Could time of whole brain radiotherapy delivery impact overall survival in patients with multiple brain metastases?
Introduction
Brain metastases occur in about 20–40% of advanced cancer patients, with lung cancers accounting for at least half of these occurrences and breast cancers accounting for 15–25% (1,2). Often, brain metastases manifest in upwards of 60–75% of patients as symptoms such as headaches, seizures, sensory deficits, and cognitive decline (1,2). Treatment modalities for brain metastases include neurosurgery, stereotactic radiosurgery (SRS), whole brain radiotherapy (WBRT), and corticosteroid use (1,2). Treatment decisions are based on patient and tumour characteristics. Those with a single brain metastasis or resectable metastases may benefit most from SRS or neurosurgery. On the other hand, symptomatic patients with many brain metastases and for whom the goal of care is often to improve quality of life (QOL), may be treated with WBRT (3). Response to WBRT in terms of symptom improvement ranges from 64% to 85%; however, since patients treated with WBRT often have poor prognosis, progression of brain metastases or other systemic disease may cause death prior to the patient receiving the full benefits of treatment (4). Thus, ways to enhance treatment efficacy for this patient population should be investigated.
Circadian rhythms are generated by the rhythmic expression of clock genes in the anterior hypothalamus that result in 24-hour patterns of hormonal secretion, body temperature fluctuation, and autonomic nervous system activity. Circadian rhythms also affect cell cycle progression. Each phase of the cell cycle is associated with different radiosensitivity (5-8), with the gap 2 (G2) and mitosis (M) phases being particularly radiosensitive (5-8). Chronotherapy aligns treatment delivery with naturally occurring circadian rhythms in an attempt to optimize treatment outcomes and potentially enhance survival (5,9,10). Numerous studies conducted with animal models have found that timing of radiotherapy delivery to discrete times in the day resulted in more radiation-induced apoptosis when cells were in the G2/M phases (7,11,12). The efficacy of chronotherapy may also be sex-specific, as previous studies conducted in animal models and human patients receiving chemotherapy observed different outcomes between males and females (13-15). A meta-analysis of three phase III trials in colorectal cancer patients concluded that optimal treatment schedules (chronomodulated or conventional infusions) to benefit OS differed depending on sex, with only males benefiting from chronomodulated therapy (13). Sex may be an important mediator of sensitivity to chronotherapy and further studies examining chronotherapy should segregate cohorts by sex to determine optimal treatment regimens for both males and females.
The efficacy of chronotherapy has also previously been studied in the context of SRS for the treatment of brain metastases in non-small cell lung cancer (NSCLC) patients (6-8,16). In these retrospective studies, the local control (LC) of treated metastases and overall survival (OS) were compared between patients treated in the morning and afternoon. One of these studies reported improved LC (97% and 67%, P=0.01), OS (9.5 and 5 months, P=0.03), and less CNS-related deaths (6% and 24%, P=0.03) in patients treated in the morning (6). However, three subsequent studies attempting to replicate these results in larger patient populations failed to observe any significant correlations between treatment time and LC or OS (7,8,16). Consequently, it is difficult to come to any conclusions regarding the efficacy of chronotherapy in SRS. No studies to date have investigated the role of chronotherapy in WBRT, which is a commonly used treatment modality for palliative brain metastases patients. As such, the present study aimed to investigate whether or not any correlations between OS and treatment time exist in cancer patients receiving WBRT. We hypothesize that time of WBRT delivery may be related to OS in patients with brain metastases.
Methods
A retrospective review of prospectively collected databases was conducted. Patients with radiologically confirmed brain metastases who received one session of WBRT between November 2004 and January 2016 were included in the study. Patients who received SRS at any point were excluded. Our institution’s Research Ethics Board approved this study.
