Is every psychiatrist an oncology psychiatrist?—Special needs for special populations: a scoping review
Highlight box
Key findings
• The current literature does not provide treatment guidelines for the management of psychiatric illness for patients with cancer, specifically gastrointestinal (GI) cancers.
• There is lack of both prospective and retrospective trials examining the management of psychiatric illness during treatment of GI malignancies.
What is known and what is new?
• Psychosocial oncology and the treatment of psychiatric illness in the context of cancer, specifically GI malignancies is vital to well-rounded, comprehensive treatment and has grown in importance as part of a multidisciplinary treatment approach for patients.
• The psychosocial oncologist is a critical member of the oncology treatment team, bringing expertise in the treatment and management of psychological complications of cancer treatment for GI malignancies.
What is the implication, and what should change now?
• Cancer centers should continue to grow their psychosocial oncology divisions so that they can provide targeted, specialized mental health treatment for patients provided by experts.
Introduction
Background
Gastrointestinal (GI) malignancies account for approximately 25% of malignancies and almost one-third of cancer death worldwide. There are many complex symptoms that patients with GI malignancies face from anxiety and depression to adjustment to an ostomy, development of sexual dysfunction and symptom management of functional neuroendocrine tumors (NETs). Psychosocial oncology, or psychiatric oncology, is a psychiatric subspecialty focused on the care of patients with cancer.
The evolution of psychiatric care for patients with cancer has played out over the last century. Until the early 1900s, a cancer diagnosis was tantamount to a death sentence. Cancer had no identifiable cause, and no treatments or cures existed. Fearing that patients would lose all hope and crumble emotionally in addition to physically, physicians hid the truth of a cancer diagnosis from patients. Telling the truth was felt to be cruel and inhumane. There was worry that cancer was contagious, and an immense amount of fear, shame, and guilt surrounded those affected. This practice is currently uncommon in the United States but persists in some cultures and communities.
In 1913, The American Cancer Society (ACS), originally named the American Society for the Control of Cancer, was formed and became the first national advocacy agency spreading cancer awareness. During these early years, treatments such as surgery, anesthesia, and tumor removal, started to emerge for some cancers. In 1937, The National Cancer Institute was formed as part of the National Institutes of Health heralding the start of cancer-focused research. In 1948, Sidney Farber achieved remission in a child with leukemia, and the success launched the search for chemotherapeutics. The 1950s brought the discovery of methotrexate as a cure for choriocarcinoma and the beginnings of psychosocial supports for patients with cancer, although the support networks were very loose and informal (1). The ACS developed its “visitor program” which connected patients who had received laryngectomy or colostomy with patients who required one of these procedures. Women who had undergone a mastectomy were joined with other women in the postoperative period through the Reach to Recovery program (2). These groups encountered hostility from the medical community. Contemporary thinking acknowledges and embraces the value of these programs, but at the time of their inception, the importance of these meaningful connections that help patients cope was unknown and unrecognized.
The first academic papers on the psychological impact of cancer surgeries were published out of Memorial Sloan Kettering Cancer Center and Massachusetts General Hospital in the mid-1950s. Clinical trials for psychosocial and psychopharmacological interventions began in the 1960s. The 1980s brought focus on identifying effective coping models and behavioral interventions to change lifestyle and reduce cancer incidence, such as smoking cessation (1,2). The Quality-of-Life Assessment was developed, and for the first time, the medical community began to study a measure of “quality-adjusted life years” in addition to a treatment’s impact on length of survival and disease-free interval (3).
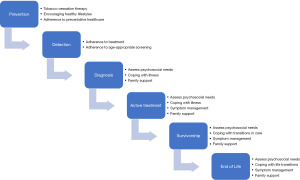
Knowing that approximately 30% of patients with cancer report psychosocial distress and mental disorders during the course of treatment (4), cancer centers employ psychosocial teams that integrate into patients’ comprehensive cancer treatment. In addition, psychiatric morbidity is associated with reduction of quality of life, impairment in social relationships, longer rehabilitation time, poor adherence to treatment, abnormal illness behavior and shorter survival and these quality measures have propelled medical teams to prioritize the mental health and well-being of patients with cancer (5). The role of a psychiatrist specializing in treating patients with cancer, a psychosocial oncologist or psycho-oncologist, spans the care continuum from prevention to end of life and is visualized in Figure 1.
Rationale and knowledge gap
While every patient with cancer would benefit from specialty psycho-oncology care, the reality is that there are not enough psychiatrists to care for all cancer patients. With this in mind, Watson et al. suggested a “stepped psychosocial care model” in which they propose interventions scaled according to the level of distress of patients (2). For those with minimal distress, their psychosocial needs can easily be met by providing information and offering support from their current cancer providers. As patient’s level of need escalates, additional supports, including social workers, peer support, spiritual care providers, palliative care physicians, and counselors are consulted. For those with moderate to severe distress surrounding their disease, having a specialty psycho-oncologist consultation and dedicated psychologist care provides the maximal amount of support in addition to the other layers that have been sequentially added (2). There is little literature focused on the psychiatric treatment of patients with GI malignancies and to our knowledge, no previous review and synthesis of this limited literature.
