [Crossref] [PubMed]
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359:1350-5. [Crossref] [PubMed]
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. [Crossref] [PubMed]
- Schneider BJ, Naidoo J, Santomasso BD, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J Clin Oncol 2021;39:4073-126. [Crossref] [PubMed]
- Bertrand A, Kostine M, Barnetche T, et al. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med 2015;13:211. [Crossref] [PubMed]
- Arnaud-Coffin P, Maillet D, Gan HK, et al. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int J Cancer 2019;145:639-48. [Crossref] [PubMed]
- Johnson DB, Nebhan CA, Moslehi JJ, et al. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol 2022;19:254-67. [Crossref] [PubMed]
- Papouin B, Mussini C, De Martin E, et al. Hepatic and digestive adverse events of immune checkpoint inhibitors (anti-CTLA-4 and, anti-PD-1/PD-L1): A clinico-pathological review. Ann Pathol 2018;38:338-51. [Crossref] [PubMed]
- Chan KK, Bass AR. Autoimmune complications of immunotherapy: pathophysiology and management. BMJ 2020;369:m736. [Crossref] [PubMed]
- Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176-81. [Crossref] [PubMed]
- De Martin E, Michot JM, Rosmorduc O, et al. Liver toxicity as a limiting factor to the increasing use of immune checkpoint inhibitors. JHEP Rep 2020;2:100170. [Crossref] [PubMed]
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N Engl J Med 2016;375:1845-55. [Crossref] [PubMed]
- Gauci ML, Baroudjian B, Bédérède U, et al. Severe immune-related hepatitis induced by immune checkpoint inhibitors: Clinical features and management proposal. Clin Res Hepatol Gastroenterol 2021;45:101491. [Crossref] [PubMed]
- Riveiro-Barciela M, Barreira-Díaz A, Vidal-González J, et al. Immune-related hepatitis related to checkpoint inhibitors: Clinical and prognostic factors. Liver Int 2020;40:1906-16. [Crossref] [PubMed]
- Haanen J, Obeid M, Spain L, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2022;33:1217-38. [Crossref] [PubMed]
- Wolchok JD, Neyns B, Linette G, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 2010;11:155-64. [Crossref] [PubMed]
- Patrinely JR Jr, McGuigan B, Chandra S, et al. A multicenter characterization of hepatitis associated with immune checkpoint inhibitors. Oncoimmunology 2021;10:1875639. [Crossref] [PubMed]
- Bhave P, Buckle A, Sandhu S, et al. Mortality due to immunotherapy related hepatitis. J Hepatol 2018;69:976-8. [Crossref] [PubMed]
- Suzman DL, Pelosof L, Rosenberg A, et al. Hepatotoxicity of immune checkpoint inhibitors: An evolving picture of risk associated with a vital class of immunotherapy agents. Liver Int 2018;38:976-87. [Crossref] [PubMed]
- Parlati L, Vallet-Pichard A, Batista R, et al. Incidence of grade 3-4 liver injury under immune checkpoints inhibitors: A retrospective study. J Hepatol 2018;69:1396-7. [Crossref] [PubMed]
- Hercun J, Vincent C, Bilodeau M, et al. Immune-Mediated Hepatitis During Immune Checkpoint Inhibitor cancer Immunotherapy: Lessons From Autoimmune Hepatitis and Liver Immunology. Front Immunol 2022. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2022.907591
- Hammers HJ, Plimack ER, Infante JR, et al. Safety and Efficacy of Nivolumab in Combination With Ipilimumab in Metastatic Renal Cell Carcinoma: The CheckMate 016 Study. J Clin Oncol 2017;35:3851-8. [Crossref] [PubMed]
- Pollack MH, Betof A, Dearden H, et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol 2018;29:250-5. [Crossref] [PubMed]
- El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492-502. [Crossref] [PubMed]
- Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol 2015;16:257-65. [Crossref] [PubMed]
- Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714-68. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020-30. [Crossref] [PubMed]
- Brown ZJ, Heinrich B, Steinberg SM, et al. Safety in treatment of hepatocellular carcinoma with immune checkpoint inhibitors as compared to melanoma and non-small cell lung cancer. J Immunother Cancer 2017;5:93. [Crossref] [PubMed]
- Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940-52. [Crossref] [PubMed]
- Duffy AG, Ulahannan SV, Makorova-Rusher O, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol 2017;66:545-51. [Crossref] [PubMed]
