
Palliative lower leg reconstruction※
Introduction
Palliative care is a multidisciplinary field that aims to relieve physical pain and psychological suffering with the goal to improve the quality of life rather than to cure or prolong life for patients who have difficulties associated with chronic, incurable, and life-threatening disease. The American College of Surgeons published a statement emphasizing the importance of palliative care and addressed the importance of using surgery as a tool to ameliorate suffering in addition to the cure of the disease (1). Instead of prioritizing treatment to extend life, treatment is focused on patient comfort and/or function.
Background
While palliative care can often refer to hospice care, it also includes surgical palliative care. Palliative surgery refers to surgery that is performed with the intent to improve the quality of life or relieve symptoms caused by advanced disease. The effectiveness of palliative surgery is determined by the presence and durability of symptom resolution and quality of life rather than classic oncologic metrics of negative margins and disease-free survival (2,3). Surgical palliation has been used in various surgical specialties including general surgery, thoracic surgery, otolaryngology, and gynecology (4,5). Although these palliative procedures may not prolong survival, these operations can improve the quality of life for these patients who may be suffering from pain, bleeding, social isolation, or other symptoms (3).
Although there is limited literature discussing the role of palliative reconstruction, plastic surgeons can play an important role in palliative care for patients. An important goal of palliative reconstruction is to improve the patient’s quality of life through pain reduction and symptomatic management. Palliative reconstruction can be clinically and ethically beneficial to certain patients depending on the patient’s values and wishes and the treatment goals (6). The fundamental ethical principles of autonomy, beneficence, nonmaleficence, and justice are important considerations in palliative reconstruction; in particular, the balance of nonmaleficence and beneficence is a critical factor in the assessment of palliative surgery and reconstruction.
Rationale and knowledge gap
Palliative lower extremity reconstruction is a complex and nebulous area of surgical practice, yet it can offer significant benefits for certain patients. Palliative lower extremity surgery may be considered for patients with terminal illnesses, most often malignancies, who have significant pain, impaired mobility, chronic wounds, infections such as osteomyelitis, chronic limb-threatening ischemia, pathologic fractures, and other symptoms and processes that cannot be managed by conservative management alone. Historically, plastic surgeons have not had a prominent role in palliative care, and thus there remains a significant knowledge gap in this field with respect to the optimal patient and procedure selection as well as the appropriate timing for initiating such interventions. Often, patients are excluded from surgical interventions due to limited life expectancies or expected gains from surgical interventions; however, in select cases, reconstructive operations may be offered as a reasonable means to an end, even in a patient with a terminal diagnosis when the patient’s goals and likely surgical outcomes are well aligned. Plastic surgeons may become increasingly involved in palliative operations through inclusion in tumor board discussions or championing by colleagues from our collaborating surgical teams (7). Plastic surgeons must also work closely with patients to frankly discuss and weigh several competing factors such as existing pain and symptoms; estimated life expectancy; the potential benefits and durability of a given palliative reconstructive procedure; and lastly, its potential risks, such as donor site morbidity, failure of the reconstruction, and what to expect from the typical postoperative recovery.
Multiple reconstructive options may be offered to patients, including amputation, limb salvage, and adjunctive procedures such as interventions for nerve pain or bleeding (8); however, it is not yet clear which choices yield the best outcomes in a palliative setting. Moreover, there is a lack of standardized protocols for palliative reconstruction, leading to significant variability in surgical decision-making and outcomes. Addressing these knowledge gaps, especially when it comes to indications for palliative reconstruction, will be critical to improving the care of patients with palliative lower extremity reconstruction needs and will require continued research and collaboration among clinicians and researchers in this field.
Objective
The aim of this paper is to review the current literature in support of palliative reconstruction in the lower extremity along with three case examples to illustrate the benefits that palliative reconstruction of the lower extremity can have for patients. Optimal patient selection, the goals and wishes of the patient, and minimizing donor site morbidity are important considerations for palliative reconstruction. We believe that palliative reconstruction can be clinically and ethically indicated for patients on a case-by-case assessment when the intended benefits of palliative reconstruction merit its risks.