Data collection
Baseline demographic and clinical information including age, gender, Karnofsky performance status (KPS), primary cancer site, dose, and fraction were prospectively collected. Treatment times and dates of death were extracted from patient medical records. OS was calculated in months from the initial treatment date to date of death. Patients that were alive at the time of analysis or lost to follow-up were censored at the date of last contact.
Segregation of treatment cohorts
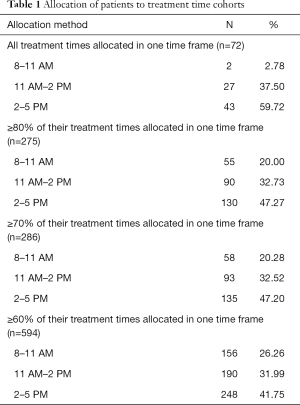
Patients were assigned to morning (8:00–11:00 AM), early afternoon (11:01AM–2:00 PM), or late afternoon (2:01–5:00 PM) cohorts based on time of radiotherapy delivery. The time intervals were selected based on a study by Bjarnason et al. (17) that investigated cell cycle progression in human oral mucosa, and found protein indicators of each cell cycle phase to peak at different time periods in the day. The protein expression of the late G1 phase, G1/S boundary, G2 phase, M phase peak at 11:00 AM, 3:00 PM, 4:00 PM, 11:00 PM, respectively. However, 11:00 PM was not included as no patients are treated as this time at our center. Treatment times before 8:00 AM were assigned to the morning cohort and those after 5:00 PM were assigned to the late afternoon cohort. To allocate a patient to a particular cohort, all (100%) of their treatment times were required to fall in one time frame. Those who did not fit this criterion were excluded from statistical analysis. Using this method, a small number of patients (n=2) were allocated to the 8:00–11:00 AM cohort. Therefore, the allocation process was repeated by assigning patients to each time cohort based on ≥80%, ≥70%, and ≥60% of their treatment times falling into one time frame. Demographic parameters were again compared for the different cohorts for each allocation method. The above analysis was again conducted by separating all patients based on whether treatment was received at consistent times of day or inconsistent times of day. Consistent treatment was defined as ≥80% of radiation treatment delivered within 2 hours of each other. Patients that did not fit this criterion were considered inconsistent.
Statistical analyses
Descriptive statistics were conducted in all patients for demographics using median and inter-quartiles for continuous variables and proportions for categorical variables. Based on different percentages of treatment times falling into one time frame (i.e., 100%, ≥80%, ≥70%, or ≥60%), baseline characteristics were compared among the three cohorts (8:00–11:00 AM, 11:01 AM–2:00 PM, and 2:01–5:00 PM) using the Kruskal-Wallis nonparametric test or Fisher exact test for continuous and categorical variables, respectively. To control the multiple comparisons on demographics (age, gender, KPS value, KPS >70, KPS >80, primary cancer site, and duration of follow-up) among three time allocations, a Bonferroni adjusted P value <0.007 was considered statistically significant.
Kaplan-Meier OS curves were plotted for the three treatment cohorts and compared using the log-rank test. Univariate Cox proportional hazard (PH) model of OS was conducted in all patients with demographic parameters and treatment time allocations. Multivariate analysis was subsequently conducted using a backward stepwise selection procedure for all variables with a P value <0.10 in univariate analysis. In multivariate analysis, P<0.05 was considered statistically significant. The R2 statistic was evaluated to determine the strength of the association between a predictive factor and OS. The 95% confidence interval (CI) and hazard ratio (HR) were also calculated. All statistical analyses were performed using statistical analysis software (SAS version 9.4 for Windows). In order to account for potential differences in circadian rhythms between genders, all statistical analyses were performed for males and females separately.