Objective
Here, we review the literature describing psychiatric illnesses commonly encountered in the care of patients with cancer. In this review, we speak directly to those psychiatric issues unique to patients with GI malignancies and provide key concepts and explore theories of care for these unique patient populations as described in the literature. We present this article in accordance with the PRISMA-ScR reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1287/rc).
Methods
We used established scoping review methodology to guide our study methods and applied the PRISMA-ScR checklist. There was no protocol for this study.
Search strategy
We conducted an electronic PubMed (pubmed.gov) search for English-language articles published up through 2022. We used the terms “psycho-oncology”, “psychosocial oncology”, “psychiatric oncology”, and “psychiatry” for psychiatric inclusion and the terms “gastrointestinal”, and “gastrointestinal malignancy” for oncologic inclusion terms. All articles were screened by a single reviewer. If the inclusion or exclusion of an article was not clear at either the title/abstract screening stage, or the full-text article review, two reviewers screened and discussed the article.
Inclusion and exclusion criteria
We included published articles, regardless of study design or outcomes reported, that described psychiatric or psychologic management of mental health symptoms in patients whose primary oncologic diagnosis was a GI malignancy.
We excluded articles that did not address specific therapeutic or pharmacologic interventions for mental illness, articles that did not offer or review treatment options, and articles that only focused on substance use disorders (outside the scope of this article).
These criteria were developed iteratively, based on increasing familiarity with the literature.
Data analysis
We extracted data based upon our group’s predetermined psychiatric conditions we wished to discuss. Data charting was performed and publication year, health domain, psychiatric illness, and publication journal specialty were collected. A descriptive format was used to synthesize and report the results.
Results
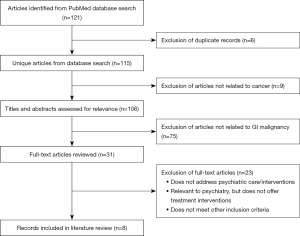
Database search resulted 115 unique manuscripts. After screening the 115 titles and abstracts, and reviewing 31 full-text articles, 8 were included as part of our review of the literature (Figure 2). The eight articles describe psychological factors arising during and after treatment for GI malignancies. The key aspects of these articles are incorporated in a narrative fashion in the next part of this review and basic content information is shown in Table 1.
Table 1
| Article title | Publication year | Journal specialty | Health domain | Psychiatric illness |
|---|---|---|---|---|
| Discrepancies Between Attainment and Importance of Life Values and Anxiety and Depression in Gastrointestinal Cancer Patients and their Spouses (6) | 2001 | Psychooncology | GI malignancy (colon, rectum, gastric, biliary, pancreatic) | Anxiety and depression |
| The role of illness perceptions in adherence to surveillance in patients with familial adenomatous polyposis (FAP) (7) | 2016 | Psychooncology | Familial adenomatous polyposis | Distress |
| Retrospective review of serotonergic medication tolerability in patients with neuroendocrine tumors with biochemically proven carcinoid syndrome (8) | 2017 | Cancer | Neuroendocrine tumors | Anxiety and depression |
| Illness perception and affective symptoms in gastrointestinal cancer patients: A moderated mediation analysis of meaning in life and coping (9) | 2019 | Psychooncology | GI malignancy (esophagus, stomach, pancreas, liver, kidney, large intestine) | Anxiety and depression |
| Illness perceptions and perceived stress in patients with advanced gastrointestinal cancer (10) | 2019 | Psychooncology | Pancreatic, hepatobiliary | Stress |
| Body image mediates the effect of stoma status on psychological distress and quality of life in patients with colorectal cancer (11) | 2020 | Psychooncology | Colorectal cancer | Anxiety, depression, psychological distress, and body image |
| A feasibility study of a peer discussion group intervention for patients with pancreatobiliary cancer and their caregivers (12) | 2022 | Palliative and Supportive Care | Pancreatic, biliary tract | Affective disorder, mood states |
| Psychological distress and resilience in patients with gastroenteropancreatic neuroendocrine tumor (13) | 2022 | Frontiers in Endocrinology | Gastroenteropancreatic neuroendocrine tumors | Distress |
GI, gastrointestinal.
Discussion
Key findings
Psychiatric symptom management patients experience a wide range of emotions and feelings over the course of their diagnosis and treatment. When symptoms lead to functional impairment, it is important to address the root causes and provide targeted symptom support. Wilson and colleagues examined a population of patients receiving palliative care for cancer and found that almost one-quarter (24.4%) of the population met criteria for a mental health disorder. A total of 10.2% met criteria for more than one disorder (14).
Attending to the psychological distress experienced by patients significantly impacts quality of life which is now recognized as an important metric in cancer care—quality-adjusted life years (3). Symptom management can be grouped into two broad categories: pharmacologic and nonpharmacologic support. Each symptom can be managed with strategies and medications from both categories. Pharmacologic interventions target many psychiatric conditions and physical symptoms experienced by patients with cancer. Nonpharmacologic support encompasses psychoeducation, different psychotherapeutic modalities, and neuromodulation. We outline general medication categories used for management of psychological and physical symptoms in Table 2 with suggestions for use. Alternative treatment interventions are outlined in Table 3 when a nonpharmacologic approach is preferred or indicated to enhance medication effects.
