Initial assessment of patients without cognitive failure admitted to palliative care: a validation study
Introduction
The transition to palliative care may be a stressful experience for patients and their families. When patients contact palliative care for the first time they may be anxious and insecure. The condition of individual patients can be very different with regard to cognitive capacity, consciousness, performance status and symptom burden. Besides the problems that the patients may have, there is also the workload of a busy service where time must be managed carefully. Patients with cognitive failure must be assessed differently from patients with communication capacity. Although many assessment tools exist for palliative care, they are very different from each other in the items they include and in their extent. Their extent may include a large number of items, such as the Problems and Needs in Palliative Care Questionnaire that included 138 items in various dimensions (1), an intermediate number such as the Memorial Symptom Assessment Scale with 32 items (2), and a small number of items such as the Edmonton Symptom Assessment System (ESAS) with 10 items (3). Some tools with a large or intermediate number of items have been shortened to make them more suitable for everyday use, such as the Problems and Needs in Palliative Care Questionnaire—short version (4) or the Condensed Memorial Symptom Assessment Scale (5). However, since multidimensional tools are usually too long and short ones are not multidimensional, none of the existing tools completely fulfilled our aim of concisely and accurately detecting patients’ problems, in various dimensions, at the first encounter with a palliative care team.
Focusing on patients without cognitive failure, a tool for the initial assessment of patients admitted to palliative care was developed through a Delphi consensus (6). From the initial 106 items in six domains and two general questions, that consensus resulted in a list of 14 problems in four domains and two general questions. The domains were physical, psychological, social and activity level. The initial spiritual and financial domains were excluded from the tool, possibly not because they were deemed minor dimensions, but because experts considered that, at the first encounter, there is no enough intimacy to address those issues, leaving them to later assessments.
After the first phase of selecting the items to be included in the tool, other phases of validation must follow. In a second phase, the tool was submitted to a factorial analysis which is described in this article.
Methods
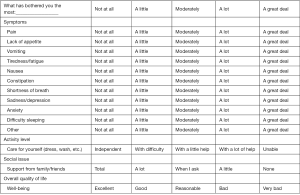
A 5-point verbal scale was added to the items selected in the first study (6) and adapted to the different kind of items, as shown in Figure S1. The tool started to be used as the assessment method of patients without cognitive failure or problems of communication admitted to our palliative care service since May 2011. Since then we decide to evaluate its utility as the usual practice of the service. That evaluation was carried out until October 2014. The researchers were the doctors and one nurse of the service who used the tool in the patients who were able to understand the assessment process when they were admitted to our palliative care service.
To investigate the pattern underlying the experience of symptoms, a principal components analysis was performed to detect independent domains (factors), based on the inter-relation between items. The factor solution was chosen based on the eigenvalue (>1) criterion. Cronbach’s alpha coefficient and item-total correlations were calculated to evaluate the internal reliability of the scale as a whole and of each subscale, determined by the factors obtained.
A final score was calculated for each patient, by adding up the points attributed to each item, and transforming the value obtained to a scale of 0 to 100. The non-existence of a gold standard tool precluded a criterion validation. The construct validity was assessed by testing whether patients with the highest scores had the shortest survival time, using a Cox regression model and the Wald test. SPSS was used for all analyses conducted and α=0.05 was used as the significance level.
As the tool was and still is used as the usual practice of the service and the study intended to evaluate that practice it was not submitted to the ethics committee of the hospital. For the same reason informed consent was not obtained from the patients. However, this study was approved by the board of directors of Institution.