Results
Patient characteristics
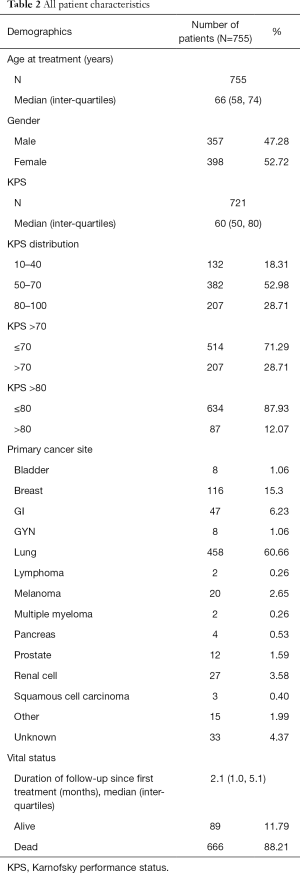
A total of 755 patients were included in the study. The median age at treatment was 66 years and there were slightly more females (52.7%). Lung (60.7%) and breast cancer (15.4%) were the most common primary cancer sites. Patients with poor KPS [10–40] represented 18.3% of patients. Patients with KPS of 50–70 and of 70–100 represented 53.0% and 28.7% of the patient population, respectively. The median time from first treatment to follow-up was 2.1 months (range of 0 to 93 months). The most common radiation dosing schedules were 20 Gy in 5 fractions (93% of patients) and 30 Gy in 10 fractions (6% of patients). Table 1 provides the number of patients within each treatment cohort using the various allocation methods and Table 2 summarizes patient characteristics.
Full table
Full table
The actuarial median OS of all 755 patients was 2.37 months (95% CI, 2.17–2.69). Of these patients, 664 died and the censored rate was 12.1%. Three patients with very long survival times (>60 months) were censored as alive at 35 months. In males and females, the actuarial median OS was 2.27 months (95% CI, 1.97–2.69) and 2.40 months (95% CI, 2.17–2.89), respectively.
All patients
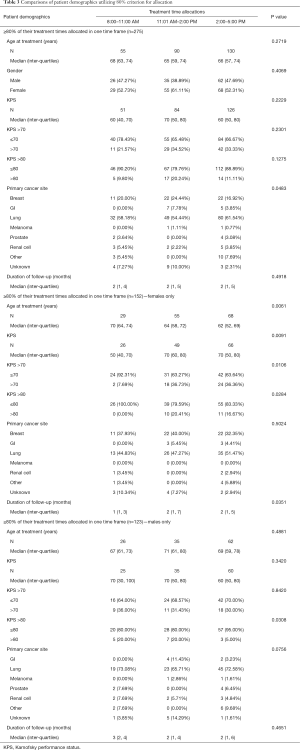
Using ≥80% of treatment times (Table 3), 275 patients were analysed and allocated to three cohorts (8:00–11:00 AM, n=55; 11:01 AM–2:00 PM, n=90; 2:01–5:00 PM, n=130). There were no statistically significant differences in baseline characteristics when allocating patients using ≥80%, ≥70% or ≥60% of their treatment times.
Full table
For patients allocated based on the ≥80% criterion, the 8:00–11:00 AM cohort (1.95 months; 95% CI, 1.31–2.89) tended to have shorter OS compared to other cohorts (11:01 AM–2:00 PM; 2.33 months; 95% CI, 1.87–3.35; 2:01–5:00 PM; 2.40 months; 95% CI, 1.81–3.55), although this was not statistically significant (P=0.2). No significant differences in OS were found in the ≥70% and ≥60% criterion cohorts.
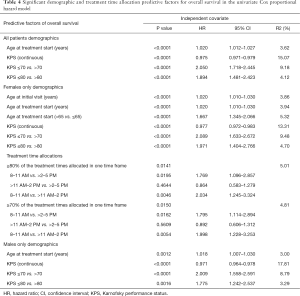
Upon univariate Cox PH model, age at the start of treatment and KPS (continuous, >70, or >80) were significantly related to OS (Table 4). In the multivariate analysis, only KPS remained significant (Table 5).
