
Hepatocellular carcinoma and sarcopenia: a narrative review※
Introduction
Background
In 2020, an estimated 905,700 people globally were diagnosed with hepatocellular carcinoma (HCC) and 830,200 individuals died (1) from the illness. As the third most common cause of cancer death worldwide, several risk factors account for HCC prevalence, including chronic infections such as hepatitis B virus (HBV) or hepatitis C virus (HCV), toxins such as alcohol and nonalcoholic fatty liver disease (NAFLD). Most individuals diagnosed with HCC have some evidence of cirrhosis, caused by exposure to risk factors (2). NAFLD, cirrhosis and HCC are associated with a higher prevalence of sarcopenia, a condition characterized by loss of muscle mass and diminished physical performance.
Rationale and knowledge gap
By identifying sarcopenia early and modifying exacerbating factors, clinicians could improve symptom burden, quality of life (QoL) and patients’ ability to tolerate anti-neoplastic therapy in HCC. We believe a review of the literature is necessary given the high prevalence of sarcopenia in HCC and CLD and the potential for improving clinical outcomes.
Both HCC and sarcopenia are associated with a chronic pro-inflammatory response that plays a prominent role in disease progression. The pro-inflammatory response typically found in patients with solid tumors is thought to drive a variable combination of anorexia, fatigue, and muscle wasting.
A meta-analysis of 57 studies involving 9,790 HCC patients (3) reported a sarcopenia pooled prevalence of 41.7% (range, 11.1% to 78.3%). Another meta-analysis revealed significant differences in the pooled prevalence depending on the methods for sarcopenia assessment and geographic location (Asia, Europe, or North America) (4). Differences in prevalence also depend on the illness trajectory. Overall, for solid tumors the prevalence of sarcopenia was 39.6% in the curative, compared to 49.2% in the palliative setting (P<0.001). In HCC the difference in prevalence between curative and palliative settings was narrower (35.4% vs. 38.2%) (5).
The objective of this narrative review is to provide clinicians with updated information to better identify, assess, and manage patients with HCC and sarcopenia. Given there is no Food and Drug Administration (FDA) approved agent for sarcopenia in cancer, an interdisciplinary multimodal approach that includes a combination of pharmacologic and non-pharmacologic therapies is emphasized. We present this article in accordance with the Narrative Review reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-23-332/rc).
Methods (Figure 1)
A literature search used PubMed as the primary database. Keywords included ‘hepatocellular carcinoma’, ‘chronic liver disease’, ‘liver cirrhosis’, ‘hepatitis’, ‘fatty liver disease’, sarcopenia’, ‘cachexia’, ‘anorexia’ in various combinations (Table 1). PubMed searches were filtered to yield articles published between 2018 and 2023. Additional articles were included after reviewing the citations of articles identified by the primary search. Older articles were included if the authors were of the opinion they were particularly important to the field.
Table 1
| Items | Specification |
|---|---|
| Date of search | January 25th 2023 |
| Databases and other sources searched | PubMed |
| Search terms used | ‘Hepatocellular carcinoma’, ‘chronic liver disease’, ‘liver cirrhosis’, ‘hepatitis’, ‘fatty liver disease’, sarcopenia’, ‘cachexia’, ‘anorexia’ |
| Timeframe | 2018–2023 |
| Inclusion criteria | Studies in English or with English translations |
| Selection process | Revoredo S and Del Fabbro E conducted the selection |
| Any additional considerations, if applicable | Additional articles were obtained after reviewing references of primarily obtained articles |
Definitions and diagnosis
Muscle wasting is common in patients with cancer and is a key component of several conditions sometimes found in the same individual, particularly in older patients. These conditions include sarcopenia, cachexia, and frailty (6). The detrimental role of excess adipose tissue in combination with muscle wasting [sarcopenic obesity (SO)] and the infiltration of fat into muscle (myosteatosis) needs to be considered in patients with muscle loss.
Sarcopenia: dual X-ray absorptiometry (DXA) or computerized tomography (CT) scan with cut-offs depending on variables such as race and sex, is a clinical diagnosis that includes loss of muscle mass (measured by imaging) and function typically occurring due to aging but may also occur because of another illness such as cancer (secondary sarcopenia).
In 2018, the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) updated the consensus definition of sarcopenia (7) to include low muscle strength as the key characteristic of sarcopenia, with low muscle quantity and quality confirming the diagnosis, and poor physical performance indicating severe sarcopenia. The EWGSOP updated their clinical algorithm for sarcopenia (Find-Assess-Confirm-Severity or F-A-C-S) and provided clinically relevant cut-off points for the variables that characterize sarcopenia, including cut-offs for physical performance. The EWGSOP2 recommends the SARC-F, a 5-item self-reported questionnaire as a screen for sarcopenia risk (8) and finding appropriate patients. The SARC-F is validated and reports on strength, walking, ability to rise from a chair, climb stairs, and frequency of falls. In a study of 256 oncology patients one third screened positive for sarcopenia using the SARC-F (9). Those with sarcopenia had higher rates of impairment, lower QoL, and increased mortality.
Thereafter, assessment of muscle strength is recommended either by hand grip strength (HGS) (using a dynamometer) or the chair stand test (time needed to rise five times from a seated position without a patient using their arms). HGS is relatively easy to measure and correlates moderately well with other muscle compartments (10). The Foundations for the National Institutes of Health (FNIH) Biomarkers Consortium has estimated cut-off points for weakness (grip strength <26 kg for men and <16 kg for women) that are similar to EWGSOP2 (11). The importance of HGS and decreased muscle mass was underscored in a study of 234 patients undergoing liver resection for malignant tumors. Compared with patients in the other groups, those with both decreased HGS and lower muscle mass experienced a longer hospital stay (P<0.001), and more frequent readmission to hospital (P=0.02) (12).
Muscle quantity or mass is confirmed by a variety of techniques, each with their advantages and limitations. DXA or CT scan with cut-offs depending on variables such as race and sex, are used most often. Magnetic resonance imaging (MRI) is also able to accurately assess sarcopenia in chronic live disease including an abbreviated MRI protocol without the need for contrast injection (13). DXA rapidly measures appendicular muscle mass with low radiation. In imaging studies with DXA, sarcopenia refers to appendicular skeletal muscle mass divided by body height squared in meters (skeletal muscle mass index) two standard deviations or more below reference values from young, healthy individuals. Cut-offs for appendicular lean mass by DXA adjusted for body mass index (BMI) (<0.789 for men and <0.512 for women) are similar for FNIH and EWGSOP2. Regional analysis of fat and fat-free tissue at the 3rd lumbar vertebra with CT strongly predicts whole-body fat and fat-free mass. This allows for the opportunistic, accurate evaluation of body composition when patients undergo CT scans for diagnosis or re-staging (14). Although most studies analyze body composition at the 3rd lumbar vertebra, a preliminary report in patients with HCC showed CT measurements of the psoas muscle were significant prognostic factors for overall survival (OS) and progression-free survival (PFS) (15).