Table 2
| Medication category | Common uses | Common medications and starting doses | Common side effects | Medication routes | Notes |
|---|---|---|---|---|---|
| SSRI | Anxiety, depression, PTSD | Fluoxetine 20 mg; sertraline 25 mg; citalopram 10 mg; paroxetine 20 mg; escitalopram 5 mg | Nausea, diarrhea, headache, sexual dysfunction | PO, IV | Avoid potent 2D6 inhibitors in patients receiving tamoxifen (fluoxetine, paroxetine, high-dose sertraline) |
| Few SSRIs are available in IV formulation and are not commonly stocked in hospital pharmacies making this route of administration unreliable | |||||
| SNRI | Anxiety, depression, PTSD, pain | Duloxetine 30 mg; venlafaxine ER 37.5 mg | Nausea, diarrhea, headache, sexual dysfunction, hypertension | PO | Avoid potent 2D6 inhibitors in patients receiving tamoxifen (venlafaxine) |
| SGA | Depression augmentation, anxiety, nausea, steroid-induced mood/psychotic symptoms | Olanzapine 2.5 mg; quetiapine 12.5 mg; risperidone 0.25 mg; aripiprazole 2.5 mg | Sedation, weight gain, dyslipidemia | PO, IV, IM | Olanzapine is available PO (tablet and dissolving tab), IV, and IM and is a potent antiemetic |
| Benzodiazepines | Anxiety, nausea, sleep | Lorazepam 0.5 mg; diazepam 2 mg; clonazepam 0.5 mg; alprazolam 0.125 mg | Sedation, fall risk | PO, PR, IV, IM | Can be given per rectum or per ostomy when the upper GI tract cannot be used |
| Psychostimulants | ADHD, depression augmentation, neoplastic related fatigue | Methylphenidate 5 mg | Elevated blood pressure | PO | Caution use in those with underlying cardiac conditions, anxiety disorders as it can worsen these |
| Anxiolytics | Anxiety, depression augmentation | Buspirone 5 mg BID | – | PO | – |
| Typical antipsychotics | Agitation, delirium, nausea | Haloperidol 0.5 mg | Sedation | PO, IV, IM | Caution use in combination with commonly prescribed antiemetics which are also potent D2 blockers and can lead to increased risk of extra pyramidal symptoms |
| TCA | Depression, anxiety, sleep, pain | Amitriptyline 25 mg; nortriptyline 10 mg; doxepin 10 mg; | Orthostasis, nausea, constipation | PO | Doxepin can be helpful for sleep at doses ~10 mg nightly |
| Nonbenzodiazepine hypnotics (“Z drugs”) | Sleep | Zolpidem 5 mg | Sedation, fall risk | PO | Use is discouraged due to frequency of adverse effects |
| Tetracyclic antidepressants | Anxiety, depression, sleep, nausea | Mirtazapine 7.5 mg | Orthostasis, weight gain, sedation, nightmares | PO (tablet and dissolving tab) | – |
| Alpha blockers | PTSD sleep symptoms | Prazosin 1 mg | Dizziness, orthostasis, hypotension, bradycardia | PO | – |
| Serotonin modulators | Sleep, depression | Trazodone 25 mg | Sedation | PO | – |
| Dopamine/norepinephrine reuptake inhibitor | Depression, tobacco cessation | Bupropion XL 150 mg (depression); bupropion SR 150 mg (smoking cessation) | Insomnia, agitation, anxiety | PO | Bupropion is an activating antidepressant that can help target low energy or poor concentration symptoms |
SSRI, selective serotonin reuptake inhibitor; PTSD, post-traumatic stress disorder; PO, orally; IV, intravenous; SNRI, serotonin and norepinephrine reuptake inhibitor; SGA, second-generation antipsychotic; IM, intramuscular; PR, per rectum; GI, gastrointestinal; ADHD, attention deficit hyperactivity disorder; BID, twice a day; TCA, tricyclic antidepressant; XL, extended release; SR, sustained release.
Table 3
| Interventions | Common uses | Common side effects | Notes |
|---|---|---|---|
| TMS | Depression | Headache | Available treatment option when the GI tract cannot be used |
| ECT | Depression, psychotic illnesses | Headache, cognitive issues | Available treatment option when the GI tract cannot be used |
| Psychotherapy | Depression, anxiety, PTSD, adjustment, existential issues, sleep (cognitive behavioral therapy-insomnia), pain (PRT, CBT for pain) | – | Available treatment option when the GI tract cannot be used |
TMS, transcranial magnetic stimulation; GI, gastrointestinal; ECT, electroconvulsive therapy; PTSD, post-traumatic stress disorder; PRT, pain reprocessing therapy; CBT, cognitive behavioral therapy.
Strengths and limitations
This is the first scoping review to describe the role of psychosocial oncology and psychiatric management of patients with GI malignancies. Additional strengths of our review include review of a topic that has limited literature presence through the use of a systematic process that is replicable and transparent. Limitations of our review include restricting psychiatric illness to those discussed in this article. There may be additional literature addressing other psychiatric diagnoses or symptoms that we are not aware of because it was beyond the defined scope of our search. We limited our literature search to the PubMed database and we did not review the quality of the literature as it is outside the methodology of the scoping review process. Additionally, as the nature of a scoping review is broad, our findings and suggestions may also be general in nature and further consideration should be taken in application to patient care.