Full table

Full table
Females
When females were analysed exclusively, 152 and 155 patients were included using ≥80% and ≥70% of treatment times, respectively. Using the 80% criterion, the 8:00–11:00 AM, 11:01 AM–2:00 PM, and 2:01–5:00 PM cohorts differed significantly in age at start of treatment, with the 8:00–11:00 AM cohort (n=29) consisting of older patients (P=0.006) (Table 3). Similar results were found when 70% criteria or 60% criterion was applied.
Significant differences in survival were found between the three cohorts using the 80% (P=0.01) and 70% criterion (P=0.01). These results were further analyzed by plotting separate Kaplan-Meier OS curves based on age (≤65 vs. >65 years) and KPS (≤70 vs. >70). In female patients >65 years of age using the 80% criterion, those in the 11:01 AM–2:00 PM cohort exhibited a longer actuarial median OS (2.12 months; 95% CI, 1.54–5.91) than the 8:00–11:00 AM (1.23 months; 95% CI, 0.53–1.97) and 2:01–5:00 PM (1.18 months, 95% CI, 0.56–2.23) cohorts (P=0.0190) (Figure 1). This was not significant for younger patients (P=0.4) (Figure 1). However, this may be explained by difference in age at start of treatment, as younger patients were more likely to receive treatment in the afternoon when compared to older patients. Therefore the difference in OS between younger and older female patients could be explained by the significant difference of age at the start of treatment between the time cohorts. When dichotomized by KPS, the OS curves did not significantly differ between cohorts (P=0.07 and P=0.52 for KPS >70 and KPS ≤70, respectively). Similar results were seen when the 70% criterion was used. The 60% criterion cohorts revealed no significant difference in OS.
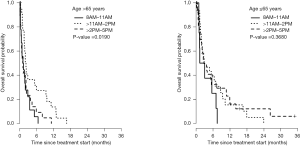
In univariate Cox PH model, age (at start of treatment or >65) and KPS (continuous, >70, or >80), and treatment time (≥80% or ≥70%) were significantly related to OS (Table 4). Patients in the 8:00–11:00 AM were more likely to have a shorter OS than the 11:01 AM–2:00 PM (P=0.005, HR =2.03, 95% CI, 1.25–3.32) or 2:01–5:00 PM cohorts (P=0.02, HR =1.77, 95% CI, 1.10–2.86). In multivariate analysis, age (>65) was considered as a confounding factor to adjust for the age effect on OS in females from different treatment cohorts. Only KPS (>70) was still significant in the final model; treatment time failed to remain significant (Table 5).
Males
For males, 123 patients were included in analysis using the 80% criterion. In Table 3, we did not find statistically significant differences in baseline characteristics when allocating patients using 80% of their treatment times (8:00–11:00 AM, n=26; 2:01–5:00 PM, n=62; 11:01 AM–2:00 PM, n=35). There were no statistically significant differences in baseline characteristics when allocating patients using 70% or 60% of their treatment times.
Males treated in the 2:01–5:00 PM cohort had the longest actuarial median OS (2.76 months; 95% CI, 1.77–3.81) and the 11:01 AM–2:00 PM cohort had the shortest actuarial median OS (2.05 months; 95% CI, 1.15–3.58). The 8:00–11:00 AM cohort had an actuarial median OS of 2.53 months (95% CI, 1.71–3.61). This was not statistically significant (P=0.59). No significant differences in OS were found in the 70% and 60% criterion cohorts either.
In the univariate Cox PH model, age at the start of treatment and KPS (continuous, >70, or >80) were significantly related to OS (Table 4); older age and KPS ≤70 remained significantly related to OS in the multivariate analysis (Table 5).
Consistent vs. inconsistent times of treatment
No significant difference in OS was found between patients who received treatment at inconsistent or consistent times of day for all patients (P=0.19), males only (P=0.22), and females only (P=0.61).