Other methods for evaluation of body composition such as electrical bioimpedance (BIA) are less accurate but have no radiation risk and are inexpensive by comparison. In a systematic review of BIA, 24 studies were selected for inclusion in the review (total number of 3,607 patients). In five studies, BIA was rated comparable to axial CT scan, calf circumference, or grip strength for sarcopenia screening, while in 14 studies, BIA-identified sarcopenia was associated with adverse clinical outcomes (16). Furthermore, the measurement of phase angle by BIA is a predictive marker for hospitalization, falls and mortality in outpatients with cirrhosis (17).
A review found ultrasound (US) to be a simple and reliable non-invasive diagnostic tool for sarcopenia in liver disease. Most of the 10 studies used large muscles of the upper and lower limbs as anatomical landmarks, half included patients with HCC (18), and the majority showed a correlation between muscle measurements and clinical outcomes. One study of HCC patients showed a significant correlation between skeletal muscle index (SMI) and the subcutaneous fat of lower limbs, using US (19). The absence of an ideal reference standard, not allowing a clear comparison among the studies, was the main limitation identified.
The EWGSOP2 suggests calf circumference measures may be used as a diagnostic proxy for older adults in settings where no other muscle mass diagnostic methods are available.
Once sarcopenia is confirmed by one of the methods outlined above, physical performance tests are needed to evaluate the severity of sarcopenia.
A recent meta-analysis reported a prevalence for severe sarcopenia ranging from 2–9% depending on region and classification system used (20). Tests of severity included but were not limited to gait speed, the Short Physical Performance Battery (SPPB), and the Timed-Up and Go (TUG) test. Tests such as HGS and SPPB are used extensively in the aging population, and their use in oncology research is growing (21). A systematic review found physical performance tests show a significant correlation with survival and could be used as a prognostic tool, particularly for older adult patients. As an example, the SPPB, a composite test that includes assessment of gait speed, a balance test, and a chair stand test is predictive of important clinical outcomes including mortality and health care utilization (22). A SPPB score <10 is predictive of decreased OS in older patients with Leukemia (23), increased post-operative complications (24), and adverse events with lower chemo completion in non-small cell lung cancer (NSCLC) (25).
A study of inpatients with liver cirrhosis found the SPPB was better at assessing function than the handgrip test and a low SPPB score was associated with adverse outcomes such as re-admission and mortality (26).
A single diagnostic for sarcopenia and adherence to published guidelines should be a future priority in order to lessen the heterogeneity between studies.
SO is a combination of reduced lean body mass (LBM) and excess adiposity. The risk and prevalence for SO increases with age (27) and varies with definitions, and cut-offs (28). Myosteatosis, the infiltration of muscle by fat, is an indicator of diminished muscle quality and is associated with decreased strength and shorter survival in cancer. Specifically in HCC, a retrospective analysis of patients with advanced HCC on immunotherapy, found sarcopenia and myosteatosis were independent poor prognostic factors (29).
Mechanisms contributing to sarcopenia (Figure 2)
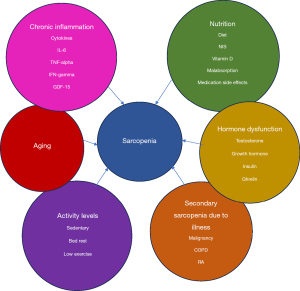
Primary sarcopenia is a result of adverse muscle changes that accumulate over a lifetime. In addition to the effects of aging, many older individuals have comorbidities or lifestyles that contribute to low muscle mass, termed ‘secondary sarcopenia’. Examples include malignancy, chronic inflammatory disease, organ dysfunction, poor nutrition, and decreased activity such as bed rest. In general, sarcopenia mechanisms can be viewed as disordered protein catabolism and anabolism, resulting in decreased protein synthesis and increased protein degradation. Potential therapeutic targets are noted in this section on mechanisms, however, these mechanisms should not be viewed in isolation since they are inter-related, e.g., inflammation may contribute to hormone resistance and autonomic dysfunction.
CLD may accompany or precede HCC and share similar mechanisms contributing to sarcopenia. The relationship between CLD and sarcopenia is complex. For example, sarcopenia is associated with non-alcoholic steatohepatitis and significant fibrosis, independent of obesity, inflammation, and insulin resistance (30). However, it is unclear whether sarcopenia is a risk factor for disease progression of NAFLD, or a complication of NAFLD due to worsening liver disease. There is also evidence that NAFLD and sarcopenia share a pathophysiological pathway along the muscle-liver-adipose axis and that a bidirectional relationship may exist whereby NAFLD contributes to sarcopenia development (31).
Muscle loss stimulates a pathophysiological pathway, along the:
- Muscle-liver-adipose tissue axis, producing insulin-resistance, decreased glycogenesis, increased lipolysis, and decreased protein synthesis. Accumulation of adipose tissue within the liver is associated with chronic low-grade inflammation mediated by adipokines and proinflammatory cytokines. In addition, altered myokine levels from decreased skeletal muscle mass promote hepatic fat accumulation and disease progression. Although muscle mass is a key determinant of whole-body insulin-mediated glucose metabolism and impacts fatty liver oxidation and energy homeostasis, the accumulation of ectopic fat in the liver and in the muscle (32) (myosteatosis) seems to be more closely associated with liver injury than loss of muscle mass.
Several mechanisms contribute to sarcopenia along the muscle-liver-adipose tissue axis, including pro-inflammatory cytokine activation, endocrine dysfunction, hyperammonemia, and autonomic nervous system dysfunction. - Hyperammonemia may be a key mediator of sarcopenia in both NAFLD and cirrhosis through mitochondrial dysfunction, increased myostatin expression and cognitive dysfunction (33). In addition, hyperammonemia is associated with elevated reactive oxygen species (34) and increased proteolysis (35) via autophagy.
- Aging is also associated with increased muscle denervation and alterations in neuromuscular junctions (36), atrophy of type IIa muscle fibers, decreased mitochondrial content and increased lipid content of type1 fibers (37). Additionally, aging is associated with decreased circulating anabolic steroids including testosterone (38) and growth hormone (GH) (39) and increased activation of the myostatin-activin A pathway (40), which modulates muscle catabolism and insulin resistance.
- Ghrelin, an endogenous brain-gut peptide is predominantly secreted by the stomach, distributed in many other tissues, and has a key role in appetite regulation (41). The administration of Ghrelin to animal models of NAFLD has beneficial effects, including amelioration of liver injury by modulating mechanisms such as dysregulated hepatic lipid metabolism, oxidative stress, apoptosis, and inflammation (42). Anamorelin, a ghrelin receptor agonist, stimulates appetite while also decreasing pro-inflammatory cytokines thought to mediate cachexia and sarcopenia (43). A post hoc analysis of a randomized controlled trial (RCT) leading to anamorelin’s approval for cancer cachexia in Japan, found LBM improved from baseline to week 12 significantly with anamorelin compared to placebo (44).
