
Treatment modalities to manage hepatocellular carcinoma patients with portal vein thrombosis: a systematic review and meta-analysis※
Highlight box
Key findings
• Local treatments may be better at reducing the odds of mortality than systemic treatments.
• Combined treatments may be better at reducing the odds of mortality than individual treatments.
What is known and what is new?
• Many treatment options have been investigated and used for patients with hepatocellular carcinoma and portal vein thrombosis (PVT).
• This paper provides a current understanding of treatment options and determines the relative effectiveness of treatment options in preventing mortality over 24 months.
What is the implication, and what should change now?
• Future strategies for hepatocellular carcinoma with PVT should look at the combination of radiation and systemic treatments either concurrently or sequentially.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most commonly diagnosed cancer and the third leading cause of cancer death worldwide, with approximately 906,000 new cases in 2020 (1). Patients are often diagnosed at advanced and incurable stages, thereby leading to a very high mortality rate (1). Among patients with HCC, portal vein thrombosis (PVT) is present in 10–40% of patients at the time of diagnosis, which can ultimately lead to extrahepatic spread (EHS), worsening portal hypertension and hepatic dysfunction (2). Without any treatment, prognosis is limited to only 3 months (3).
Many treatments have been investigated and used for the treatment of patients with HCC and PVT. Radiation therapy is a localized cancer treatment that kills cancer cells and shrinks tumors by damaging their DNA through radiation (4). Sorafenib, a Raf kinase inhibitor, is a systemic treatment that targets receptor tyrosine kinase pathways that are commonly unregulated by cancer and disrupts cancer cell cycle reproduction (5). Transarterial chemoembolization (TACE), a local treatment, acts by delivering chemotherapeutic and embolic agents into the arterial blood supply of the tumor (6). However, this treatment may not be suitable for patients with segmental or main PVT, as it can induce hepatic necrosis and liver failure (7). Another form of local regional therapy is transarterial radioembolization (TARE, aka SIRT, aka Y90), which involves the targeted delivery of Yttrium-90 to the tumor and surrounding liver parenchyma (8). This modality has shown promising results for selected patients with PVT and relatively preserved liver function in combination with ablative doses of radiation (8).
While relative outcomes of different treatment modalities for primary HCC as a whole has been the subject of several recent systematic reviews and network meta-analyses (9,10), how these modalities compare for the treatment of HCC patients with PVT remains poorly defined. Given the variable clinical presentation of patients with PVT, as well as specific characteristics to and expertise of each cancer center, there is currently a heterogenous approach in treating patients with PVT. A 2018 review by Finn et al. synthesized the evidence of treatment for patients with Child-Pugh scores of A and B and advanced HCC with either macrovascular invasion or EHS (11). They reported on 14 studies; three were randomized controlled trials (RCTs) and 11 were observational studies. Sample sizes ranged from 10 to 691, and the mean/median age was over 50 years old across all trials. In two RCTs, sorafenib had superior overall survival (OS) compared to best supportive care. However, observational studies, which evaluated loco-regional therapies alone or in combination with other treatments, were limited by very low quality of evidence. Therefore, no conclusions were made for the other treatments they assessed, including TACE, TARE, stereotactic ablative radiotherapy (SABR) [also known as stereotactic body radiation therapy (SBRT)] and no therapy. Thus, an updated systematic review is needed as new research has been published in the past five years. The aim of this systematic review was to synthesize existing evidence regarding the effectiveness of systemic and locoregional approaches to treating advanced HCC and PVT. We present this article in accordance with the PRISMA reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-23-463/rc).
Methods
This review was registered a priori on PROSPERO (CRD42022290708) and written in accordance to PRISMA reporting guidelines (12).
Search strategy
Citations from January 2007 to January 2022 were searched in the databases of PubMed, Embase and Cochrane CENTRAL. Studies published prior to 2007 were excluded in order to report only on modern treatment modalities, as one of the earliest landmark studies prompting the use of kinase inhibition was published in 2008 (13). The search terms included liver cell carcinoma, hepatocellular carcinoma, irradiation, radiotherapy, sorafenib, radiofrequency ablation, radiofrequency, TACE, transarterial chemoembolization, TARE, transarterial radioembolization, hepatic arterial infusion, HAIC, transcatheter chemoembolization, y-90, y90, yttrium 90, thrombosis, thrombus, portal vein thrombosis, portal vein, liver vein thrombosis, liver vein, venous thromboembolism, venous and PVT. No language restrictions were applied. The search strategy is reported in Supplementary file (Appendix 1).