Discussion
WBRT is a commonly used treatment modality for advanced cancer patients with poor prognosis and multiple brain metastases. Prior studies have examined how the time of SRS delivery can impact LC and OS, with inconsistent results (6-8,16). The aim of the present study was to determine whether treatment time affects OS in patients receiving WBRT for brain metastases. A subgroup analysis of elderly female patients (>65 years) revealed that those treated between 11:01 AM and 2:00 PM had significantly longer OS when compared to the other time cohorts upon univariate analysis only. However, this may be due to the significant differences in age at start of treatment between the three time allocations. Time of radiotherapy delivery had no impact on OS in male patients or female patients. Median survival for patients treated with WBRT for brain metastases ranges from 2.7–5.5 months (11,12,18,19), which is comparable, but slightly higher than the actuarial median OS found in our patient population (2.4 months). The median OS may have been lower than previous studies as we only included patients who received 1 session of WBRT, whereas patients who received multiple sessions of WBRT would have had longer survival.
Previous studies have examined chronotherapy in SRS treatment for NSCLC patients using various time frames to separate patients. Rahn et al. were the first to do this, using a cut-off point of 12:30PM as it equally separated patients into two cohorts and simplified notation for clinical and statistical applications (6). To confirm the positive findings of this study, Kabolizadeh et al. separated patients treated before and after 12:00PM (16). Subsequent studies by Badiyan et al. utilized receiver operating characteristic (ROC) curves to find the optimal cutoff point (11:41 and 11:42 AM) corresponding to the most significant difference in OS between cohorts (7,8). Despite the similar time frames, treatment time was predictive of LC or OS exclusively in the study by Rahn et al. (6). As this study had 97 patients (only 48 assessed for LC), whereas the remaining studies analysed much larger groups of patients, low patient numbers could account for their positive findings. Additionally, as the time frames used were in close proximity of each other, cell cycle changes may have produced very minute differences in outcomes and thus were undetectable. Also, if three or more distinct time frames corresponding to differing treatment outcomes were to exist, separating patients using only two time frames may not be an adequate method of detecting these differences in outcomes. While previous studies of chronotherapy for brain metastases segregated patients using cohorts that maximized survival differences, the present study utilized time frames that corresponded to previously reported phases of the cell cycle in human oral epithelium (17).
Gender differences in response to chronotherapy have been reported in previous studies. A meta-analysis by Giacchetti et al. of three international phase III trials in colorectal cancer patients receiving chemotherapy observed that males received survival benefits from chronomodulated chemotherapy, while females experienced worse OS (13). Additionally, Bjarnason et al. prospectively evaluated the incidence of oral mucositis induced by radiotherapy in head and neck cancer patients and observed less mucositis in males when treated in the morning compared to the afternoon, while the opposite trend was detected in women (14). In a study by Ahowesso et al., the degree of irinotecan-induced circadian disruptions was compared between male and female mice. In female mice, the degree of circadian disruption varied more drastically depending on time of administration. Conversely, the response to treatment in male mice remained the same regardless of administration time (15). Therefore, we hypothesize that the differences observed in females resulted from their increased susceptibility to the effects of chronotherapy due to underlying gender-specific circadian mechanisms. Future studies should investigate the efficacy of chronotherapy in females with more homogenous patient populations.
Age may potentially influence the effectiveness of chronotherapy in the radiation setting. The study by Hsu et al. on prostate cancer patients observed a significant correlation between evening radiation and higher incidence of late gastrointestinal toxicities only for patients ≥70 years old (P<0.0001) (20). A possible explanation for this age-dependent effect is that the circadian rhythm of melatonin dampens with age, resulting in reduced amplitude and duration of melatonin peaks (21). In the elderly population, females may exhibit even lower levels of melatonin secretion in comparison to males due to post-menopausal changes (22-24). Prior research has shown that melatonin provides a protective effect against radiation-induced oxidative stress by scavenging for free radicals (25-30). This radiation-induced damage to DNA in the brain can persist weeks to months post-radiation (25-30). In fact, levels of oxidative stress markers have been associated with increased all-cause mortality, as well as cancer mortality (31). As elderly women exhibit reduced melatonin levels due to aging and post-menopausal changes, they may have less protection from oxidative stress. Thus, when treated with radiotherapy at optimal timing, malignant tissues may be more susceptible to radiation damage.