- Pro-inflammatory cytokines such as interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α) and interferon (IFN) gamma are associated with the pathogenesis of sarcopenia. IL-6 increases the production of ubiquitin and E3 ubiquitin ligase proteins leading to increased, possibly preferential ubiquination of the myosin heavy chain, with dissociation of myosin from the contractile apparatus and subsequent degradation into peptides by the proteasomes. The combination of TNF-α and IFN gamma is thought to suppress the nuclear transcription factor MyoD thereby decreasing transcription of the myosin heavy chain (45). In addition, cytokines involved in the pathogenesis of sarcopenia such as adiponectin, leptin, IL-6, IL-8, IL-10, TNF, soluble receptor of TNF (sTNFr)-1 and sTNFr-2 may serve as potential biomarkers to diagnose and determine the severity of sarcopenia (46).
- Growth differentiation factor-15 (GDF-15), a member of the TGF-β superfamily has emerged as a mediator of weight loss and appetite suppression. In the context of cancer related sarcopenia, GDF-15 is believed to induce muscle apoptosis via the Bcl2-/caspase 3 pathway (47). Overexpression of GDF-15 following activation of the mTOR1 signaling is also associated with aging and sarcopenia (48), supporting findings that GDF-15 tends to increase with age and with decreased muscle mass. GDF-15 administration in animal models enhances energy expenditure, inducing weight loss (49) and decreases NAFLD. Plasma GDF15 levels are associated with intrahepatic fat content, increased in youth with NAFLD and therefore have potential as a biomarker for NAFLD (50). A phase II clinical trial using GDF-15 as both marker and therapeutic target for the GDF-15 inhibitor, Ponsegromab is underway in cachectic patients with advanced lung, pancreatic and colon cancer (51) (HCC patients are ineligible). While it is anticipated that participants may gain weight, the effect on muscle mass and function is uncertain.
- Autonomic dysfunction and activation of the sympathetic nervous system is common in patients with advanced cancer, leading to symptoms such as gastroparesis and hypermetabolism. The therapeutic role of beta-adrenergic receptor blockers has long been of interest in modulating sarcopenia (52). In a phase II clinical trial, espindolol, a nonselective beta-receptor antagonist, partial beta-2 receptor agonist, and 5-HT1A receptor antagonist, promoted weight gain, and improved HGS in patients with NSCLC and colorectal cancer (53).
- Alteration of the gut microbiome is an emerging mechanism identified in cirrhosis, promoting sarcopenia and increased protein and amino acid metabolism (54). Excess alcohol use, a common cause of liver cirrhosis (55) is also associated with alterations of the gut microbiome and overall decreased protein synthesis.
- Anti-neoplastic therapies may also contribute to sarcopenia. Tyrosine kinase inhibitors (TKIs) have a proven role in treating HCC, however, they may exacerbate muscle loss. In one study, 54 out of 67 patients had decreased muscle mass 1–3 months after receiving either sorafenib or lenvatinib therapy for HCC, regardless of tumor progression (56). A meta-analysis that included four studies of TKI therapy for HCC, reported poorer prognosis with sarcopenia vs. non-sarcopenia patients (57). In addition, based on a meta-analysis of 48 studies with a variety of malignancies, the association between treatment toxicity and low skeletal muscle mass, is stronger in kinase inhibitors than conventional chemotherapy or check point inhibitors (58). A multicenter Japanese group has developed ‘the management of sorafenib score (MS score)’ to stratify patients’ survival according to three parameters (skeletal muscle mass, disease control of sorafenib, and post-sorafenib therapy). Patients with an MS score ≥2 showed longer survival than those with an MS score ≤1 (16.4 vs. 8.4 months; P<0.001) (59).
Sarcopenia and prognosis in HCC
Several prognostic scoring systems exist for patients with HCC; however, sarcopenia provides additional independent prognostic information.
Cirrhosis and HCC are associated with poorer clinical outcomes when sarcopenia is present, and interestingly, sarcopenia may increase the risk of cirrhosis transitioning to HCC. In a study of 492 patients with cirrhosis and no evidence of HCC at baseline, male patients with sarcopenia had a significantly higher risk of developing HCC (60).
A meta-analysis found sarcopenia was highly associated with risk of mortality in patients with cirrhosis (61). Sarcopenia also increases risk for complications in cirrhosis, such as hepatic encephalopathy and sepsis, and is associated with worse outcomes after liver transplantation, including increased length of stay, dependence on mechanical ventilation, organ injury, and post-operative mortality (62). Sarcopenia assessed radiologically by musculature at the level of the third lumbar vertebra can be combined with the Model for End-Stage Liver Disease score (MELD) to improve the prognostic model in patients with HCC awaiting transplantation (63). Furthermore, the combination of sarcopenia and obesity (as measured by visceral obesity on CT scan) is associated with increased post-transplant mortality in acutely ill patients with cirrhosis, and remains significant even after adjusting for age, sex, diabetes, encephalopathy, HCC, and MELD (64).
Treatment modalities for HCC are also adversely affected by sarcopenia. A systematic review and meta-analysis of 57 studies and 9,790 patients with HCC found 41.7% of patients had sarcopenia (defined using CT/MRI with various cut-off values). Those with sarcopenia had decreased OS, increased rates of tumor recurrence following hepatectomy or living donor liver transplant, decreased tumor response, and increased rates of drug-related severe adverse events. The study evaluated several treatments including radiofrequency ablation, hepatectomy, intra-arterial therapy, radiotherapy, sorafenib, levantinib, and immune checkpoint inhibitors. Most forms of therapy demonstrated decreased survival with the presence of sarcopenia. The presence of cirrhosis and Child Pugh class B increased the hazard of mortality from sarcopenia, suggesting an additive or synergistic effect of HCC and cirrhosis in patients with sarcopenia (3).
A multi-center study of more than 1172 patients undergoing hepatic resection for HCC, compared ‘Textbook Outcomes’ (TO) between patients with sarcopenia (29%) and non-sarcopenia (43%). TO were defined as no 30-day morality, no 30-day readmission, negative margins, no prolonged hospital stay, and no major complications. Sarcopenia was an independent predictor of TO (P<0.001). These positive short term post-operative outcomes were in turn, independent predictors of OS and recurrence-free survival (P<0.001) (65). A prospective study in 171 non-cirrhosis liver cancer patients determined the relationship between the combined measures of muscle mass (measured by pre-operative CT scan) and muscle strength (measured by handgrip) and clinical outcomes (major complications and 90-d readmission rate). Patients with both low muscle mass and decreased strength had significantly higher postoperative complications, 90-day readmission rate (21.7%, P=0.037) and hospitalization costs than other groups (66).