- Yau T, Kang YK, Kim TY, et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol 2020;6:e204564. [Crossref] [PubMed]
- Kelley RK, Abou-Alfa GK, Bendell JC, et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. J Clin Oncol 2017;35:4073. [Crossref]
- Bruix J, Chan SL, Galle PR, et al. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J Hepatol 2021;75:960-74. [Crossref] [PubMed]
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382:1894-905. [Crossref] [PubMed]
- Zen Y, Yeh MM. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug-induced liver injury. Mod Pathol 2018;31:965-73. [Crossref] [PubMed]
- Kim KW, Ramaiya NH, Krajewski KM, et al. Ipilimumab associated hepatitis: imaging and clinicopathologic findings. Invest New Drugs 2013;31:1071-7. [Crossref] [PubMed]
- Kleiner DE, Berman D. Pathologic changes in ipilimumab-related hepatitis in patients with metastatic melanoma. Dig Dis Sci 2012;57:2233-40. [Crossref] [PubMed]
- Cohen JV, Dougan M, Zubiri L, et al. Liver biopsy findings in patients on immune checkpoint inhibitors. Mod Pathol 2021;34:426-37. [Crossref] [PubMed]
- De Martin E, Michot JM, Papouin B, et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol 2018;68:1181-90. [Crossref] [PubMed]
- Johncilla M, Misdraji J, Pratt DS, et al. Ipilimumab-associated Hepatitis: Clinicopathologic Characterization in a Series of 11 Cases. Am J Surg Pathol 2015;39:1075-84. [Crossref] [PubMed]
- Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer 2017;5:95. [Crossref] [PubMed]
- Gauci ML, Baroudjian B, Zeboulon C, et al. Immune-related hepatitis with immunotherapy: Are corticosteroids always needed? J Hepatol 2018;69:548-50. [Crossref] [PubMed]
- Sangro B, Chan SL, Meyer T, et al. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J Hepatol 2020;72:320-41. [Crossref] [PubMed]
- Danlos FX, Voisin AL, Dyevre V, et al. Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. Eur J Cancer 2018;91:21-9. [Crossref] [PubMed]
- Cortellini A, Buti S, Santini D, et al. Clinical Outcomes of Patients with Advanced Cancer and Pre-Existing Autoimmune Diseases Treated with Anti-Programmed Death-1 Immunotherapy: A Real-World Transverse Study. Oncologist 2019;24:e327-37. [Crossref] [PubMed]
- Kähler KC, Eigentler TK, Gesierich A, et al. Ipilimumab in metastatic melanoma patients with pre-existing autoimmune disorders. Cancer Immunol Immunother 2018;67:825-34. [Crossref] [PubMed]
- Abdel-Wahab N, Shah M, Lopez-Olivo MA, et al. Use of Immune Checkpoint Inhibitors in the Treatment of Patients With Cancer and Preexisting Autoimmune Disease: A Systematic Review. Ann Intern Med 2018;168:121-30. [Crossref] [PubMed]
- Tison A, Quéré G, Misery L, et al. Safety and Efficacy of Immune Checkpoint Inhibitors in Patients With Cancer and Preexisting Autoimmune Disease: A Nationwide, Multicenter Cohort Study. Arthritis Rheumatol 2019;71:2100-11. [Crossref] [PubMed]
- Fountzilas E, Lampaki S, Koliou GA, et al. Real-world safety and efficacy data of immunotherapy in patients with cancer and autoimmune disease: the experience of the Hellenic Cooperative Oncology Group. Cancer Immunol Immunother 2022;71:327-37. [PubMed]
- Mack CL, Adams D, Assis DN, et al. Diagnosis and Management of Autoimmune Hepatitis in Adults and Children: 2019 Practice Guidance and Guidelines From the American Association for the Study of Liver Diseases. Hepatology 2020;72:671-722. [Crossref] [PubMed]
- Montano Loza AJ, Czaja AJ. Current therapy for autoimmune hepatitis. Nat Clin Pract Gastroenterol Hepatol 2007;4:202-14. [Crossref] [PubMed]
- Revill PA, Chisari FV, Block JM, et al. A global scientific strategy to cure hepatitis B. Lancet Gastroenterol Hepatol 2019;4:545-58. [Crossref] [PubMed]
- Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017;2:161-76. [Crossref] [PubMed]
- Tsai KN, Kuo CF, Ou JJ. Mechanisms of Hepatitis B Virus Persistence. Trends Microbiol 2018;26:33-42. [Crossref] [PubMed]
- Tsai E. Review of Current and Potential Treatments for Chronic Hepatitis B Virus Infection. Gastroenterol Hepatol (N Y) 2021;17:367-76. [PubMed]
- Tsukuda S, Watashi K. Hepatitis B virus biology and life cycle. Antiviral Res 2020;182:104925. [Crossref] [PubMed]