Studies have observed that patients with poorer performance status exhibited abolished or altered circadian rhythmicity in cortisol and hematologic variables (32,33). Therefore, the performance status of patients could potentially reduce the impact of treatment timing on outcomes. In our study, a close to significant difference in survival was seen in females with KPS >70 (P=0.07), however no survival curve could be created for the 8:00–11:00 AM cohort due to low patient numbers. In females with KPS ≤70, survival did not differ based on treatment time. Our results reflect the potential impact of poor performance status in the efficacy of chronotherapy, but further research must be done to validate this.
Dexamethasone is a synthetic glucocorticoid commonly prescribed in conjunction with WBRT in order to alleviate symptoms related to intracerebral edema and toxicities of radiotherapy (34). Endogenous glucocorticoids act as an entraining signal in peripheral tissues, synchronizing peripheral clocks with the circadian pacemaker, the suprachiasmatic nuclei (SCN) (35,36). Treatment with dexamethasone can cause phase shifts in circadian rhythms by inducing the expression of core circadian genes, Per1 and Per2 (37,38). Therefore, concurrent use of dexamethasone with WBRT may alter the role and optimal timing of chronotherapy. Future investigations should take this limitation into account.
As our study was a retrospective analysis of prospective data, we were unable to allocate patients equally into each time cohort, resulting in a deficit of patients in the 8:00–11:00 AM cohort. As well, many of the patients included in the paper had good KPS and may have been eligible for chemotherapy; however, the systemic therapy that patients were receiving at time of WBRT was not able to be collected for all patients due to the retrospective nature of this study and therefore limited our findings. In addition, our study population was quite heterogeneous which limits the conclusions that can be made from our investigations. Future studies examining this topic should be conducted prospectively to allow for equal allocation into treatment cohorts with more homogenous patient groups and to allow the collection of more patient characteristics parameters. Furthermore, we only considered age and KPS as a prognostic factor despite validated prognostic models including factors such as number of brain metastases and the presence of extracranial metastases. Finally, patients’ OS may have been affected by progression of systemic disease rather than reflect the degree of control of brain metastases. In future studies, this should be addressed by using LC as the primary endpoint, as well as collecting information on cause of death.
Conclusions
The present study was the first to examine the influence of the timing of WBRT on OS in cancer patients. We conclude that chronotherapy in the context of WBRT may potentially affect OS in elderly women, as treatment delivery between 11:01 AM–2:00 PM was associated with longer OS, but only upon univariate analysis and may be due to significant differences between treatment cohorts. The heterogeneous nature of our patient population limits the conclusions that can be made regarding the impact of chronotherapy for patients with brain metastases. Chronotherapy should be further explored in this setting due to the poor prognosis of patients receiving WBRT, as mechanisms to improve survival in this patient population are urgently needed. Future studies should focus on LC as the primary endpoint and account for differences in age, tumor type, performance status and gender during analysis. In addition, the impact of extra-cranial disease needs to be included in the analysis of OS.
Acknowledgements
We thank the generous support of Bratty Family Fund, Michael and Karyn Goldstein Cancer Research Fund, Joey and Mary Furfari Cancer Research Fund, Pulenzas Cancer Research Fund, Joseph and Silvana Melara Cancer Research Fund, and Ofelia Cancer Research Fund.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by our institution’s Research Ethics Board (No. 080-2016).
References
- Soffietti R, Cornu P, Delattre JY, et al. EFNS Guidelines on diagnosis and treatment of brain metastases: report of an EFNS Task Force. Eur J Neurol 2006;13:674-81. [Crossref] [PubMed]
- Pérez-Larraya JG, Hildebrand J. Brain metastases. In: Biller J, Ferro JM. editors. Handbook of Clinical Neurology. Volume 121. Amsterdam: Elsevier BV, 2014:1143-57.