Results
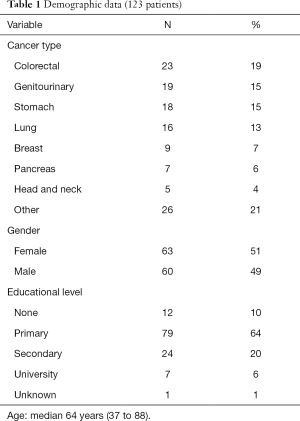
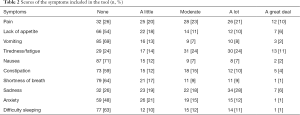
In the period under study, the tool was used with 123 patients; 60 (49%) of them were male and 63 (51%) were female, with a median age of 64 (37 to 88). Diagnoses and educational levels can be seen in Table 1. The scores for the various items are shown in Tables 2-5. The patients’ opinions on how difficult it was to reply to the questionnaire were: not at all—77 (63%); somewhat—24 (20%); very—7 (6%); unknown—15 (12%). The median survival time was 19 days (1 to 428).
Full table
Full table

Full table

Full table

Full table
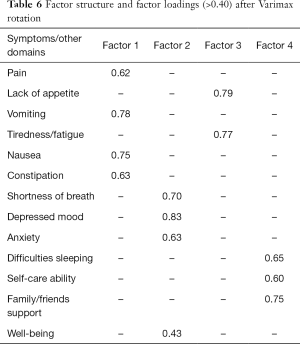
No floor or ceiling effects were found in the items evaluated. A four-factor structure was found through the principal components analysis, explaining 60.1% of the total variance. The factor loadings of the symptoms on these factors are given in Table 6.
Full table
The first factor explained 24.3% of the variance and included the items “pain”, “vomiting”, “nausea” and “constipation”, reflecting a physical dimension which relates to the main gastrointestinal complaints. The second factor explained 15.9% of the variance and included the anxiety-related symptoms “shortness of breath”, “depressed mood”, “anxiety” and “well-being”. The third factor explained 10.5% of the variance and was related to the items “lack of appetite” and “tiredness/fatigue”. The fourth factor explained 9.4% of the variance, including the items “difficulties sleeping”, “self-care ability” and “family/friends support”.
The scale presented good reliability, with a Cronbach’s alpha of 0.72. For each of the four subscales, Cronbach’s alpha was 0.67, 0.65, 0.63 and 0.42. In each factor, items presented item-total correlations close to or higher than 0.40, except in the fourth factor, which presented lower values (between 0.22 and 0.29).
The hypothesis under study for the validity of construct was supported by the statistical analysis performed. A hazard ratio of 1.016 (P=0.019) was obtained in the Cox regression model including the final score as an explanatory variable of survival time, which means that for each increment of 1% in the total score, there was an increased risk of death of 1.6%.
Discussion
After the number of items was reduced using the Delphi method, a manageable number of symptoms/problems was reached (6). However, the study of an assessment tool is an endless task. For example, in a study on 15 years of validation studies on the ESAS, the authors concluded that there was a need for further validation studies (7). With respect to the present tool, after the item selection study, this is the first study that has been performed to see how this tool works in practice. The initial choices were to carry out a factorial analysis and a validity of construct.
Validity of criterion could not be tested as there is no gold standard that the scores obtained in this study can be compared to. Age, sex and education were not used to evaluate validity of construct because they are not expected to yield different symptom scores among palliative care patients. As such, and given that all patients were deceased prior to May 2015, survival time was considered the most adequate variable to provide information on validity of construct. The results confirmed the hypothesis that patients with the highest scores had the shortest survival time. This relationship was also seen in other studies (8). Although the association between symptom burden and survival was evaluated for construct validation, this tool should not be used for prognostication, but rather for an initial assessment of patients admitted to palliative care.
Although the educational level of most patients was quite low, the majority of them had no difficulty in replying to the questionnaire. This is a very important topic because it means that it is clear, which contributes to the reliability of the answers given.