- Lok AS, Zoulim F, Dusheiko G, et al. Hepatitis B cure: From discovery to regulatory approval. J Hepatol 2017;67:847-61. [Crossref] [PubMed]
- Iannacone M, Guidotti LG. Immunobiology and pathogenesis of hepatitis B virus infection. Nat Rev Immunol 2022;22:19-32. [Crossref] [PubMed]
- Shin EC, Sung PS, Park SH. Immune responses and immunopathology in acute and chronic viral hepatitis. Nat Rev Immunol 2016;16:509-23. [Crossref] [PubMed]
- Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol 2006;1:23-61. [Crossref] [PubMed]
- Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560-99. [Crossref] [PubMed]
- Fisicaro P, Barili V, Montanini B, et al. Targeting mitochondrial dysfunction can restore antiviral activity of exhausted HBV-specific CD8 T cells in chronic hepatitis B. Nat Med 2017;23:327-36. [Crossref] [PubMed]
- Zhang X, Zhou Y, Chen C, et al. Hepatitis B virus reactivation in cancer patients with positive Hepatitis B surface antigen undergoing PD-1 inhibition. J Immunother Cancer 2019;7:322. [Crossref] [PubMed]
- Loomba R, Liang TJ, Hepatitis B. Reactivation Associated With Immune Suppressive and Biological Modifier Therapies: Current Concepts, Management Strategies, and Future Directions. Gastroenterology 2017;152:1297-309. [Crossref] [PubMed]
- Shi Y, Zheng M. Hepatitis B virus persistence and reactivation. BMJ 2020;370:m2200. [Crossref] [PubMed]
- Lau GK, Leung YH, Fong DY, et al. High hepatitis B virus (HBV) DNA viral load as the most important risk factor for HBV reactivation in patients positive for HBV surface antigen undergoing autologous hematopoietic cell transplantation. Blood 2002;99:2324-30. [Crossref] [PubMed]
- Myint A, Tong MJ, Beaven SW. Reactivation of Hepatitis B Virus: A Review of Clinical Guidelines. Clin Liver Dis (Hoboken) 2020;15:162-7. [Crossref] [PubMed]
- Bessone F, Dirchwolf M. Management of hepatitis B reactivation in immunosuppressed patients: An update on current recommendations. World J Hepatol 2016;8:385-94. [Crossref] [PubMed]
- Yoo S, Lee D, Shim JH, et al. Risk of Hepatitis B Virus Reactivation in Patients Treated With Immunotherapy for Anti-cancer Treatment. Clin Gastroenterol Hepatol 2022;20:898-907. [Crossref] [PubMed]
- Irshad M, Mankotia DS, Irshad K. An insight into the diagnosis and pathogenesis of hepatitis C virus infection. World J Gastroenterol 2013;19:7896-909. [Crossref] [PubMed]
- Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001;345:41-52. [Crossref] [PubMed]
- De Keukeleire SJ, Vermassen T, Nezhad ZM, et al. Managing viral hepatitis in cancer patients under immune checkpoint inhibitors: should we take the risk? Immunotherapy 2021;13:409-18. [Crossref] [PubMed]
- Riezu-Boj JI, Larrea E, Aldabe R, et al. Hepatitis C virus induces the expression of CCL17 and CCL22 chemokines that attract regulatory T cells to the site of infection. J Hepatol 2011;54:422-31. [Crossref] [PubMed]
- Roehlen N, Crouchet E, Baumert TF. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020;9:875. [Crossref] [PubMed]
- Lee HL, Bae SH, Jang B, et al. Reactivation of Hepatitis C Virus and Its Clinical Outcomes in Patients Treated with Systemic Chemotherapy or Immunosuppressive Therapy. Gut Liver 2017;11:870-7. [Crossref] [PubMed]
- Torres HA, Hosry J, Mahale P, et al. Hepatitis C virus reactivation in patients receiving cancer treatment: A prospective observational study. Hepatology 2018;67:36-47. [Crossref] [PubMed]
- Tapia Rico G, Chan MM, Loo KF. The safety and efficacy of immune checkpoint inhibitors in patients with advanced cancers and pre-existing chronic viral infections (Hepatitis B/C, HIV): A review of the available evidence. Cancer Treat Rev 2020;86:102011. [Crossref] [PubMed]
- Mahale P, Kontoyiannis DP, Chemaly RF, et al. Acute exacerbation and reactivation of chronic hepatitis C virus infection in cancer patients. J Hepatol 2012;57:1177-85. [Crossref] [PubMed]
- Ramsey SD, Unger JM, Baker LH, et al. Prevalence of Hepatitis B Virus, Hepatitis C Virus, and HIV Infection Among Patients With Newly Diagnosed Cancer From Academic and Community Oncology Practices. JAMA Oncol 2019;5:497-505. [Crossref] [PubMed]