- Tsao MN. Brain metastases: advances over the decades. Ann Palliat Med 2015;4:225-32. [PubMed]
- Tsao MN, Lloyd N, Wong RK, et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev 2012.CD003869. [PubMed]
- Mormont MC, Levi F. Cancer chronotherapy: principles, applications, and perspectives. Cancer 2003;97:155-69. [Crossref] [PubMed]
- Rahn DA 3rd, Ray DK, Schlesinger DJ, et al. Gamma knife radiosurgery for brain metastasis of nonsmall cell lung cancer: is there a difference in outcome between morning and afternoon treatment? Cancer 2011;117:414-20. [Crossref] [PubMed]
- Badiyan SN, Ferraro DF, Yaddanapudi S, et al. Impact of time of day of gamma knife radiosurgery on survival for non-small cell lung cancer brain metastases. Radiother Oncol 2012;103:S491-2. [Crossref]
- Badiyan SN, Ferraro DJ, Yaddanapudi S, et al. Impact of time of day on outcomes after stereotactic radiosurgery for non-small cell lung cancer brain metastases. Cancer 2013;119:3563-9. [Crossref] [PubMed]
- Lévi F. Circadian chronotherapy for human cancers. Lancet Oncol 2001;2:307-15. [Crossref] [PubMed]
- Innominato PF, Lévi FA, Bjarnason GA. Chronotherapy and the molecular clock: Clinical implications in oncology. Adv Drug Deliv Rev 2010;62:979-1001. [Crossref] [PubMed]
- Priestman TJ, Dunn J, Brada M, et al. Final results of the Royal College of Radiologists' trial comparing two different radiotherapy schedules in the treatment of cerebral metastases. Clin Oncol (R Coll Radiol) 1996;8:308-15. [Crossref] [PubMed]
- Chatani M, Matayoshi Y, Masaki N, et al. Radiation therapy for brain metastases from lung carcinoma. Prospective randomized trial according to the level of lactate dehydrogenase. Strahlenther Onkol 1994;170:155-61. [PubMed]
- Giacchetti S, Dugué PA, Innominato PF, et al. Sex moderates circadian chemotherapy effects on survival of patients with metastatic colorectal cancer: a meta-analysis. Ann Oncol 2012;23:3110-6. [Crossref] [PubMed]
- Bjarnason GA, Mackenzie RG, Nabid A, et al. Comparison of toxicity associated with early morning versus late afternoon radiotherapy in patients with head-and-neck cancer: a prospective randomized trial of the National Cancer Institute of Canada Clinical Trials Group (HN3). Int J Radiat Oncol Biol Phys 2009;73:166-72. [Crossref] [PubMed]
- Ahowesso C, Li XM, Zampera S, et al. Sex and dosing-time dependencies in irinotecan-induced circadian disruption. Chronobiol Int 2011;28:458-70. [Crossref] [PubMed]
- Kabolizadeh P, Wegner R, Bernard M, et al. The Effect of Treatment Time on Outcome in Non-small Cell Lung Cancer Brain Metastases Treated with Stereotactic Radiosurgery. Radiation Oncology 2011;81:S301.