Explanation of findings for psychiatric symptom management
Anxiety
Anxiety is the most common psychiatric symptom reported by patients with cancer. Symptoms of anxiety may represent an underlying psychiatric disorder, a physiologic phenomenon, or a reaction response to psychosocial stressors or existential distress. Underlying psychiatric disorders may include generalized anxiety, panic, or specific phobias. Wilson and colleagues found that 13.9% of a population of patients receiving palliative care for cancer met criteria for an anxiety disorder (14). Patients who do not meet criteria for an anxiety disorder do report the symptom of anxiety for many reasons. Psychosocial stressors often include diagnosis, prognosis, understanding the treatment plan, lack of understanding, concerns around the financial implications of care, and feeling isolated or having limited social supports. The importance of identifying and treating anxiety has many implications, and can lead to hindering adherence to treatment and increasing costs of care (15-18).
There are certain points during the timeline of cancer treatment in which anxiety symptoms are ubiquitous. The first notable time for universal anxiety is at diagnosis; education and psychosocial for the patient and family or friends can help alleviate and build rapport with the treatment team. For most patients and families, the “shock” of diagnosis subsides over a matter of weeks to months. Transitions in treatment are another anxiety-provoking time point, and thoughtful discussions to prepare patients of impending shifts or completions of therapies allow for time to psychologically adjust and plan. Providing anticipatory guidance of what is to come can minimize anxiety and alleviate uncertainty. Termination of active treatment and remission with surveillance monitoring is often full of feelings of abandonment and worry. This is a time where patients feel unmoored because they have been consistently coming to the treatment center, having regular appointments, lab work and contact points with the medical team. Moving to surveillance requires acceptance of significant uncertainty and patience. Again, talking with patients and planning for the completion of treatment will permit them to set expectations for the next stage of their illness management. Throughout the course of care, waiting for test results and undergoing imaging scans can lead to worry and stress colloquially referred to as “scanxiety”, which is commonly present in the days or weeks before a scan and remains present until results are known. Providing psychoeducation about the normal and expected feelings and emotions connected with monitoring the illness and providing psychosocial support can alleviate the anxiety and distress at these common triggering times during a cancer diagnosis.
Non-pharmacological interventions can be effective for management of anxiety, and encouraging patients to engage with a therapist or psychologist provides significant benefits for most patients. Based on an individual patient’s needs, the type of therapy can be adjusted, but cognitive behavioral therapy (CBT), acceptance and commitment therapy (ACT) and dialectic behavioral therapy (DBT) are all effective for anxiety. Techniques such as meditation, journaling, breathwork, personal connection, and exercise should be recommended to patients to lessen anxiety.
Pharmacologic management of anxiety disorders includes selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), second-generation antipsychotics (SGAs), buspirone, benzodiazepines, and different therapy modalities (see Table 2). In general, it is recommended to start with a trial of an SSRI and anticipate that symptoms typically improve over a matter of several weeks to a month. Short-term symptom relief might be obtained with a short course of a benzodiazepine to permit treatment adherence and lag time for the SSRI to take effect. Using benzodiazepines for long-term anxiolysis leads to potential problems with addiction, cognitive issues and sedation and is not recommended for most patients.
Special considerations for patients with abnormal GI tract functioning include using tablets that can be crushed and placed through a gastrostomy tube or using dissolvable mirtazapine. Benzodiazepines come in liquid formulations which can be effectively used when patients are unable to swallow pills, but remain a poor long-term option for anxiolysis.
Depression
Depression is common among patients with cancer, but is often underreported. Approximately one fifth of patients receiving palliative care for cancer meet clinical criteria for major depressive disorder (14). It is important to recognize and diagnose depression so that treatment can be provided in a timely manner. A simple two-question screen should be utilized and is considered positive if questions answer affirmatively to one or both questions. This screen, the Patient Heath Questionnaire-2 has a 91% sensitivity and 86% specificity for depression (19). It asks patients how often in the past two weeks has the individual “felt down, depressed or hopeless” and had “little interest or pleasure in doing things” and how frequently they have been bothered by those symptoms.
Identifying and treating depression has many implications. Untreated depression can worsen physical symptoms associated with cancer including fatigue, pain, poor appetite and sleep disturbances (20-22). Patients with physical illness and depression receive fewer medication interventions for their symptoms than patients who are physically well and depressed (23). Additionally, untreated depression has been shown to hinder adherence to treatment and increases the costs of care (15-18,24). Untreated depression also increases disability and mortality leading to poorer prognosis as shown in non-oncological diseases (stroke, myocardial infarction, etc.) (25-30). Missing a diagnosis of a mental health condition deprives the patient of access to effective treatment options. The 2011 systematic review by Rayner and colleagues supported that antidepressants are effective in treating patients with depression receiving palliative care; medication treatment was superior to placebo within 4–5 weeks and continued use further improved results (31).
While it is important to recognize and treat a depressive disorder, it may be difficult to tease apart the somatic symptoms driven by physical illness and treatment side effects from those stemming from an underlying psychiatric illness. Additionally, having a life-limiting illness invokes fear, sadness, and worry; this can be normal. Balancing treating a psychiatric illness and supporting patients experiencing the normal grieving process from a serious illness is a challenging tightrope to navigate. We do not want to medicalize a normal process of coping at risk of stigmatizing patients or missing the opportunity to appropriately treat and support symptoms.