In HCC treated with a programmed death receptor-1 (PD-1) inhibitor, higher levels of inflammation, lower albumin and a shorter PFS are found in patients with sarcopenia than those without sarcopenia (67). In 138 patients with advanced HCC (>80% with extra-hepatic metastasis) patients with sarcopenia had poorer PFS and OS (P=0.002) than those without sarcopenia. In addition, patients with myosteatosis exhibited poorer PFS and OS (P<0.001) than those without myosteatosis (29). The value of measuring myosteatosis is supported by another study of 611 patients undergoing transarterial chemoembolization (TACE) at a tertiary care center. Although there was no difference in TACE response between sarcopenia and non-sarcopenia patients, myosteatosis was associated with poor TACE response and reduced survival (15.9 vs. 27.1 months, P<0.001) (68).
Sarcopenia combined with markers of inflammation/nutrition
Several markers of nutrition status and inflammation have been combined with sarcopenia in attempts to improve prognostication for CLD and HCC. Caution is required in attributing poor clinical outcomes directly to the combination of inflammation and sarcopenia because inflammatory markers such as C-reactive protein (CRP) may also reflect HCC growth and invasiveness. The lymphocyte-monocyte ratio (LMR) is reported to be an independent risk factor for OS and recurrence free survival in HCC patients after hepatectomy (69). The prognostic nutritional index (PNI), a marker of nutritional status and inflammation, is calculated using albumin and lymphocyte count. When combined, PNI and sarcopenia are stronger prognostic factors for postoperative complications in patients with HCC than either alone (70).
Treatment
Given the multifactorial nature of sarcopenia, therapeutic interventions should be individualized and address the many underlying causes pertinent to each patient. Most professional organizations endorse a multimodal approach. For example, the Clinical Oncology Society of Australia (COSA) recommends management of sarcopenia include nutritional therapy, exercise and physical activity, plus physical and psychological symptom management. The European Society for Parenteral and Enteral Nutrition (ESPEN) recommend exercise and nutritional counseling. No pharmacological intervention has been approved, although replacement therapy (testosterone and vitamin D) may be helpful.
Pharmacologic therapy
While there is no single agent approved for sarcopenia or cancer cachexia, several promising therapies reported improved outcomes in clinical trials.
The purported mechanisms for modulating muscle mass in oncology patients by drugs such as anamorelin, espindolol and a GDF-15 inhibitor, were discussed earlier in this review. Since anamorelin is approved for cancer cachexia in Japan and increases weight LBM and appetite, this oral medication has the greatest potential to be available more widely for wasting conditions. However, as regards its potential therapeutic benefit for sarcopenia specifically, caution should be exercised because anamorelin did not improve HGS in a large international RCT, despite significant improvement in LBM (71).
A recent review focused on pharmacologic interventions for sarcopenia included testosterone, selective androgen receptor modulators (SARMs), estrogen, dehydroepiandrosterone (DHEA), insulin-like growth factor-1 (IGF-1), GH, GH secretagogue (GHS), drugs targeting myostatin and activin receptor pathway, vitamin D, angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs), or β-blockers. The review concluded that some drugs have been effective in improving muscle mass, but unfortunately this was not accompanied by clinically relevant improvements in physical performance (72).
Other promising drugs noted in pre-clinical development include metformin, exerkines (73) (IL-6, TNF-α, IL-15, fibroblast growth factor 21, irisin, apelin) or senolytics (dasatinib and quercetin, ruxolitinib). Agents currently available to clinicians and shown to be safe include testosterone and vitamin D.
Androgens
An umbrella review of meta-analyses concluded testosterone replacement can be justified in daily practice for older men with muscle weakness (74). Another meta-analysis reported anabolic androgens combined with exercise may enhance outcomes (75). Although no trials have specifically evaluated the effect of androgens on sarcopenia in patients with HCC, there is evidence testosterone replacement therapy improves outcomes in men with cirrhosis and in men with advanced solid tumors. A 12-month double blind placebo-controlled trial of intramuscular testosterone increased muscle mass, bone mass and hemoglobin with no increase in adverse effects (76). A preliminary double blind placebo-controlled trial in men with advanced solid tumors found intramuscular testosterone improved fatigue scores and performance status compared to placebo injections, by week 10 (77).
Vitamin D
Given the low risk of side-effects or harm, and meta-analyses showing sarcopenic adults have lower blood 25(OH) D concentrations (78), vitamin D supplementation is a reasonable recommendation for older people with low serum levels. Based on meta-analyses vitamin D has a significant effect on muscle strength and physical performance, especially in women with low baseline values (<25 nmol/L). Vitamin D deficiency is common in CLD, associated with increased risk of sarcopenia (79) and poorer prognosis when sarcopenia and severe vitamin D deficiency co-exist (80). The results of clinical trials using vitamin D are inconsistent, in part because vitamin D is often combined with interventions such as exercise or protein supplementation, making it difficult to determine the benefit of vitamin D alone (81). A meta-analysis of vitamin D in combination with whey protein and exercise found benefit for lean muscle mass and physical function (82) while another reported combination therapy with vitamin D supplementation, protein supplementation and exercise significantly increased grip strength and trended toward increasing muscle mass (83). The form of supplementation may also play a role; for example, calcifediol may have a positive effect on muscle strength parameters (with less evidence on physical performance) (84).
Non-pharmacologic therapy
Non-pharmacologic interventions such as exercise (85) and nutrition are key components of multimodal therapy and may be most effective delivered in combination, and by an interdisciplinary team.
Nutrition
In patients with cancer, many older individuals may be consuming insufficient calories and protein. Dieticians should provide individualized counseling with a focus on maintaining adequate calorie and protein intake in patients with HCC or CLD. A study of 320 Canadian patients with advanced cancer and weight loss found the majority consumed diets insufficient to maintain weight even in healthy individuals (86). The study also found higher consumption of protein and energy correlated with greater weight gain. When considering the relationship between nutrition and sarcopenia, macronutrient content may be especially important. A scoping review reported energy dense ‘fat and fish’ diets, are associated with reduced risk of low muscle mass in patients with cancer (87).
While there are no specific recommendations for HCC, ESPEN has practical guidelines for clinical nutrition in liver disease (88). For patients with malnutrition and muscle depletion, ESPEN recommends 30–35 kcal/kg/d and 1.5 g/kg/d of protein and 0.25 g/kg/d of branched chain amino acids (BCAAs) for those who are protein intolerant (i.e., develop encephalopathy). Furthermore, a meta-analysis concluded that a late-evening snack high in protein or carbohydrates improved nitrogen balance and decreased protein and fat catabolism (89).
Exercise
Some professional organizations include exercise in their guidelines for managing cancer-associated sarcopenia, while others such as the American Society for Clinical Oncology (ASCO) have found insufficient evidence for recommendations (90). COSA, ESPEN, and the European Society for Clinical Oncology (ESMO) recommend individualized prescriptions, including resistance exercise in addition to aerobic exercise for maintaining muscle strength and mass (91).
Although there are no direct comparisons between aerobic and resistance exercise in HCC, studies in other solid tumors and hematological malignancies have demonstrated the value of resistance exercise. A meta-analysis of 34 resistance-training trials in patients with cancer or survivors, showed greater LBM increase compared to controls (P=0.004). In prostate cancer (92) younger men and those with lower baseline levels of physical function, derived greater benefits regarding muscle strength and physical function. Although most exercise interventions are aerobic based in patients with liver cirrhosis, a 12-week resistance training program improved muscle size and physical performance with significantly increased 6-minute walk distances (93).