Eligibility criteria
Two reviewers (Liu B, Grindrod N) independently screened articles for potential eligibility. If consensus could not be reached, disagreements were resolved by the senior author (Lock M). Studies were included if they reported on the mortality of any non-palliative treatment for patients with Child-Pugh score of A or B cirrhosis and HCC with PVT. Only multi-arm comparative studies with propensity score matched analysis were included to reduce the amount of bias among patients receiving different treatments.
Data extraction
For each study, the patient and treatment characteristics were recorded. All-cause mortality at prespecified timepoints of 6, 12, 18 and 24 months was extracted from each study’s Kaplan-Meier curve using an online digitizer (14) to estimate event data. Additionally, each study was assessed by two reviewers (Liu B, Grindrod N) for study quality using the Cochrane Risk of Bias in Non-Randomized Studies of Interventions tool (ROBINS-1) (15). Study quality assessment was presented graphically using the online robvis visualization tool (16).
Statistical analysis
Odds ratios and corresponding 95% confidence intervals (CIs) were calculated for each study and each timepoint then graphically displayed in forest plots. A random-effects DerSimonian-Laird model was used to calculate summary odds ratio and corresponding 95% CI for studies reporting on (I) radiation therapy vs. other; and (II) sorafenib vs. other. Type I error was set at 0.05. All analyses were conducted using StataBE 17.0. Due to the small number of studies, publication bias was not assessed.
Results
A total of 1,606 articles were identified from database search, of which 1,247 were screened after removing duplicate publications. Ultimately, six articles (17-22) were included in this review (Figure 1). Study sample size ranged from 63 to 985. The mean and median age was over 50 years old across all trials, with a mean and median follow-up time period of greater than 50 months. Individual study demographics are presented in Table 1. All studies had a moderate overall risk of bias (Figure 2). Each study had a moderate risk of bias in domain four of the ROBINS-I tool, which measures bias due to deviations from intended interventions. The primary bias in domain four for the studies of Chu et al., Cho et al., and Martelletti et al. was due to insufficient information to answer the sections of 4.3–4.6 (which assess the effect of starting and adhering to interventions) and, therefore, domain four had a moderate risk of bias (17-19). For Im et al., they reported that 63% of patients receiving RT also received additional treatment, which may impact the survival analysis (20). Nakazawa et al. reported that 90% of patients who took sorafenib discontinued treatment due to adverse events, which can lead to bias as defined in Section 4.5 of ROBINS-I. This section assesses whether participants adhered to the assigned intervention (21). For Li et al. some patients underwent repeat TACE, and there was differential loss to follow-up in both arms, leading to moderate risk of bias in domains 4 and 5 (bias in the classification of interventions and bias due to missing data) (22).
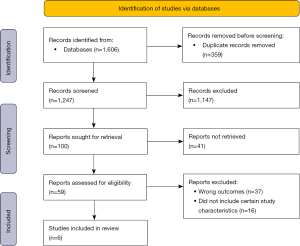
Table 1
| Study | Sample size | Study design | Comparative treatments | Age (years) | Time period | Size of hepatic lesions (cm) | Levels of AFP (ng/mL) | Child-Pugh score | Adjusted covariates |
|---|---|---|---|---|---|---|---|---|---|
| Chu et al. [2020] | 307 | Propensity score-matched analysis | (I) TACE + sorafenib; (II) TACE + RT | (I) 56.4±10.8*; (II) 55.6±9.3* | 72 months | (I) 10.6±4.2; (II) 9.2±4 | ≥400: (I) 60; (II) 113 | (I) A: 91, B: 13; (II) A: 182, B: 21 | Age, AFP, CPS, tumour size, tumour number, dose, number of lesions |