Surgical procedure
Palliative reconstruction in malignancies of the head and neck, trunk, pelvis, and skin
Palliative surgery and palliative reconstruction has been used in various fields including head and neck, thoracic, breast, pelvic, and skin malignancies (9). With recent advances in neoadjuvant and adjuvant oncologic treatment, survival rates and durations have increased even for patients with advanced cancers, necessitating solutions to issues that would otherwise plague a patient’s end of life (10). Palliative reconstruction can provide this solution, making resections possible that may not otherwise be compatible with life; this has been demonstrated in reconstruction following radical chest wall resections (10,11), resections of exophytic neck tumors leading to exposure of major vessels (12), and resections of the calvarium with exposed brain and meninges underneath (13-18), among other scenarios. In situations where a complete resection of the tumor cannot be achieved, palliative reconstruction may still offer several benefits, including closing ulcerated and open wounds, facilitating patient wound care, and improving the quality of life of the palliative patient (10).
Palliative reconstruction has also evolved into an essential component of cancer treatment in patients with thoracic malignancies and breast cancer. Unlike patients who undergo thoracic and breast cancer resections with curative intent, the perioperative morbidity must be weighed more significantly in palliative patients before proceeding with extensive tumor resections that risk destabilizing the chest wall and compromising respiratory function (19,20). Full-thickness chest wall resections have been shown to have low morbidity and mortality rates but necessitate plastic surgery solutions for the resulting defects (21). Often, palliative chest wall and breast cancer resections may leave some tumor behind, with a focus on debulking and improving symptom control rather than aiming for negative margins through radical resections. However, plastic surgery techniques can be immensely valuable at expanding the limits of reasonable resections even in the palliative setting and may allow surgeons to address problems should they arise following extirpative surgeries (22).
Different reconstructive options can be utilized to address functional and aesthetic concerns and to provide stability for the chest wall and preserve normal respiratory dynamics (19). Given the excellent blood supply to the skin and soft tissues of the trunk as well as the proximity and reach of several pedicled flaps such as the omentum, pectoralis major, latissimus dorsi, and rectus abdominis, trunk defects can usually be reconstructed with local and regional options without often requiring free tissue transfer (11,19,23,24). As surgical efficiency and monitoring techniques have improved, microsurgery has gained popularity due to several clinical advantages over local and regional options as it has become much less cumbersome and more feasible of an option even in end-of-life care (25). When free flaps are needed, surgeons generally advocate for using bread and butter options such as the latissimus dorsi, anterolateral thigh, tensor fascia lata, transverse rectus abdominis, or deep inferior epigastric artery perforator flap, as these are generally reliable, most familiar to surgeons, and have acceptable donor site morbidities, especially when considering a shorter expected duration of life postoperatively (11,19,20,26). Similar to the head and neck literature, cases have been reported with more advanced reconstructive approaches including use of arteriovenous loops and multiple free flap reconstructions for extensive defects; patient selection is paramount in these scenarios where the reconstruction carries additive risks due to incremental increases in procedure complexity and the increased quantity of potential points of failure (25). For patients with shoulder girdle tumors, forequarter amputation is indicated and allows for “spare parts” harvest of massive fasciocutaneous free flaps of the forearm and arm to reconstruct the resulting shoulder and thoracic defects (11,27,28). Adjuncts to any type of autologous tissue reconstruction may be required when the chest wall is destabilized or respiration is affected; synthetic and biologic meshes or rib and sternal plates may be required, especially in a non-radiated thorax given it remains more supple and less rigid, and thus at risk of paradoxical respiration (19,29). Daigeler and colleagues proposed the first reconstructive algorithm for patients with palliative chest wall resections, suggesting many of the aforementioned approaches based on core variables such as trunk defect size and location (30).
Plastic surgery reconstruction can help improve the quality of life and mitigate negative effects of advanced tumors in a palliative setting. Although most skin cancers are curable, locally advanced and metastatic tumors may not be curable. Oftentimes, these advanced tumors can cause pain, disfigurement, infection, and even life-threatening bleeding. Although the disease may not be curable, palliative resection and then reconstruction to provide adequate soft tissue coverage can be indicated to help reduce pain, wound drainage, odor, bleeding, and risk of infection (16,31,32). In addition to the aforementioned indications, palliative reconstruction has also been performed for large gynecologic resections (33).