A combination of exercise interventions may be optimal for improving outcomes in HCC. A prospective observational study of patients with HCC and CLD evaluated the effect of rehabilitation on skeletal muscle mass using a combination of stretching, strength, balance, and endurance training. Patients instructed by physical therapists on exercise had increased SMI and improved survival compared to patients not exercising (94). Similarly, individuals undergoing TACE treatment for HCC had increased muscle mass when they completed in-hospital combination exercises for 20–40 min/day (95).
Aerobic exercise is important for outcomes related to sarcopenia and in the elderly, may be indistinguishable from resistance exercise in the ability to prevent sarcopenia (96). Also, perioperative cardiopulmonary function and change in anerobic threshold (the point during exercise at which oxygen demand outstrips oxygen delivery and metabolism starts to become anaerobic) were found to more important than muscle mass for long-term survival in patients undergoing first hepatectomy for HCC (97). Perioperative exercise (stretching and walking) was prescribed based on individual patients’ anaerobic threshold. The authors concluded that exercise involving >5,000 steps per day is necessary for postoperative walking.
Overall, a recent systematic review of diet and/or exercise interventions in cirrhosis of 22 controlled trials and 5 single arm trials showed improvement in body composition, with the largest improvements occurring when diet and exercise interventions were combined (98).
Symptoms
High symptom burden may impact therapy for nutrition and physical performance. Nutrition impact symptoms (NIS) limit an individual’s ability to consume food, exacerbating weight loss. Symptoms such as nausea, depression, and pain are often identified in oncology patients because of their disease or anti-neoplastic treatment side-effects (99). The opportunity for improving NIS and thereby increasing protein and caloric intake is likely present in patients with HCC, although there are no studies specifically addressing NIS in HCC. Specific medications for NIS are often inexpensive and readily available, but beyond the scope of this review.
Furthermore, addressing symptom burden is an essential component for optimizing physical function. For example, a relationship between symptoms and sarcopenia related outcome measures such as SPPB was reported in 359 older oncology patients from two tertiary medical centers. Each unit increase in a composite symptom score was associated with greater instrumental activity of daily living (IADL) impairment (4.8%), physical activity limitations (4.4%), falls (2.9%), and SPPB ≤9 (2.5%) (P<0.05) (100).
Psychological
Patients with sarcopenia and cancer experience stressors that affect their mental health, including decreased cognitive function, psychological and spiritual distress, increased dependency, and changes in body image. In patients with HCC, health-related quality of life (HRQoL) decreases over time, with a particular emphasis on the mental health component (101). In patients with incurable gastrointestinal or lung cancer, sarcopenia diagnosed by CT imaging was associated with worse QoL and greater depression symptoms (102). These findings underscore the need to adopt a comprehensive approach to managing patients with sarcopenia, addressing their symptom burden, including mood.
Conclusions
The association of sarcopenia with adverse clinic outcomes such as decreased OS, increased side-effects of anti-neoplastic therapy and decreased physical performance suggests that sarcopenia should be a priority for clinicians managing patients with HCC. Sarcopenia is present in almost half of patients with HCC and given its prevalence in cirrhosis, may be frequently present at the initial cancer diagnosis. Clinical trials are required to determine whether therapeutic interventions targeting sarcopenia can improve clinical outcomes such as survival, treatment side-effects and QoL. This review highlights current guidelines regarding screening, assessing, and managing patients with sarcopenia and how this may apply specifically to patients with HCC. Although no single agent is approved, the multifactorial nature of sarcopenia suggests a multimodal combination of pharmacological and non-pharmacological therapies may be most effective.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Sukeshi Patel Arora and Sherri Rauenzahn Cervantez) for the series “Comprehensive Care for Patients with Hepatocellular Carcinoma: Insights from the 2022 San Antonio Liver Cancer Symposium” published in Annals of Palliative Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-23-332/rc
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-23-332/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-23-332/coif). The series “Comprehensive Care for Patients with Hepatocellular Carcinoma: Insights from the 2022 San Antonio Liver Cancer Symposium” was commissioned by the editorial office without any funding or sponsorship. EDF is supported by grant 1R01AG061558-01A1 from the National Institutes of Health and receives UpToDate royalty for chapter. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
※Special series on Comprehensive Care for Patients with Hepatocellular Carcinoma: Insights from the 2022 San Antonio Liver Cancer Symposium.
References
- Rumgay H, Arnold M, Ferlay J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol 2022;77:1598-606. [Crossref] [PubMed]
- European Association for the Study of the Liver. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. Erratum in: J Hepatol 2019;70:817. [Crossref]
- Guo Y, Ren Y, Zhu L, et al. Association between sarcopenia and clinical outcomes in patients with hepatocellular carcinoma: an updated meta-analysis. Sci Rep 2023;13:934. [Crossref] [PubMed]
- Jiang C, Wang Y, Fu W, et al. Association between sarcopenia and prognosis of hepatocellular carcinoma: A systematic review and meta-analysis. Front Nutr 2022;9:978110. [Crossref] [PubMed]
- Surov A, Wienke A. Prevalence of sarcopenia in patients with solid tumors: A meta-analysis based on 81,814 patients. JPEN J Parenter Enteral Nutr 2022;46:1761-8. [Crossref] [PubMed]
- Gingrich A, Volkert D, Kiesswetter E, et al. Prevalence and overlap of sarcopenia, frailty, cachexia and malnutrition in older medical inpatients. BMC Geriatr 2019;19:120. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:601. [Crossref] [PubMed]
- Malmstrom TK, Miller DK, Simonsick EM, et al. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle 2016;7:28-36. [Crossref] [PubMed]
- Williams GR, Al-Obaidi M, Dai C, et al. SARC-F for screening of sarcopenia among older adults with cancer. Cancer 2021;127:1469-75. [Crossref] [PubMed]
- Luengpradidgun L, Chamroonkul N, Sripongpun P, et al. Utility of handgrip strength (HGS) and bioelectrical impedance analysis (BIA) in the diagnosis of sarcopenia in cirrhotic patients. BMC Gastroenterol 2022;22:159. [Crossref] [PubMed]
- Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014;69:547-58. [Crossref] [PubMed]
- Berardi G, Antonelli G, Colasanti M, et al. Association of Sarcopenia and Body Composition With Short-term Outcomes After Liver Resection for Malignant Tumors. JAMA Surg 2020;155:e203336. [Crossref] [PubMed]
- Beer L, Bastati N, Ba-Ssalamah A, et al. MRI-defined sarcopenia predicts mortality in patients with chronic liver disease. Liver Int 2020;40:2797-807. [Crossref] [PubMed]
- Mourtzakis M, Prado CM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997-1006. [Crossref] [PubMed]
- Wu CH, Liang PC, Hsu CH, et al. Total skeletal, psoas and rectus abdominis muscle mass as prognostic factors for patients with advanced hepatocellular carcinoma. J Formos Med Assoc 2021;120:559-66. [Crossref] [PubMed]
- Aleixo GFP, Shachar SS, Nyrop KA, et al. Bioelectrical Impedance Analysis for the Assessment of Sarcopenia in Patients with Cancer: A Systematic Review. Oncologist 2020;25:170-82. [Crossref] [PubMed]
- Román E, Poca M, Amorós-Figueras G, et al. Phase angle by electrical bioimpedance is a predictive factor of hospitalisation, falls and mortality in patients with cirrhosis. Sci Rep 2021;11:20415. [Crossref] [PubMed]
- Becchetti C, Berzigotti A. Ultrasonography as a diagnostic tool for sarcopenia in patients with cirrhosis: Examining the pros and cons. Eur J Intern Med 2023;S0953-6205(23)00214-5.