| Li et al. [2016] | 839 | Propensity score-matched analysis | (I) TACE + RT; (II) TACE | (I) 51.7±10*; (II) 51±10.4* | 60 months | (I) ≤5: 20, >5: 92; (II) ≤5: 96, >5: 639 | (I) ≤400: 45, >400: 67; (II) ≤400: 266, >400: 469 | (I) A: 105, B: 7; (II) A: 688, B: 47 | Age, AFP, CPS, tumour size, tumour number, dose, fractions, number of lesions |
| Im et al. [2017] | 985 | Propensity score-matched analysis | (I) TACE + HAIC, TACE or HAIC; (II) RT | 54 [23–84]^ | 60 months | <10: 583; ≥10: 402 | <400: 450, ≥400: 535 | A: 753, B: 232 | Age, AFP, CPS, tumour size, tumour number, dose, fractions, ECOG score |
| Cho et al. [2016] | 63 | Propensity score-matched analysis | (I) Y90; (II) sorafenib | (I) 63.7±11.1*; (II) 60.3±10.4* | 60 months | NR | (I) ≤20: 5, 20–200: 7, >200: 20; (II) ≤20: 6, 20–200: 5, >200: 20 | (I) A: 28, B: 4; (II) A: 22, B: 9 | Age, lesion size, CPS, AFP, cause of cirrhosis, previous treatments |
| Martelletti et al. [2021] | 65 | Propensity score-matched analysis | (I) Sorafenib; (II) TARE | (I) 75 [62–81]^; (II) 73 [63–82]^ | 54 months | (I) 48 [40–70]^; (II) 55 [36.5–72.5]^ | (I) 907; (II) 326 | NR | Age, lesion size, AFP |
| Nakazawa et al. [2014] | 97 | Propensity score-matched analysis | (I) Sorafenib; (II) RT | (I) 70 [61–70]^; (II) 67 [61–70]^ | 108 months | NR | (I) 680; (II) 43 | NR | Age, AFP, cause of cirrhosis |
*, mean ± SD; ^, median [IQR]. AFP, alpha-fetoprotein; TACE, transarterial chemoembolization; RT, radiotherapy; HAIC, hepatic arterial infusion chemotherapy; NR, not reported; CPS, Child-Pugh score; ECOG, Eastern Cooperative Oncology; IQR, interquartile range.
Individual study results
Individual study results at pre-specified timepoints of 6, 12, 18 and 24 months are presented in Figure 3. At 6 months, Y90 has an improved survival compared to sorafenib, and TACE + RT had an improved survival compared to TACE. At 12 months, TACE + RT had an improved survival compared to RT alone. At 18 months, TARE had an improved survival compared to sorafenib, and TACE + RT had an improved survival compared to TACE. At 24 months, sorafenib had an improved survival compared to Y90, and TACE + RT had an improved survival compared to TACE.
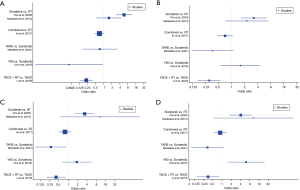
Radiotherapy (RT) vs. other
When comparing RT to all other treatments, similar OS was observed longitudinally. There was a trend that RT yielded better survival at the early stages of 6 months [odds ratio (OR) 0.70, 95% CI: 0.28–1.76]. There was no concern for heterogeneity (Figure 4).
Sorafenib vs. other
Sorafenib was associated with higher odds for mortality at 6 months (OR 2.20, 95% CI: 1.11–4.39). No significant differences were noted at the other timepoints, of 12, 18 and 24 months. There was no concern for heterogeneity (Figure 5).
Discussion
In this systematic review evaluating the effectiveness of locoregional and systemic treatments in patients with advanced HCC with PVT, we summarized results from 6 propensity score matched observational studies that included a total of 2,356 patients. We found that local treatments may be better at reducing the odds of mortality compared to systemic treatments. Furthermore, combined treatments may be better at reducing the odds of mortality than individual treatments.
Our results suggest that sorafenib is associated with higher odds of mortality at 6 months contrasts the findings by Finn et al., which concluded that in patients with advanced HCC and Child-Pugh A liver function, sorafenib was the only treatment that has been shown to improve OS in randomized studies (11). This observation may be due to the heterogeneous patient population amongst individuals with HCC and PVT; different populations may ultimately lead to a different effect estimate of sorafenib and, therefore, a different conclusion. Our meta-analysis includes four observational studies, all using propensity-score matched analysis, and all conducted recently. This should provide the most controlled and recent estimate available of the value of sorafenib in this patient population.