- Pu D, Yin L, Zhou Y, et al. Safety and efficacy of immune checkpoint inhibitors in patients with HBV/HCV infection and advanced-stage cancer: A systematic review. Medicine (Baltimore) 2020;99:e19013. [Crossref] [PubMed]
- Ruf B, Heinrich B, Greten TF. Immunobiology and immunotherapy of HCC: spotlight on innate and innate-like immune cells. Cell Mol Immunol 2021;18:112-27. [Crossref] [PubMed]
- Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol 2022;76:862-73. [Crossref] [PubMed]
- Haber PK, Puigvehí M, Castet F, et al. Evidence-Based Management of Hepatocellular Carcinoma: Systematic Review and Meta-analysis of Randomized Controlled Trials (2002-2020). Gastroenterology 2021;161:879-98. [Crossref] [PubMed]
- Verset G, Borbath I, Karwal M, et al. Pembrolizumab Monotherapy for Previously Untreated Advanced Hepatocellular Carcinoma: Data from the Open-Label, Phase II KEYNOTE-224 Trial. Clin Cancer Res 2022;28:2547-54. [Crossref] [PubMed]
- Kudo M, Finn RS, Edeline J, et al. Updated efficacy and safety of KEYNOTE-224: a phase II study of pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. Eur J Cancer 2022;167:1-12. [Crossref] [PubMed]
- Abou-Alfa GK, Lau G, Kudo M, et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid 2022;1:EVIDoa2100070.
- Ho WJ, Danilova L, Lim SJ, et al. Viral status, immune microenvironment and immunological response to checkpoint inhibitors in hepatocellular carcinoma. J Immunother Cancer 2020;8:e000394. [Crossref] [PubMed]
- Ravi S, Spencer K, Ruisi M, et al. Ipilimumab administration for advanced melanoma in patients with pre-existing Hepatitis B or C infection: a multicenter, retrospective case series. J Immunother Cancer 2014;2:33. [Crossref] [PubMed]
- Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013;59:81-8. [Crossref] [PubMed]
- Bodsworth NJ, Cooper DA, Donovan B. The influence of human immunodeficiency virus type 1 infection on the development of the hepatitis B virus carrier state. J Infect Dis 1991;163:1138-40. [Crossref] [PubMed]
- Kim MK, Ahn JH, Kim SB, et al. Hepatitis B reactivation during adjuvant anthracycline-based chemotherapy in patients with breast cancer: a single institution's experience. Korean J Intern Med 2007;22:237-43. [Crossref] [PubMed]
- Lau GK, Yiu HH, Fong DY, et al. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology 2003;125:1742-9. [Crossref] [PubMed]
- Huang YH, Hsiao LT, Hong YC, et al. Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol 2013;31:2765-72. [Crossref] [PubMed]
- Raimondo G, Pollicino T, Cacciola I, et al. Occult hepatitis B virus infection. J Hepatol 2007;46:160-70. [Crossref] [PubMed]
- Tavakolpour S, Alavian SM, Sali S, Hepatitis B. Reactivation During Immunosuppressive Therapy or Cancer Chemotherapy, Management, and Prevention: A Comprehensive Review-Screened. Hepat Mon 2016;16:e35810. [Crossref] [PubMed]
- Jang JW, Choi JY, Bae SH, et al. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemo-lipiodolization. Hepatology 2006;43:233-40. [Crossref] [PubMed]
- Hsu C, Hsiung CA, Su IJ, et al. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin's lymphoma: a randomized trial. Hepatology 2008;47:844-53. [Crossref] [PubMed]
- Li H, Zhang HM, Chen LF, et al. Prophylactic lamivudine to improve the outcome of HBsAg-positive lymphoma patients during chemotherapy: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol 2015;39:80-92. [Crossref] [PubMed]
- Ghany MG, Morgan TR, Hepatitis C. Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology 2020;71:686-721. [Crossref] [PubMed]
- Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep 2013;62:362-5. [PubMed]
- Carrat F, Fontaine H, Dorival C, et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet 2019;393:1453-64. [Crossref] [PubMed]
- Martinello M, Hajarizadeh B, Grebely J, et al. Management of acute HCV infection in the era of direct-acting antiviral therapy. Nat Rev Gastroenterol Hepatol 2018;15:412-24. [Crossref] [PubMed]
- Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, et al. Oral Direct-Acting Agent Therapy for Hepatitis C Virus Infection: A Systematic Review. Ann Intern Med 2017;166:637-48. [Crossref] [PubMed]
- Curry MP, O'Leary JG, Bzowej N, et al. Sofosbuvir and Velpatasvir for HCV in Patients with Decompensated Cirrhosis. N Engl J Med 2015;373:2618-28. [Crossref] [PubMed]