- Sakai M, Kawaguchi T, Koya S, et al. Subcutaneous Fat Thickness of the Lower Limb is Associated with Trunk Muscle Mass in Patients with Hepatocellular Carcinoma: A Simple Assessment for Sarcopenia Using Conventional Ultrasonography. Kurume Med J 2022;67:97-105. [Crossref] [PubMed]
- Petermann-Rocha F, Balntzi V, Gray SR, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 2022;13:86-99. [Crossref] [PubMed]
- Liu MA, DuMontier C, Murillo A, et al. Gait speed, grip strength, and clinical outcomes in older patients with hematologic malignancies. Blood 2019;134:374-82. [Crossref] [PubMed]
- Pavasini R, Guralnik J, Brown JC, et al. Short Physical Performance Battery and all-cause mortality: systematic review and meta-analysis. BMC Med 2016;14:215. [Crossref] [PubMed]
- Klepin HD, Geiger AM, Tooze JA, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood 2013;121:4287-94. [Crossref] [PubMed]
- Hanada M, Yamauchi K, Miyazaki S, et al. Short-Physical Performance Battery (SPPB) score is associated with postoperative pulmonary complications in elderly patients undergoing lung resection surgery: A prospective multicenter cohort study. Chron Respir Dis 2020;17:1479973120961846. [Crossref] [PubMed]
- Collins JT, Noble S, Chester J, et al. The value of physical performance measurements alongside assessment of sarcopenia in predicting receipt and completion of planned treatment in non-small cell lung cancer: an observational exploratory study. Support Care Cancer 2018;26:119-27. [Crossref] [PubMed]
- Essam Behiry M, Mogawer S, Yamany A, et al. Ability of the Short Physical Performance Battery Frailty Index to Predict Mortality and Hospital Readmission in Patients with Liver Cirrhosis. Int J Hepatol 2019;2019:8092865. [Crossref] [PubMed]
- Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol 2018;14:513-37. [Crossref] [PubMed]
- Donini LM, Busetto L, Bauer JM, et al. Critical appraisal of definitions and diagnostic criteria for sarcopenic obesity based on a systematic review. Clin Nutr 2020;39:2368-88. [Crossref] [PubMed]
- Chen BB, Liang PC, Shih TT, et al. Sarcopenia and myosteatosis are associated with survival in patients receiving immunotherapy for advanced hepatocellular carcinoma. Eur Radiol 2023;33:512-22. [Crossref] [PubMed]
- Koo BK, Kim D, Joo SK, et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol 2017;66:123-31. [Crossref] [PubMed]
- Ha NB, Lai JC. The bidirectional relationship between nonalcoholic fatty liver disease and sarcopenia. Hepatol Int 2022;16:489-91. [Crossref] [PubMed]
- Zambon Azevedo V, Silaghi CA, Maurel T, et al. Impact of Sarcopenia on the Severity of the Liver Damage in Patients With Non-alcoholic Fatty Liver Disease. Front Nutr 2022;8:774030. [Crossref] [PubMed]
- Nardelli S, Gioia S, Faccioli J, et al. Sarcopenia and cognitive impairment in liver cirrhosis: A viewpoint on the clinical impact of minimal hepatic encephalopathy. World J Gastroenterol 2019;25:5257-65. [Crossref] [PubMed]
- Allen SL, Quinlan JI, Dhaliwal A, et al. Sarcopenia in chronic liver disease: mechanisms and countermeasures. Am J Physiol Gastrointest Liver Physiol 2021;320:G241-57. [Crossref] [PubMed]
- Wu J, Ding P, Wu H, et al. Sarcopenia: Molecular regulatory network for loss of muscle mass and function. Front Nutr 2023;10:1037200. [Crossref] [PubMed]
- Paez HG, Pitzer CR, Alway SE. Age-Related Dysfunction in Proteostasis and Cellular Quality Control in the Development of Sarcopenia. Cells 2023;12:249. [Crossref] [PubMed]
- St-Jean-Pelletier F, Pion CH, Leduc-Gaudet JP, et al. The impact of ageing, physical activity, and pre-frailty on skeletal muscle phenotype, mitochondrial content, and intramyocellular lipids in men. J Cachexia Sarcopenia Muscle 2017;8:213-28. [Crossref] [PubMed]
- Traustadóttir T, Harman SM, Tsitouras P, et al. Long-Term Testosterone Supplementation in Older Men Attenuates Age-Related Decline in Aerobic Capacity. J Clin Endocrinol Metab 2018;103:2861-9. [Crossref] [PubMed]
- Liu H, Zang P, Lee II, et al. Growth hormone secretagogue receptor-1a mediates ghrelin’s effects on attenuating tumour-induced loss of muscle strength but not muscle mass. J Cachexia Sarcopenia Muscle 2021;12:1280-95. [Crossref] [PubMed]
- Lee SJ, Bhasin S, Klickstein L, et al. Challenges and Future Prospects of Targeting Myostatin/Activin A Signaling to Treat Diseases of Muscle Loss and Metabolic Dysfunction. J Gerontol A Biol Sci Med Sci 2023;78:32-7. [Crossref] [PubMed]
- Jiao ZT, Luo Q. Molecular Mechanisms and Health Benefits of Ghrelin: A Narrative Review. Nutrients 2022;14:4191. [Crossref] [PubMed]
- Francisco V, Sanz MJ, Real JT, et al. Adipokines in Non-Alcoholic Fatty Liver Disease: Are We on the Road toward New Biomarkers and Therapeutic Targets? Biology (Basel) 2022;11:1237. [Crossref] [PubMed]
- Naito T, Uchino J, Kojima T, et al. A multicenter, open-label, single-arm study of anamorelin (ONO-7643) in patients with cancer cachexia and low body mass index. Cancer 2022;128:2025-35. [Crossref] [PubMed]
- Takayama K, Takiguchi T, Komura N, et al. Efficacy and safety of anamorelin in patients with cancer cachexia: Post-hoc subgroup analyses of a placebo-controlled study. Cancer Med 2023;12:2918-28. [Crossref] [PubMed]
- Chamberlain JS. Cachexia in cancer--zeroing in on myosin. N Engl J Med 2004;351:2124-5. [Crossref] [PubMed]
- da Costa Teixeira LA, Avelar NCP, Peixoto MFD, et al. Inflammatory biomarkers at different stages of Sarcopenia in older women. Sci Rep 2023;13:10367. [Crossref] [PubMed]
- Zhang W, Sun W, Gu X, et al. GDF-15 in tumor-derived exosomes promotes muscle atrophy via Bcl-2/caspase-3 pathway. Cell Death Discov 2022;8:162. [Crossref] [PubMed]