The scale attached to each symptom did not ask about the intensity but rather about the degree of disturbance they caused. There are other tools whose focus is the impact of the symptom rather than its intensity, as in the Rotterdam Symptom Checklist (9). Other tools check the intensity and the impact of each symptom, like The Memorial Symptom Assessment Scale (2). After all, in palliative care, the most important aspect is the degree of disturbance caused by the particular symptoms. Therefore, the comparisons with tools that measure only the intensity may not be accurate. Nevertheless, when comparing the frequency of the symptoms in this study with their frequency in another study that we carried out at national level (10), it can be seen that fatigue, sadness, anxiety, anorexia and pain are the most frequent symptoms in both studies. Pain is a more important symptom in the present study, ranking in second place together with sadness, while pain was ranked in fifth place in the other study.
Only a small percentage of patients lack support from their families or friends. Most patients have a high level of dependency, as was also seen in the previous study (11). However, most see their well-being at least as reasonable, meaning that many patients have a positive attitude regardless of the many difficulties they face.
This tool has some similarities with the ESAS, but has also important differences. Our tool includes dimensions that the ESAS does not, namely the social dimension and the activity level, as well as symptoms such as vomiting, constipation, and difficulty in sleeping. The ESAS does not include also the open question “what bothers you the most?” The differences can be explained by the different aims of the tools: this tool was developed for the initial assessment of patients admitted to palliative care and the ESAS for the daily assessment of patients.
At this time the tool was only validated in cancer patients, but it would be interesting to validate it in non-malignant advanced diseases. This tool is in accordance with the recommended characteristics that an assessment tool should have (12). It is simple to administer and easy to explain, complete and analyze. However, this tool should be seen as a screening tool, a starting point for the impeccable assessment and treatment of the problems that palliative care patients may have, as the World Health Organization (WHO) recommends (13). Many other problems can arise in each patient, but this tool seems to be a good assessment basis.
Acknowledgements
This work was supported in part by the North Section of the Portuguese League against Cancer.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Osse BH, Vernooij MJ, Schadé E, et al. Towards a new clinical tool for needs assessment in the palliative care of cancer patients: the PNPC instrument. J Pain Symptom Manage 2004;28:329-41. [Crossref] [PubMed]
- Portenoy RK, Thaler HT, Kornblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer 1994;30A:1326-36. [Crossref] [PubMed]
- Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 1991;7:6-9. [PubMed]
- Osse BH, Vernooij-Dassen MJ, Schadé E, et al. A practical instrument to explore patients' needs in palliative care: the Problems and Needs in Palliative Care questionnaire—short version.
- Chang VT, Hwang SS, Kasimis B, et al. Shorter symptom assessment instruments: the Condensed Memorial Symptom Assessment Scale (CMSAS). Cancer Invest 2004;22:526-36. [Crossref] [PubMed]
- Gonçalves F. Initial assessment of patients without cognitive failure admitted in palliative care. Am J Hosp Palliat Care 2014;31:33-7. [Crossref] [PubMed]
- Nekolaichuk C, Watanabe S, Beaumont C. The Edmonton Symptom Assessment System: a 15-year retrospective review of validation studies (1991--2006). Palliat Med 2008;22:111-22. [Crossref] [PubMed]
- Mohan A, Goyal A, Singh P, et al. Survival in small cell lung cancer in India: prognostic utility of clinical features, laboratory parameters and response to treatment. Indian J Cancer 2006;43:67-74. [PubMed]
- de Haes JC, Olschewski M, Fayers P, et al. The Rotterdam Symptom Checklist (RSCL). Northern Centre for Health Care Research, University of Groningen, The Netherlands,1996.
- Gonçalves F, Almeida A, Antunes C, et al. Symptoms other than pain in palliative care in Portugal. Am J Hosp Palliat Care 2015;32:335-40. [Crossref] [PubMed]
- Gonçalves F, Almeida A, Antunes C, et al. A cross-sectional survey of the activity of palliative care teams in Portugal. Am J Hosp Palliat Care 2013;30:648-51. [Crossref] [PubMed]
- Donnelly S, Walsh D. Quality of life assessment in advanced cancer. Palliat Med 1996;10:275-83. [Crossref] [PubMed]
- WHO Definition of Palliative Care. Available online: www.who.int/cancer/palliative/definition/en.