Management of depressive disorders includes SSRIs, SNRIs, SGAs, tricyclic antidepressants (TCAs), neuromodulation, and different psychotherapy modalities (see Table 2). In general, it is recommended to start with a trial of an SSRI due to tolerability, side effect profiles, and drug-drug interactions as compared to other antidepressants. Evidence suggests that antidepressants are effective in treating depression in cancer patients receiving palliative care. The superiority of medication over placebo is noted to separate in 4–5 weeks and continues to separate with continued use (31). In patients with co-morbid sleep difficulties, poor appetite, nausea, and/or anxiety symptoms, we recommend using mirtazapine which is a tetracyclic antidepressant acting on presynaptic alpha2-adrenergic receptors, norepinephrine, and serotonin and is effective in targeting these symptoms.
Fatigue is a common symptom in patients with cancer that can contribute to depression, and using a stimulant, such as methylphenidate, can augment mood and work effectively with a person’s antidepressant. Some of the non-pharmacologic strategies reviewed for anxiety can also be effective in supporting patients struggling with depression.
Special considerations for patients with abnormal GI tract functioning include using medications that can be crushed and administered through a gastrostomy tube or using dissolvable mirtazapine.
Sleep
Many patients with cancer experience poor sleep. Whether these issues pre-exist their cancer diagnosis, are exacerbated by anxiety symptoms, or related to physiologic effects of treatment, helping patients with restorative sleep is an important foundation to establish for the success of other psychologic interventions. It is impossible to overstate the importance of consistent and restorative sleep for patients with cancer.
Management of sleep disorders is two-fold: behavioral and medication-assisted. The first-line treatment intervention consists of behavioral modifications. Discussing sleep hygiene with all patients is essential: going to bed and getting out of bed at the same time every day, getting adequate exercise during the day, avoiding screentime for 90 minutes before bed, avoiding caffeinated and alcoholic beverages in the late afternoon or evening, keep the bedroom dark and quiet, take a warm shower or bath, if unable to fall asleep get out of bed and engage a relaxing activity and return to bed when sleepy. Patients can be referred for CBT for insomnia (CBT-I) which involves working with a therapist on developing better sleep habits. Other strategies, such as meditation and progressive muscle relaxation, have anecdotal evidence to recommend them.
If therapy alone is not helpful, a medication alone or in combination with therapy may be beneficial. Over-the-counter antihistamines such as doxylamine or diphenhydramine can help with sleep onset. Doxepin is a generic tricyclic antidepressant that at low dose almost exclusively impacts the histamine receptors and therefore can be a helpful sleep aid without adding anticholinergic burden that over-the-counter antihistamines do. We recommend starting doxepin at 3 mg and titrating to a dose of approximately 10 mg nightly. Trazodone is an antidepressant commonly used to help patients with sleep and also functions as an antihistamine at low dose. We recommend starting patients on 25–50 mg nightly and titrating to 100–150 mg nightly. Doses beyond 150 mg do not confer additional sleep benefit. Benzodiazepines and nonbenzodiazepine hypnotics (Z drugs) should be utilized for refractory sleep difficulties and thought of as temporizing measures and should not be used long-term.
Special considerations for patients with abnormal GI tract functioning include using medications that can be administered through a gastrostomy tube, using a liquid formulation or dissolvable tablets.
Post-traumatic stress disorder (PTSD)
Trauma symptoms may develop related to specific aspects of the cancer diagnosis and treatment for some patients. For others, previous traumatic experiences or symptoms may be exacerbated by the stress, anxiety, or circumstantial familiarity related to cancer treatment. We can work to reduce the burden of trauma symptoms by speaking openly and honestly with our patients, ensuring that we share information at a pace and in a manner that they communicate is comfortable for them. Anticipating difficult conversations, procedures, hospitalizations and providing time and space for patients to discuss their thoughts and feelings can lessen traumatic symptoms.
Management of trauma symptoms begins with a trial of an SSRI which is titrated to effect over weeks. If sleep is impacted, utilizing prazosin, starting at 1 mg nightly, can be helpful. Prazosin is particularly helpful for managing nightmares and the autonomic reactions to nightmares. Mirtazapine is also indicated for trauma-induced sleep symptoms in addition to anxiety or depressive symptoms, if present. While benzodiazepines may be tempting to reduce anxiety symptoms driven by trauma, or to facilitate sleep, this class of medication is not recommended for use. Benzodiazepines can impair recovery and potentiate distressing symptoms. Finally, psychotherapy is recommended, and various modalities can be used. Referral to a specialist is recommended. Having a cadre of therapists skilled in working with cancer patients is very helpful.
Distress
While not an official psychiatric diagnosis, distress is a common feeling experienced by patients with cancer. The Commission on Cancer recognizes distress screening as a vital measure for cancer center accreditation utilizing the National Comprehensive Cancer Network (NCCN) distress thermometer measurement tool (32). Distress represents many different concerns for patients including physical, emotional, social, practical, and spiritual in nature. We recommend management of distress in a transdiagnostic manner addressing the symptoms patients are experiencing and mitigating practical barriers to care as best able.