The basic principles of the reconstructive ladder are used to select an adequate reconstructive procedure for palliative reconstruction. While the options for reconstruction range from simple options such as skin grating to complex reconstruction with microvascular tissue transfer, the safest and least challenging option to provide sufficient coverage should be chosen (34). According to Vogt and Jokuszies, indications for palliative plastic surgery reconstruction include removal of visible tumors, protection of vital structures, infections, bleeding, pain, wound closure, hygiene, and improvement of bleeding (35). Advanced ulcerating tumors can cause pain, bleeding, including life-threatening erosive bleeding, infection, fistula formation, odor, and chronic wounds, which can compromise a patient’s quality of life. This can lead to a loss of self-esteem, function, and the ability to carry out activities of daily living, and result in social isolation. Although these tumors may be incurable, palliative reconstruction can help by reducing tumor mass, closing ulcerating wounds, and minimizing other burdensome symptoms of terminal disease which can improve quality of life (35).
Palliative reconstruction in malignancies of the lower extremity
Palliative reconstruction in the lower extremity has unique considerations. As with any reconstruction, it is important to assess the patient’s current functional status, overall goals, and clinical prognosis. For such patients where a curative resection cannot be obtained, improving the quality of life is paramount and palliative reconstruction can help these patients maintain their quality of life and independence (10). Although this may be applicable to any surgery, it is important to emphasize that the role of palliative reconstruction is patient-specific. According to Nthumba, proper patient selection is perhaps the most important consideration for palliative surgical intervention; the patient should remain the focus at all times and the goals of intervention should be driven by the needs of the patient and family (3). Careful patient selection is paramount in palliative reconstruction and important considerations include perioperative recovery, the ability to tolerate a large operation, and the patient’s goals and wishes (34). Patients must be assessed from a global standpoint on a case-by-case basis to determine if the benefits of palliative reconstruction would outweigh potential risks.
For palliative surgery in the extremities, it is often a question of amputation versus limb salvage. Even if the patient only has a short lifespan, limb salvage to preserve ambulation may be preferable to amputation (35). For example, a patient with an advanced soft-tissue sarcoma of the groin who may be able to obtain a potentially curative resection with clear surgical margins with a radical resection involving a hemipelvectomy, may prefer limb salvage with palliative limb reconstruction in order to preserve ambulation and quality of life (10,35). Moreover, limb salvage has been shown to have lower lifetime medical costs and high rates of independent mobility compared to amputation (10).
Masaoka describes a case of palliative reconstruction for a 73-year-old patient with metastatic plantar sarcoma, who had a palliative free tissue transfer for a right plantar wound (36). Although this patient had metastatic sarcoma, the right plantar wound significantly limited his quality of life by causing difficulty walking as well as pain with ambulation. Palliative reconstruction was performed successfully with a free anterolateral thigh flap and the patient was able to ambulate without pain, which maintained his independence and quality of life despite the palliative intent. When assessing whether palliative reconstruction is a viable option for a given patient, it is important to weigh the extent of the intervention against the benefits which should be in line with the patient’s specific goals (35).
Not only is careful patient selection paramount in palliative reconstruction, but the intervention should also not have a negative impact on remaining lifespan (35). When considering options for palliative reconstruction, any additional donor site morbidity should be minimized to reduce complications, hospitalization, and pain. One technique that has been used for palliative reconstruction of the lower extremity with no donor site morbidity is the fillet flap. By using the “spare parts” concept, the distal portions of these resected extremities can be harvested as fillet flaps to reconstruct massive oncologic defects of the lower extremity for both curative and palliative intent (37,38). Not only do fillet flaps have no associated donor site, but they have also been shown to be oncological sound, have acceptable incidence of major complications and improve quality of life (37). Although survival was unchanged, using fillet flap palliative reconstruction allowed for closed and healed wounds as well as an improvement in the quality of life (39).
Case examples of palliative reconstruction of the lower extremity
To further illustrate the benefits of palliative reconstruction of the lower extremity, we present three case examples that demonstrate how palliative reconstruction can improve quality of life, independence, and ambulation in select patients.