It is important to note that sorafenib was the only systemic therapy approved worldwide for advanced/metastatic HCC from about 2008 to 2018, and for that reason it has been extensively used around the world. Multiple trials in that period failed to supplant sorafenib. After 2018, however, newer treatments have shown promise over sorafenib. The REFLECT trial demonstrated lenvatinib (tyrosine kinase inhibitor) was non-inferior to sorafenib but had better response rates and PFS (23). Immunotherapy combinations of atezolizumab/bevacizumab and durvalumab/tremelimumab have also proven to be effective (24,25). Additionally, the results of other trials assessing ipilimumab/nivolumab, which may outperform sorafenib in efficacy and toxicity, are eagerly awaited (26,27).
However, based on the results of our meta-analysis, there may be a role for localized treatment, as patients receiving local therapy had generally improved survival at 6 months. There have been significant treatment advances over the past decade in the localized treatments of RT, TACE, and TARE; these advances have conferred greater efficacy and safety and may be promising for the treatment of patients with HCC and PVT (28). Our results, based on recent studies, support further investigation of localized treatment in this patient population. No significant differences were observed in meta-analyses looking at endpoints of 12 months and beyond, likely due to crossover or additional treatment. Patients who may live this long may receive additional treatment; therefore, survival beyond 12 months could be more reflective of additional treatment than the initial treatment.
It is also worth mentioning that novel surgical techniques in liver resection may also prove to be promising in this setting (29).
This study was not without limitations. First, the number of patients afflicted with HCC and PVT is, on an epidemiological level, still very small. Only observational studies are included in this meta-analysis, each having some risk of bias. In the absence of RCTs, these observational studies with propensity score matched analyses may be our best evidence. Secondly, due to the lack of standardized reporting of outcomes, other than OS, no other data (i.e., quality of life, progression free survival, treatment-related toxicities) was available for data extraction and meta-analysis. Future studies should aim to report on these other important endpoints as well to provide a better understanding of the differential results of varying treatment options beyond mortality. Additionally, trials with direct comparisons are not available for newer systemic treatments that have become standard of care (e.g., atezolizumab/bevacizumab and lenvatinib). Although these systemic treatments have shown important advances, many of the conclusions regarding the benefits of local treatments likely still apply despite the change in systemic treatments. Finally, the results of this meta-analysis are ultimately frail due to the small number of patients; in the counterfactual scenario where additional studies are available, a future meta-analysis could have a different conclusion.
Conclusions
In conclusion, this systematic review and meta-analysis reports on six studies with a total sample size of 2,356 patients. Localized treatments may yield superior OS at 6 months. Further investigations should be conducted to further understand the efficacy of localized treatments for patients with HCC and PVT.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Edward L.W. Chow and Candice Johnstone) for the series “Palliative Radiotherapy Column”, published in Annals of Palliative Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-23-463/rc
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-23-463/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-23-463/coif). The series “Palliative Radiotherapy Column” was commissioned by the editorial office without any funding sponsorship. C.B.S. serves as the co-Editor-in-Chief of Annals of Palliative Medicine. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This article was registered a priori on PROSPERO (CRD42022290708).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
※Special series on Palliative Radiotherapy Column.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Quirk M, Kim YH, Saab S, et al. Management of hepatocellular carcinoma with portal vein thrombosis. World J Gastroenterol 2015;21:3462-71. [Crossref] [PubMed]