- Bjarnason GA, Jordan RC, Sothern RB. Circadian variation in the expression of cell-cycle proteins in human oral epithelium. Am J Pathol 1999;154:613-22. [Crossref] [PubMed]
- Wong E, Tsao M, Zhang L, et al. Survival of patients with multiple brain metastases treated with whole-brain radiotherapy. CNS Oncol 2015;4:213-24. [Crossref] [PubMed]
- Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys 2010;77:655-61. [Crossref] [PubMed]
- Hsu FM, Hou WH, Huang CY, et al. Differences in toxicity and outcome associated with circadian variations between patients undergoing daytime and evening radiotherapy for prostate adenocarcinoma. Chronobiol Int 2016;33:210-9. [Crossref] [PubMed]
- Reiter RJ. The ageing pineal gland and its physiological consequences. Bioessays 1992;14:169-75. [Crossref] [PubMed]
- Obayashi K, Saeki K, Tone N, et al. Lower melatonin secretion in older females: gender differences independent of light exposure profiles. J Epidemiol 2015;25:38-43. [Crossref] [PubMed]
- Toffol E, Kalleinen N, Haukka J, et al. Melatonin in perimenopausal and postmenopausal women: associations with mood, sleep, climacteric symptoms, and quality of life. Menopause 2014;21:493-500. [Crossref] [PubMed]
- Vakkuri O, Kivelä A, Leppäluoto J, et al. Decrease in melatonin precedes follicle-stimulating hormone increase during perimenopause. Eur J Endocrinol 1996;135:188-92. [Crossref] [PubMed]
- Sisodia R, Kumari S, Verma RK, et al. Prophylactic role of melatonin against radiation induced damage in mouse cerebellum with special reference to Purkinje cells. J Radiol Prot 2006;26:227-34. [Crossref] [PubMed]
- Sokolovic D, Djindjic B, Nikolic J, et al. Melatonin reduces oxidative stress induced by chronic exposure of microwave radiation from mobile phones in rat brain. J Radiat Res 2008;49:579-86. [Crossref] [PubMed]
- Pandi-Perumal SR. Melatonin antioxidative defense: therapeutical implications for aging and neurodegenerative processes. Neurotox Res 2013;23:267-300. [Crossref] [PubMed]
- Djordjevic B, Sokolovic D, Kocic G, et al. The effect of melatonin on the liver of rats exposed to microwave radiation. Bratisl Lek Listy 2015;116:96-100. [PubMed]
- Reiter RJ, Guerrero JM, Garcia JJ, et al. Reactive oxygen intermediates, molecular damage, and aging. Relation to melatonin. Ann N Y Acad Sci 1998;854:410-24. [Crossref] [PubMed]
- Kucuktulu E. Protective effect of melatonin against radiation induced nephrotoxicity in rats. Asian Pac J Cancer Prev 2012;13:4101-5. [Crossref] [PubMed]
- Schöttker B, Brenner H, Jansen EH, et al. Evidence for the free radical/oxidative stress theory of ageing from the CHANCES consortium: a meta-analysis of individual participant data. BMC Med 2015;13:300. [Crossref] [PubMed]
- Bailleul F, Lévi F, Reinberg A, et al. Interindividual differences in the circadian hematologic time structure of cancer patients. Chronobiol Int 1986;3:47-54. [Crossref] [PubMed]
- Touitou Y, Lévi F, Bogdan A, et al. Rhythm alteration in patients with metastatic breast cancer and poor prognostic factors. J Cancer Res Clin Oncol 1995;121:181-8. [Crossref] [PubMed]
- Sturdza A, Millar BA, Bana N, et al. The use and toxicity of steroids in the management of patients with brain metastases. Support Care Cancer 2008;16:1041-8. [Crossref] [PubMed]
- Kino T. Circadian rhythms of glucocorticoid hormone actions in target tissues: potential clinical implications. Sci Signal 2012;5:pt4. [PubMed]
- Pezük P, Mohawk JA, Wang LA, et al. Glucocorticoids as entraining signals for peripheral circadian oscillators. Endocrinology 2012;153:4775-83. [Crossref] [PubMed]
- Balsalobre A, Brown SA, Marcacci L, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 2000;289:2344-7. [Crossref] [PubMed]
- Wu X, Yu G, Parks H, et al. Circadian mechanisms in murine and human bone marrow mesenchymal stem cells following dexamethasone exposure. Bone 2008;42:861-70. [Crossref] [PubMed]