Case 1
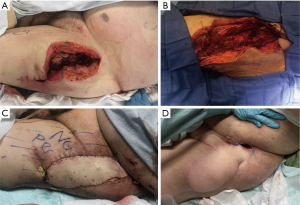
A 78-year-old ambulatory male with a history of coronary arterial disease, hypertension, and atrial fibrillation presented via transfer from an outside hospital with an abscess of the left superior thigh for a higher level of care (Figure 1). Prior to the abscess, the patient had a history of a palpable mass in this area for several months. Upon arrival, an initial incision and drainage was performed to decompress the abscess and pathology was consistent with chondrosarcoma (Figure 1A). Potentially due to receiving antibiotics prior to admission, cultures were negative. Despite evidence concerning for metastatic spread to the lungs, the patient underwent wide local excision of the primary oncologic focus, resulting in a significant soft tissue defect (Figure 1B). This was initially managed with a negative pressure dressing until final margins resulted. Once negative margins were confirmed, the defect was reconstructed with a contralateral chimeric anterolateral thigh flap with vastus lateralis muscle. The muscle was used to fill the large defect, and the skin and subcutaneous tissue were used to resurface the skin defect (Figure 1C). The surgery was well tolerated, and the postoperative course was uneventful other than the development of two superficial areas of dehiscence, one of which healed with expectant management and the other which required surgical closure (Figure 1D). Ultimately, several months after the patient had fully healed his wounds, he succumbed to stage 4 sarcoma. Notably, the benefit of palliative reconstruction for this patient was that it facilitated his ability to ambulate and remain functional (i.e., likely improving quality of life) in the last few months of his life. Of note, the patient reported satisfaction with having undergone palliative surgery and subjective improvement in quality of life.
Case 2
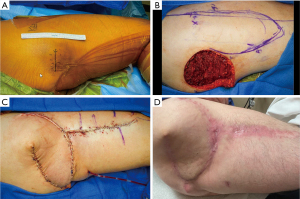
A 52-year-old otherwise healthy male presented with a mass of the right superior posterior thigh and inferior gluteal region (Figure 2). A biopsy was performed and consistent with osteosarcoma. Despite concern for metastatic spread on imaging, the decision was made in collaboration with the orthopedic oncology team to perform wide local excision in an effort to control the primary tumor focus (Figure 2A). Given the size of the defect and the exposure of the sciatic nerve, immediate reconstruction with a pedicled tensor fascia lata flap was performed (Figure 2B,2C). The surgery was well tolerated and the postoperative recovery was unremarkable (Figure 2D). He was later treated with systemic therapy for his stage 4 disease. After surgical resection and palliative reconstruction, the patient was able to ambulate and function appropriately, which demonstrates the utility of palliative lower extremity reconstruction in preserving patient ambulation, and independence and providing effective palliation. After healing was complete, the patient was treated with adjuvant chemotherapy for his metastatic disease, which continues currently.
Case 3
A 36-year-old male with high-grade chondroblastic osteosarcoma underwent neoadjuvant chemotherapy followed by curative surgery with a right extended external hemipelvectomy with hemisacrectomy (Figure 3), resulting in a 25 cm × 20 cm pelvic defect (Figure 3A-3C). Tumor thrombus was identified in the right common iliac vein with extension to the inferior vena cava (IVC), which informed the design of the reconstruction, as these were not suitable recipient vessels. Per hematology and vascular surgery recommendations, the patient received anticoagulation treatment but was not an ideal candidate for an IVC filter. Prior to resection, a lower extremity fillet flap was harvested; however, given the intraoperative findings of tumor involvement of the planned recipient vessels, flap harvest was modified to include the entire length of the femoral vessels in order to maximize pedicle length (Figure 3D). A vascular tunneler was used to tunnel the femoral vessels subcutaneously in order to perform an end-to-side anastomosis to the ipsilateral axillary vessels. Due to the extent and length of the procedure, a temporary negative pressure dressing was applied and flap inset was staged. Due to significant volume resuscitation, edema resulted in venous congestion due to kinking of the femoral vein where the pedicle exited the flap and entered the inferior aspect of the subcutaneous tunnel. Operative exploration was performed and the subcutaneous tunnel was unroofed to allow for a more favorable lie for the vein to improve outflow. Flap inset and placement of acellular dermal matrix were later performed. The patient’s postoperative course was otherwise uncomplicated and he was discharged to acute rehabilitation after two weeks. The patient was able to ambulate with assistance at the three-month follow-up visit (Figure 3E). Most recently, the patient remains active but with incurable metastatic disease.

Conclusions
Palliative lower extremity reconstruction has an important role in maintaining quality of life, ambulation, and independence for oncologic patients. Careful patient selection, balancing the risks and benefits guided by the patient’s goals, and minimizing donor site morbidity are important considerations in palliative reconstruction of the lower extremity.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Marios Papadakis) for the series “Palliative Reconstructive Surgery” published in Annals of Palliative Medicine. The article has undergone external peer review.
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-23-358/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-23-358/coif). The series “Palliative Reconstructive Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Consent was provided for use of all images and case-related information.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
※Special series on Palliative Reconstructive Surgery.