Cancer is unlike many other medical diagnoses because it can feel like a threat to life and a threat to the self. This contributes to the existential distress that many patients experience at some point during their journey. Patients are faced with reflecting on their identity and mortality. Part of the job of the psychosocial oncologist is to guide patients through this journey. Issues explored by the psycho-oncologist to understand who the patient is, what brings them meaning, and what their experience since diagnosis has been like are outlined in Table 4.
Table 4
| Encounter type | Questions |
|---|---|
| Initial encounter | Tell me about your cancer |
| - What symptoms were you experiencing that led to you seeking an evaluation? | |
| - Who told you the diagnosis? | |
| - Where were you when you received your diagnosis? | |
| - How did you feel when you heard the diagnosis? | |
| What are you most fearful of? | |
| What are you most hopeful for? | |
| Who were you before your diagnosis? | |
| Who are you now after your diagnosis? | |
| Who do you want to be? | |
| Who are you responsible for? | |
| Who do you lean on for support? | |
| Do you identify with any particular spiritual practice or faith? | |
| Follow up encounters | What are you most proud of in life so far? |
| What legacy do you want to live now and what legacy do you want to leave? | |
| Since your diagnosis, what has changed in your life for the better? | |
| Since your diagnosis, what has changed that you would change again? | |
| What makes for a “good” life? | |
| What makes for a “good” death? | |
| Do you have unfinished business? |
There are other common physical symptoms, such as pain and nausea, that often have a psychiatric component or are exacerbated by psychiatric symptoms. Exploring the full range of physical and psychiatric symptoms and targeting treatment to the underlying cause maximizes patient wellbeing.
Substance use disorders
The impact of substance use disorders on patients with cancer and the management of these disorders during cancer treatment is nuanced, important and challenging. This topic is beyond the scope of this article, but is discussed in detail in another article in this issue.
Unique GI cancer-related issues
While many psychological and physical issues that arise during cancer treatment are common across the cancer spectrum, there are disease-specific themes that arise for patients with for GI malignancies. Here we aim to identify unique issues affecting this population and offer targeted interventions where applicable.
Ostomy and body image
Patients with GI malignancy may require an ostomy at some point during their care. When patients are told of the need to consider an ostomy, many meet the information with dismay and refusal. Patients cite concerns over permanence, body image, and daily functioning as reasons against an ostomy. In fact, ostomies have been found to lead to reduced social functioning, increased levels of depressive symptoms, and negative impact on body image (33). Patients with ostomies have poorer quality of life than those without an ostomy (34). Some patients have been so averse to ostomy placement that they have chosen to forego all cancer treatment, preferring to die than live with an ostomy. Issues with body image are not unique to patients with GI cancer; dissatisfaction with body image is a strong predictor of depression in rheumatologic disease and breast cancer, and it would be reasonable to expect that this true for patients with GI cancers given the negative impact of a stoma on body image of patients with colorectal cancer (33,35,36).
Sexual dysfunction
Patients with lower GI cancers like rectal and anal cancer, often experience sexual dysfunction during and following treatment (37,38). Evidence suggests that sexual dysfunction affects both men and women. Women experience reduced lubrication and dyspareunia and men experience increased rates of erectile dysfunction, and decreased libido is common across the board. Risk factors for sexual dysfunction include age, gender, neoadjuvant chemo/radiotherapy, advanced tumor stage, surgical interventions and presence of a stoma. These risk factors are magnified by the psychosocial stressors previously discussed (38).
Management of sexual dysfunction includes targeting specific symptoms. For men experiencing erectile dysfunction, use of a phosphodiesterase-5 inhibitor can be considered. For women experiencing reduced lubrication, artificial lubricants should be utilized. Dyspareunia is commonly seen following pelvis radiation leading to vaginal dryness, narrowing, or shortening. Using a vaginal dilator during and following treatment can prevent or treat these changes by preventing scarring and increasing blood flow to the area. Other target-specific therapies such as estrogen supplementation, lubrication, nerve blocks, and physical therapy can be used. Consideration for psychotherapy, especially when mental health factors contribute to sexual functioning and body image can be helpful for some patients. Referral to therapists that specialize in sexual health and intimate partner relationships may be warranted.
NETs
NET, a relatively rare neoplasm, most commonly arises in the GI tract and lungs. NETs are considered functional when they produce and release metabolically active substances. One of the substances that can be released is serotonin, resulting in the clinical carcinoid syndrome. This excess systemic serotonin manifests with diarrhea, flushing, abdominal pain, and neuropsychiatric symptoms. The neuropsychiatric symptoms manifest at least in part due to systemic conversion of tryptophan to serotonin, depleting the central nervous system of tryptophan stores by approximately 60% (39). This relative serotonin deficit at the central nervous system level increases the risk of developing psychiatric conditions. Anxiety is described affecting 30–40% of patients with NET (40). With tumor-directed treatment, the severity of psychiatric illness can lessen. Moderate-to-severe anxiety symptoms decreased by 10% in the beginning months of treatment in a population-based study by Hallet and colleagues (41). Depression is seen in up to 90% of patients with NET (42). Psychosis is described in case reports and appears to be a relatively rare complication of the underlying neoplastic process (43-46). Eating disorders, cognitive impairment, and sexual dysfunction are also described in case reports (40).