- Tang H, Inoki K, Brooks SV, et al. mTORC1 underlies age-related muscle fiber damage and loss by inducing oxidative stress and catabolism. Aging Cell 2019;18:e12943. [Crossref] [PubMed]
- Wang D, Townsend LK, DesOrmeaux GJ, et al. GDF15 promotes weight loss by enhancing energy expenditure in muscle. Nature 2023;619:143-50. [Crossref] [PubMed]
- Galuppo B, Agazzi C, Pierpont B, et al. Growth differentiation factor 15 (GDF15) is associated with non-alcoholic fatty liver disease (NAFLD) in youth with overweight or obesity. Nutr Diabetes 2022;12:9. [Crossref] [PubMed]
- Study of the Efficacy and Safety of Ponsegromab in Patients With Cancer, Cachexia and Elevated GDF-15 (PROACC-1). Available online: https://www.clinicaltrials.gov/ct2/show/NCT05546476
- Dev R, Hui D, Chisholm G, et al. Hypermetabolism and symptom burden in advanced cancer patients evaluated in a cachexia clinic. J Cachexia Sarcopenia Muscle 2015;6:95-8. [Crossref] [PubMed]
- Stewart Coats AJ, Ho GF, Prabhash K, et al. Espindolol for the treatment and prevention of cachexia in patients with stage III/IV non-small cell lung cancer or colorectal cancer: a randomized, double-blind, placebo-controlled, international multicentre phase II study (the ACT-ONE trial). J Cachexia Sarcopenia Muscle 2016;7:355-65. [Crossref] [PubMed]
- Nishikawa H, Enomoto H, Nishiguchi S, et al. Liver Cirrhosis and Sarcopenia from the Viewpoint of Dysbiosis. Int J Mol Sci 2020;21:5254. [Crossref] [PubMed]
- Prokopidis K, Witard OC. Understanding the role of smoking and chronic excess alcohol consumption on reduced caloric intake and the development of sarcopenia. Nutr Res Rev 2022;35:197-206. [Crossref] [PubMed]
- Uchikawa S, Kawaoka T, Namba M, et al. Skeletal Muscle Loss during Tyrosine Kinase Inhibitor Treatment for Advanced Hepatocellular Carcinoma Patients. Liver Cancer 2020;9:148-55. [Crossref] [PubMed]
- March C, Omari J, Thormann M, et al. Prevalence and role of low skeletal muscle mass (LSMM) in hepatocellular carcinoma. A systematic review and meta-analysis. Clin Nutr ESPEN 2022;49:103-13. [Crossref] [PubMed]
- Surov A, Pech M, Gessner D, et al. Low skeletal muscle mass is a predictor of treatment related toxicity in oncologic patients. A meta-analysis. Clin Nutr 2021;40:5298-310. [Crossref] [PubMed]
- Yamasaki T, Saeki I, Yamauchi Y, et al. Management of Systemic Therapies and Hepatic Arterial Infusion Chemotherapy in Patients with Advanced Hepatocellular Carcinoma Based on Sarcopenia Assessment. Liver Cancer 2022;11:329-40. [Crossref] [PubMed]
- Feng Z, Zhao H, Jiang Y, et al. Sarcopenia associates with increased risk of hepatocellular carcinoma among male patients with cirrhosis. Clin Nutr 2020;39:3132-9. [Crossref] [PubMed]
- Tantai X, Liu Y, Yeo YH, et al. Effect of sarcopenia on survival in patients with cirrhosis: A meta-analysis. J Hepatol 2022;76:588-99. [Crossref] [PubMed]
- Warner Ii ER, Satapathy SK. Sarcopenia in the Cirrhotic Patient: Current Knowledge and Future Directions. J Clin Exp Hepatol 2023;13:162-77. [Crossref] [PubMed]
- Alsebaey A, Sabry A, Rashed HS, et al. MELD-Sarcopenia is Better than ALBI and MELD Score in Patients with Hepatocellular Carcinoma Awaiting Liver Transplantation. Asian Pac J Cancer Prev 2021;22:2005-9. [Crossref] [PubMed]
- Ha NB, Montano-Loza AJ, Carey EJ, et al. Sarcopenic visceral obesity is associated with increased post-liver transplant mortality in acutely ill patients with cirrhosis. Am J Transplant 2022;22:2195-202. [Crossref] [PubMed]
- Wu DH, Liao CY, Wang DF, et al. Textbook outcomes of hepatocellular carcinoma patients with sarcopenia: A multicenter analysis. Eur J Surg Oncol 2023;49:802-10. [Crossref] [PubMed]
- Yang J, Wang D, Ma L, et al. Sarcopenia negatively affects postoperative short-term outcomes of patients with non-cirrhosis liver cancer. BMC Cancer 2023;23:212. [Crossref] [PubMed]
- Guo Y, Ren Y, Wu F, et al. Prognostic impact of sarcopenia in patients with hepatocellular carcinoma treated with PD-1 inhibitor. Therap Adv Gastroenterol 2022;15:17562848221142417. [Crossref] [PubMed]
- Bannangkoon K, Hongsakul K, Tubtawee T, et al. Association of myosteatosis with treatment response and survival in patients with hepatocellular carcinoma undergoing chemoembolization: a retrospective cohort study. Sci Rep 2023;13:3978. [Crossref] [PubMed]
- Liao C, Li G, Bai Y, et al. Prognostic value and association of sarcopenic obesity and systemic inflammatory indexes in patients with hepatocellular carcinoma following hepatectomy and the establishment of novel predictive nomograms. J Gastrointest Oncol 2021;12:669-93. [Crossref] [PubMed]
- Hayashi H, Shimizu A, Kubota K, et al. Combination of sarcopenia and prognostic nutritional index to predict long-term outcomes in patients undergoing initial hepatectomy for hepatocellular carcinoma. Asian J Surg 2023;46:816-23. [Crossref] [PubMed]
- Temel JS, Abernethy AP, Currow DC, et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol 2016;17:519-31. [Crossref] [PubMed]
- Rolland Y, Dray C, Vellas B, et al. Current and investigational medications for the treatment of sarcopenia. Metabolism 2023; Epub ahead of print. [Crossref] [PubMed]
- Chow LS, Gerszten RE, Taylor JM, et al. Exerkines in health, resilience and disease. Nat Rev Endocrinol 2022;18:273-89. [Crossref] [PubMed]
- De Spiegeleer A, Beckwée D, Bautmans I, et al. Pharmacological Interventions to Improve Muscle Mass, Muscle Strength and Physical Performance in Older People: An Umbrella Review of Systematic Reviews and Meta-analyses. Drugs Aging 2018;35:719-34. [Crossref] [PubMed]