References
- ACS. Statement of Principles of Palliative Care. [cited 2023 Mar 27]. Available online: https://www.facs.org/about-acs/statements/principles-of-palliative-care/
- Hanna J, Blazer DG, Mosca PJ. Overview of palliative surgery: principles and priorities. J Palliative Care Med 2012;2:132. [Crossref]
- Nthumba PM. Palliative reconstructive surgery: contextualizing palliation in resource-poor settings. Plast Surg Int 2014;2014:275215. [Crossref] [PubMed]
- Badgwell B, Krouse RS. The role of general surgery in the palliative care of patients with cancer. In: Cherny N, Fallon M, Kaasa S, et al. editors. 5 edition. Oxford textbook of palliative medicine. Oxford: Oxford University Press, 2015:790-8.
- Chan JY, To VS, Wong ST, et al. Quality of dying in head and neck cancer patients: the role of surgical palliation. Eur Arch Otorhinolaryngol 2013;270:681-8. [Crossref] [PubMed]
- Teven CM, Lettieri SC, Rebecca AM. The Case for Palliative Reconstruction. Plast Reconstr Surg 2021;148:868e-70e. [Crossref] [PubMed]
- Farzaliyev F, Steinau HU, Ring A, et al. Outcome of Surgery as Part of Palliative Care of Patients with Symptomatic Advanced or Metastatic Extra-Abdominal High-Grade Soft Tissue Sarcoma. Palliat Med Rep 2023;3:64-70. [Crossref] [PubMed]
- LiBrizzi CL. Indications and outcomes of palliative major amputation in patients with metastatic cancer. Surg Oncol 2022;40:101700. [Crossref] [PubMed]
- Deo SVS, Kumar N, Rajendra VKJ, et al. Palliative Surgery for Advanced Cancer: Clinical Profile, Spectrum of Surgery and Outcomes from a Tertiary Care Cancer Centre in Low-Middle-Income Country. Indian J Palliat Care 2021;27:281-5. [Crossref] [PubMed]
- Daigeler A, Harati K, Kapalschinski N, et al. Plastic surgery for the oncological patient. Front Surg 2014;1:42. [Crossref] [PubMed]
- Beahm EK, Chang DW. Chest wall reconstruction and advanced disease. Semin Plast Surg 2004;18:117-29. [Crossref] [PubMed]
- Amrillaeva V, Dralle H, Weber F, et al. The internal mammary artery perforator flap for neck reconstruction after palliative resection of advanced anaplastic thyroid cancer: a case report. J Med Case Rep 2023;17:8. [Crossref] [PubMed]
- Rankin T, Mailey B, Suliman A, et al. Palliative reconstructive surgery may improve quality of life in high functioning noncurable head and neck oncologic patients. Ann Plast Surg 2015;74:S52-6. [Crossref] [PubMed]
- Wang HT, Erdmann D, Olbrich KC, et al. Free flap reconstruction of the scalp and calvaria of major neurosurgical resections in cancer patients: lessons learned closing large, difficult wounds of the dura and skull. Plast Reconstr Surg 2007;119:865-72. [Crossref] [PubMed]
- Neumann ED, León Vintró X, Vega García C, et al. Oral cavity colon adenocarcinoma metastases: case report with surgical approach and review of more than 30 years literature. Oral Maxillofac Surg 2021;25:99-101. [Crossref] [PubMed]
- Jang DW, Teng MS, Ojo B, et al. Palliative surgery for head and neck cancer with extensive skin involvement. Laryngoscope 2013;123:1173-7. [Crossref] [PubMed]
- Gasteratos K, Alser O, Hart J, et al. Palliative Reconstructive Surgery for Advanced Maxillofacial Osteosarcoma in the Peak of COVID-19 Pandemic: A Matter of Ethical Decision-making. Plast Reconstr Surg Glob Open 2021;9:e3545. [Crossref] [PubMed]
- Miglani A, Patel VM, Stern CS, et al. Palliative Reconstruction for the Management of Incurable Head and Neck Cancer. J Reconstr Microsurg 2016;32:226-32. [Crossref] [PubMed]
- Harati K, Kolbenschlag J, Behr B, et al. Thoracic Wall Reconstruction after Tumor Resection. Front Oncol 2015;5:247. [Crossref] [PubMed]