There is theoretical concern with serotonin-producing NETs that serotonergic medications could lead to adverse side effects. However, these therapies appear to be safe in this population of patients (8,47). Therefore, management of the psychiatric complications of NETs involves cancer-directed treatment in addition to managing the psychologic conditions as described earlier in this paper.
Study implications
Our study reveals a need for more rigorous studies examining the management of psychiatric conditions in patients with cancer, specifically GI malignancies. This will help guide care of these patients across different settings and contexts.
Conclusions
Cancer affects all aspects of an individual’s life, creating new psychosocial stressors and exacerbating pre-existing psychiatric symptoms. This scoping review utilizes a descriptive format to summarize the results of our review of the available literature as it pertains to psychiatric care of patients with cancer. Attending to the mental well-being of a patient with cancer, as well as that of the caregivers, effectively improves quality of life and can improve treatment adherence, leading to improved medical outcomes. Psychosocial care is the responsibility of the entire care team. The role of a psychosocial oncologist as part of the collaborative multidisciplinary treatment team provides nuanced care with attention to unique cancer-related issues that arise during the disease course. The psycho-oncologist brings expertise in combining targeted therapeutic strategies with pharmacologic interventions to address the multi-dimensional symptomatology patients experience. Using a layered approach, patients with mild symptoms can be supported by the general team, while those with moderate to severe symptoms require specialty psychiatric consultation. The field has grown substantially since its inception a little over 70 years ago and continues to mature, expand, and search for meaningful ways to impact patient and family lives.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Palliative Medicine, for the series “Palliative Care in GI Malignancies”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the PRISMA-ScR reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-22-1287/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-22-1287/coif). The series “Palliative Care in GI Malignancies” was commissioned by the editorial office without any funding or sponsorship. K.A. served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Holland JC. History of psycho-oncology: overcoming attitudinal and conceptual barriers. Psychosom Med 2002;64:206-21. [Crossref] [PubMed]
- Watson M, Dunn J, Holland JC. Review of the history and development in the field of psychosocial oncology. Int Rev Psychiatry 2014;26:128-35. [Crossref] [PubMed]
- Testa MA, Simonson DC. Assessment of quality-of-life outcomes. N Engl J Med 1996;334:835-40. [Crossref] [PubMed]
- Mitchell AJ, Chan M, Bhatti H, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol 2011;12:160-74. [Crossref] [PubMed]
- Grassi L. Psychiatric and psychosocial implications in cancer care: the agenda of psycho-oncology. Epidemiol Psychiatr Sci 2020;29:e89. [Crossref] [PubMed]
- Nordin K, Wasteson E, Hoffman K, et al. Discrepancies between attainment and importance of life values and anxiety and depression in gastrointestinal cancer patients and their spouses. Psychooncology 2001;10:479-89. [Crossref] [PubMed]
- Eriksson LE, Fritzell K, Rixon L, et al. The role of illness perceptions in adherence to surveillance in patients with familial adenomatous polyposis (FAP). Psychooncology 2016;25:699-706. [Crossref] [PubMed]
- Shi DD, Yuppa DP, Dutton T, et al. Retrospective review of serotonergic medication tolerability in patients with neuroendocrine tumors with biochemically proven carcinoid syndrome. Cancer 2017;123:2735-42. [Crossref] [PubMed]
- Krok D, Telka E, Zarzycka B. Illness perception and affective symptoms in gastrointestinal cancer patients: A moderated mediation analysis of meaning in life and coping. Psychooncology 2019;28:1728-34. [Crossref] [PubMed]
- Miceli J, Geller D, Tsung A, et al. Illness perceptions and perceived stress in patients with advanced gastrointestinal cancer. Psychooncology 2019;28:1513-9. [Crossref] [PubMed]
- Song L, Han X, Zhang J, et al. Body image mediates the effect of stoma status on psychological distress and quality of life in patients with colorectal cancer. Psychooncology 2020;29:796-802. [Crossref] [PubMed]
- Yanai Y, Makihara RA, Matsunaga N, et al. A feasibility study of a peer discussion group intervention for patients with pancreatobiliary cancer and their caregivers. Palliat Support Care 2022;20:527-34. [Crossref] [PubMed]
- Song L, Cao Y, Li J, et al. Psychological distress and resilience in patients with gastroenteropancreatic neuroendocrine tumor. Front Endocrinol (Lausanne) 2022;13:947998. [Crossref] [PubMed]
- Wilson KG, Chochinov HM, Skirko MG, et al. Depression and anxiety disorders in palliative cancer care. J Pain Symptom Manage 2007;33:118-29. [Crossref] [PubMed]