- Falqueto H, Júnior JLR, Silvério MNO, et al. Can conditions of skeletal muscle loss be improved by combining exercise with anabolic-androgenic steroids? A systematic review and meta-analysis of testosterone-based interventions. Rev Endocr Metab Disord 2021;22:161-78. [Crossref] [PubMed]
- Sinclair M, Grossmann M, Hoermann R, et al. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: A randomised controlled trial. J Hepatol 2016;65:906-13. [Crossref] [PubMed]
- Del Fabbro E, Garcia JM, Dev R, et al. Testosterone replacement for fatigue in hypogonadal ambulatory males with advanced cancer: a preliminary double-blind placebo-controlled trial. Support Care Cancer 2013;21:2599-607. [Crossref] [PubMed]
- Luo J, Quan Z, Lin S, et al. The association between blood concentration of 25- hydroxyvitamin D and sarcopenia: a meta-analysis. Asia Pac J Clin Nutr 2018;27:1258-70. [PubMed]
- Saeki C, Kanai T, Nakano M, et al. Low Serum 25-Hydroxyvitamin D Levels Are Related to Frailty and Sarcopenia in Patients with Chronic Liver Disease. Nutrients 2020;12:3810. [Crossref] [PubMed]
- Saeki C, Kanai T, Ueda K, et al. Prognostic significance of sarcopenia and severe vitamin D deficiency in patients with cirrhosis. JGH Open 2023;7:351-7. [Crossref] [PubMed]
- Prokopidis K, Giannos P, Katsikas Triantafyllidis K, et al. Effect of vitamin D monotherapy on indices of sarcopenia in community-dwelling older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 2022;13:1642-52. [Crossref] [PubMed]
- Chang MC, Choo YJ. Effects of Whey Protein, Leucine, and Vitamin D Supplementation in Patients with Sarcopenia: A Systematic Review and Meta-Analysis. Nutrients 2023;15:521. [Crossref] [PubMed]
- Cheng SH, Chen KH, Chen C, et al. The Optimal Strategy of Vitamin D for Sarcopenia: A Network Meta-Analysis of Randomized Controlled Trials. Nutrients 2021;13:3589. [Crossref] [PubMed]
- Barbagallo M, Veronese N, Di Prazza A, et al. Effect of Calcifediol on Physical Performance and Muscle Strength Parameters: A Systematic Review and Meta-Analysis. Nutrients 2022;14:1860. [Crossref] [PubMed]
- Bowen TS, Schuler G, Adams V. Skeletal muscle wasting in cachexia and sarcopenia: molecular pathophysiology and impact of exercise training. J Cachexia Sarcopenia Muscle 2015;6:197-207. [Crossref] [PubMed]
- Nasrah R, Kanbalian M, Van Der Borch C, et al. Defining the role of dietary intake in determining weight change in patients with cancer cachexia. Clin Nutr 2018;37:235-41. [Crossref] [PubMed]
- Curtis AR, Livingstone KM, Daly RM, et al. Associations between Dietary Patterns and Malnutrition, Low Muscle Mass and Sarcopenia in Adults with Cancer: A Scoping Review. Int J Environ Res Public Health 2022;19:1769. [Crossref] [PubMed]
- Bischoff SC, Bernal W, Dasarathy S, et al. ESPEN practical guideline: Clinical nutrition in liver disease. Clin Nutr 2020;39:3533-62. [Crossref] [PubMed]
- Chen CJ, Wang LC, Kuo HT, et al. Significant effects of late evening snack on liver functions in patients with liver cirrhosis: A meta-analysis of randomized controlled trials. J Gastroenterol Hepatol 2019;34:1143-52. [Crossref] [PubMed]
- Roeland EJ, Bohlke K, Baracos VE, et al. Management of Cancer Cachexia: ASCO Guideline. J Clin Oncol 2020;38:2438-53. [Crossref] [PubMed]
- Arends J, Strasser F, Gonella S, et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines☆. ESMO Open 2021;6:100092. [Crossref] [PubMed]
- Lopez P, Newton RU, Taaffe DR, et al. Moderators of resistance-based exercise programs’ effect on sarcopenia-related measures in men with prostate cancer previously or currently undergoing androgen deprivation therapy: An individual patient data meta-analysis. J Geriatr Oncol 2023;14:101535. [Crossref] [PubMed]
- Aamann L, Dam G, Borre M, et al. Resistance Training Increases Muscle Strength and Muscle Size in Patients With Liver Cirrhosis. Clin Gastroenterol Hepatol 2020;18:1179-1187.e6. [Crossref] [PubMed]
- Hashida R, Kawaguchi T, Koya S, et al. Impact of cancer rehabilitation on the prognosis of patients with hepatocellular carcinoma. Oncol Lett 2020;19:2355-67. [Crossref] [PubMed]
- Koya S, Kawaguchi T, Hashida R, et al. Effects of in-hospital exercise on sarcopenia in hepatoma patients who underwent transcatheter arterial chemoembolization. J Gastroenterol Hepatol 2019;34:580-8. [Crossref] [PubMed]
- Bolotta A, Filardo G, Abruzzo PM, et al. Skeletal Muscle Gene Expression in Long-Term Endurance and Resistance Trained Elderly. Int J Mol Sci 2020;21:3988. [Crossref] [PubMed]
- Kaibori M, Matsui K, Yoshii K, et al. Perioperative exercise capacity in chronic liver injury patients with hepatocellular carcinoma undergoing hepatectomy. PLoS One 2019;14:e0221079. [Crossref] [PubMed]
- Johnston HE, Takefala TG, Kelly JT, et al. The Effect of Diet and Exercise Interventions on Body Composition in Liver Cirrhosis: A Systematic Review. Nutrients 2022;14:3365. [Crossref] [PubMed]
- Khorasanchi A, Nemani S, Pandey S, et al. Managing Nutrition Impact Symptoms in Cancer Cachexia: A Case Series and Mini Review. Front Nutr 2022;9:831934. [Crossref] [PubMed]
- Pandya C, Magnuson A, Flannery M, et al. Association Between Symptom Burden and Physical Function in Older Patients with Cancer. J Am Geriatr Soc 2019;67:998-1004. [Crossref] [PubMed]
- Verma M, Paik JM, Younossi I, et al. The impact of hepatocellular carcinoma diagnosis on patients’ health-related quality of life. Cancer Med 2021;10:6273-81. [Crossref] [PubMed]
- Nipp RD, Fuchs G, El-Jawahri A, et al. Sarcopenia Is Associated with Quality of Life and Depression in Patients with Advanced Cancer. Oncologist 2018;23:97-104. [Crossref] [PubMed]