- Bae BK, Kim JC. The response of thrombosis in the portal vein or hepatic vein in hepatocellular carcinoma to radiation therapy. Radiat Oncol J 2016;34:168-76. [Crossref] [PubMed]
- Chandra RA, Keane FK, Voncken FEM, et al. Contemporary radiotherapy: present and future. Lancet 2021;398:171-84. [Crossref] [PubMed]
- Keating GM. Sorafenib: A Review in Hepatocellular Carcinoma. Target Oncol 2017;12:243-53. [Crossref] [PubMed]
- Raoul JL, Forner A, Bolondi L, et al. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev 2019;72:28-36. [Crossref] [PubMed]
- Han K, Kim JH, Ko GY, et al. Treatment of hepatocellular carcinoma with portal venous tumor thrombosis: A comprehensive review. World J Gastroenterol 2016;22:407-16. [Crossref] [PubMed]
- Cardarelli-Leite L, Chung J, Klass D, et al. Ablative Transarterial Radioembolization Improves Survival in Patients with HCC and Portal Vein Tumor Thrombus. Cardiovasc Intervent Radiol 2020;43:411-22. [Crossref] [PubMed]
- Chow R, Simone CB 2nd, Jairam MP, et al. Radiofrequency ablation vs radiation therapy vs transarterial chemoembolization vs yttrium 90 for local treatment of liver cancer - a systematic review and network meta-analysis of survival data. Acta Oncol 2022;61:484-94. [Crossref] [PubMed]
- Malik A, Jairam MP, Chow R, et al. Radiofrequency ablation versus stereotactic body radiation therapy for hepatocellular carcinoma: a meta-regression. Future Oncol 2023;19:279-87. [Crossref] [PubMed]
- Finn RS, Zhu AX, Farah W, et al. Therapies for advanced stage hepatocellular carcinoma with macrovascular invasion or metastatic disease: A systematic review and meta-analysis. Hepatology 2018;67:422-35. [Crossref] [PubMed]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Plot digitizer. (n.d.). Retrieved April 27, 2022. Available online: http://plotdigitizer.sourceforge.net/
- Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [Crossref] [PubMed]
- Risk of bias tools—Robvis (Visualization tool). (n.d.). Retrieved May 11, 2022. Available online: https://www.riskofbias.info/welcome/robvis-visualization-tool
- Chu HH, Kim JH, Shim JH, et al. Chemoembolization Plus Radiotherapy Versus Chemoembolization Plus Sorafenib for the Treatment of Hepatocellular Carcinoma Invading the Portal Vein: A Propensity Score Matching Analysis. Cancers (Basel) 2020;12:1116. [Crossref] [PubMed]
- Cho YY, Lee M, Kim HC, et al. Radioembolization Is a Safe and Effective Treatment for Hepatocellular Carcinoma with Portal Vein Thrombosis: A Propensity Score Analysis. PLoS One 2016;11:e0154986. [Crossref] [PubMed]
- Martelletti C, Ricotti A, Gesualdo M, et al. Radioembolization vs sorafenib in locally advanced hepatocellular carcinoma with portal vein tumor thrombosis: A propensity score and Bayesian analysis. J Dig Dis 2021;22:496-502. [Crossref] [PubMed]
- Im JH, Yoon SM, Park HC, et al. Radiotherapeutic strategies for hepatocellular carcinoma with portal vein tumour thrombosis in a hepatitis B endemic area. Liver Int 2017;37:90-100. [Crossref] [PubMed]
- Nakazawa T, Hidaka H, Shibuya A, et al. Overall survival in response to sorafenib versus radiotherapy in unresectable hepatocellular carcinoma with major portal vein tumor thrombosis: propensity score analysis. BMC Gastroenterol 2014;14:84. [Crossref] [PubMed]
- Li XL, Guo WX, Hong XD, et al. Efficacy of the treatment of transarterial chemoembolization combined with radiotherapy for hepatocellular carcinoma with portal vein tumor thrombus: A propensity score analysis. Hepatol Res 2016;46:1088-98. [Crossref] [PubMed]
- Yamashita T, Kudo M, Ikeda K, et al. REFLECT-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset. J Gastroenterol 2020;55:113-22. [Crossref] [PubMed]
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382:1894-905. [Crossref] [PubMed]
- Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (Pts) with unresectable hepatocellular carcinoma (Uhcc): HIMALAYA. J Clin Oncol 2022;40:abstr 379.
- Finn RS, Ikeda M, Zhu AX, et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol 2020;38:2960-70. [Crossref] [PubMed]
- Yau T, Kang YK, Kim TY, et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol 2020;6:e204564. [Crossref] [PubMed]
- Khan AR, Wei X, Xu X. Portal Vein Tumor Thrombosis and Hepatocellular Carcinoma - The Changing Tides. J Hepatocell Carcinoma 2021;8:1089-115. [Crossref] [PubMed]
- Benatatos N, Papadopoulou I, Assimakopoulos SF, et al. Surgical management in hepatocellular carcinoma with portal vein tumour thrombosis: is this the end of the road or a chance to expand the criteria for resectability? Prz Gastroenterol 2022;17:257-65. [Crossref] [PubMed]