- Kuerer HM, Beahm EK, Swisher SG, et al. Surgery for inoperable breast cancer. Am J Surg 2002;183:160-1. [Crossref] [PubMed]
- Durgan DM, De La Cruz Ku G, Thomas M, et al. Chest wall resection for breast cancer: 21st century Mayo clinic experience. J Surg Oncol 2022;126:962-9. [Crossref] [PubMed]
- el-Tamer M, Chaglassian T, Martini N. Resection and debridement of chest-wall tumors and general aspects of reconstruction. Surg Clin North Am 1989;69:947-64. [Crossref] [PubMed]
- Contant CM, van Geel AN, van der Holt B, et al. The pedicled omentoplasty and split skin graft (POSSG) for reconstruction of large chest wall defects. A validity study of 34 patients. Eur J Surg Oncol 1996;22:532-7. [Crossref] [PubMed]
- Newing RK, Pribaz JJ, Bennett RC, et al. Omental transposition and skin graft in the management of chest wall recurrence of carcinoma of the breast. Aust N Z J Surg 1979;49:546-51. [Crossref] [PubMed]
- Gazyakan E, Engel H, Lehnhardt M, et al. Bilateral double free-flaps for reconstruction of extensive chest wall defect. Ann Thorac Surg 2012;93:1289-91. [Crossref] [PubMed]
- Sillah NM, Shah J, Fukudome E, et al. Use of Lumbar Perforator Recipient Vessels for Salvage Chest Wall Reconstruction: A Case Report. Plast Reconstr Surg Glob Open 2016;4:e642. [Crossref] [PubMed]
- Zachary LS, Gottlieb LJ, Simon M, et al. Forequarter amputation wound coverage with an ipsilateral, lymphedematous, circumferential forearm fasciocutaneous free flap in patients undergoing palliative shoulder-girdle tumor resection. J Reconstr Microsurg 1993;9:103-7. [Crossref] [PubMed]
- Cordeiro PG, Cohen S, Burt M, et al. The total volar forearm musculocutaneous free flap for reconstruction of extended forequarter amputations. Ann Plast Surg 1998;40:388-96. [Crossref] [PubMed]
- Dudek W, Schreiner W, Horch RE, et al. Sternal resection and reconstruction for secondary malignancies. J Thorac Dis 2018;10:4230-5. [Crossref] [PubMed]
- Daigeler A, Harati K, Goertz O, et al. Thoracic Wall Reconstruction in Advanced Breast Tumours. Geburtshilfe Frauenheilkd 2014;74:548-56. [Crossref] [PubMed]
- Santos PJ, Prendergast C, Leis A. Giant Anterior Chest Wall Basal Cell Carcinoma: An Approach to Palliative Reconstruction. Case Rep Oncol Med 2016;2016:5067817. [Crossref] [PubMed]
- Fujioka M, Yakabe A. Palliative surgery for advanced fungating skin cancers. Wounds 2010;22:247-50. [PubMed]
- Tashiro J, Yamaguchi S, Ishii T, et al. Salvage total pelvic exenteration with bilateral v-y advancement flap reconstruction for locally recurrent rectal cancer. Case Rep Gastroenterol 2013;7:175-81. [Crossref] [PubMed]
- Kandi LA, Hammond JB, Struve SL, et al. Ethical Considerations in Palliative Reconstruction. Plast Reconstr Surg 2022;150:938e-9e. [Crossref] [PubMed]
- Vogt PM, Jokuszies A. Advanced malignant soft tissue tumors: plastic reconstructive options for palliative treatment. Chirurg 2010;81:1108-14. [Crossref] [PubMed]
- Masaoka K, Tokuhara S, Tsuchiya K, et al. Palliative Free Flap Surgery for Plantar Sarcoma: A Case Report and Literature Review. Cureus 2022;14:e30488. [Crossref] [PubMed]
- Ver Halen JP, Yu P, Skoracki RJ, et al. Reconstruction of massive oncologic defects using free fillet flaps. Plast Reconstr Surg 2010;125:913-22. [Crossref] [PubMed]
- Kreutz-Rodrigues L, Mohan AT, Moran SL, et al. Extremity free fillet flap for reconstruction of massive oncologic resection-Surgical technique and outcomes. J Surg Oncol 2020;121:465-73. [Crossref] [PubMed]
- Tran NV, Evans GR, Kroll SS, et al. Free filet extremity flap: indications and options for reconstruction. Plast Reconstr Surg 2000;105:99-104. [Crossref] [PubMed]