- Boulanger L, Zhao Y, Bao Y, et al. A retrospective study on the impact of comorbid depression or anxiety on healthcare resource use and costs among diabetic neuropathy patients. BMC Health Serv Res 2009;9:111. [Crossref] [PubMed]
- Cukor D, Rosenthal DS, Jindal RM, et al. Depression is an important contributor to low medication adherence in hemodialyzed patients and transplant recipients. Kidney Int 2009;75:1223-9. [Crossref] [PubMed]
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 2000;160:2101-7. [Crossref] [PubMed]
- Jindal RM, Neff RT, Abbott KC, et al. Association between depression and nonadherence in recipients of kidney transplants: analysis of the United States renal data system. Transplant Proc 2009;41:3662-6. [Crossref] [PubMed]
- Mitchell AJ. Are one or two simple questions sufficient to detect depression in cancer and palliative care? A Bayesian meta-analysis. Br J Cancer 2008;98:1934-43. [Crossref] [PubMed]
- Barkwell DP. Ascribed meaning: a critical factor in coping and pain attenuation in patients with cancer-related pain. J Palliat Care 1991;7:5-14. [Crossref] [PubMed]
- Laird BJ, Boyd AC, Colvin LA, et al. Are cancer pain and depression interdependent? A systematic review. Psychooncology 2009;18:459-64. [Crossref] [PubMed]
- Hotopf M, Mayou R, Wadsworth M, et al. Temporal relationships between physical symptoms and psychiatric disorder. Results from a national birth cohort. Br J Psychiatry 1998;173:255-61. [Crossref] [PubMed]
- Kendrick T, Dowrick C, McBride A, et al. Management of depression in UK general practice in relation to scores on depression severity questionnaires: analysis of medical record data. BMJ 2009;338:b750. [Crossref] [PubMed]
- Unützer J, Schoenbaum M, Katon WJ, et al. Healthcare costs associated with depression in medically Ill fee-for-service medicare participants. J Am Geriatr Soc 2009;57:506-10. [Crossref] [PubMed]
- Hays RD, Wells KB, Sherbourne CD, et al. Functioning and well-being outcomes of patients with depression compared with chronic general medical illnesses. Arch Gen Psychiatry 1995;52:11-9. [Crossref] [PubMed]
- Wells KB, Stewart A, Hays RD, et al. The functioning and well-being of depressed patients. Results from the Medical Outcomes Study. JAMA 1989;262:914-9. [Crossref] [PubMed]
- Frasure-Smith N, Lespérance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA 1993;270:1819-25. [Crossref] [PubMed]
- Hata M, Yagi Y, Sezai A, et al. Risk analysis for depression and patient prognosis after open heart surgery. Circ J 2006;70:389-92. [Crossref] [PubMed]
- House A, Knapp P, Bamford J, et al. Mortality at 12 and 24 months after stroke may be associated with depressive symptoms at 1 month. Stroke 2001;32:696-701. [Crossref] [PubMed]
- Lloyd-Williams M, Shiels C, Taylor F, et al. Depression--an independent predictor of early death in patients with advanced cancer. J Affect Disord 2009;113:127-32. [Crossref] [PubMed]
- Rayner L, Price A, Evans A, et al. Antidepressants for the treatment of depression in palliative care: systematic review and meta-analysis. Palliat Med 2011;25:36-51. [Crossref] [PubMed]
- Donovan KA, Grassi L, McGinty HL, et al. Validation of the distress thermometer worldwide: state of the science. Psychooncology 2014;23:241-50. [Crossref] [PubMed]
- Sharpe L, Patel D, Clarke S. The relationship between body image disturbance and distress in colorectal cancer patients with and without stomas. J Psychosom Res 2011;70:395-402. [Crossref] [PubMed]
- Pachler J, Wille-Jørgensen P. Quality of life after rectal resection for cancer, with or without permanent colostomy. Cochrane Database Syst Rev 2012;12:CD004323. [Crossref] [PubMed]
- Bloom JR, Stewart SL, Chang S, et al. Then and now: quality of life of young breast cancer survivors. Psychooncology 2004;13:147-60. [Crossref] [PubMed]
- Fobair P, Stewart SL, Chang S, et al. Body image and sexual problems in young women with breast cancer. Psychooncology 2006;15:579-94. [Crossref] [PubMed]
- Celentano V, Cohen R, Warusavitarne J, et al. Sexual dysfunction following rectal cancer surgery. Int J Colorectal Dis 2017;32:1523-30. [Crossref] [PubMed]
- Sörensson M, Asplund D, Matthiessen P, et al. Self-reported sexual dysfunction in patients with rectal cancer. Colorectal Dis 2020;22:500-12. [Crossref] [PubMed]
- Kvols LK. Metastatic carcinoid tumors and the malignant carcinoid syndrome. Ann N Y Acad Sci 1994;733:464-70. [Crossref] [PubMed]
- La Salvia A, Portigliatti Pomeri A, Persano I, et al. Serotoninergic brain dysfunction in neuroendocrine tumor patients: A scoping review. Compr Psychiatry 2021;109:152244. [Crossref] [PubMed]
- Hallet J, Davis LE, Mahar AL, et al. Patterns of Symptoms Burden in Neuroendocrine Tumors: A Population-Based Analysis of Prospective Patient-Reported Outcomes. Oncologist 2019;24:1384-94. [Crossref] [PubMed]
- Fröjd C, Larsson G, Lampic C, et al. Health related quality of life and psychosocial function among patients with carcinoid tumours. A longitudinal, prospective, and comparative study. Health Qual Life Outcomes 2007;5:18. [Crossref] [PubMed]
- Boujan N, Géraud C. Neuropsychiatric symptoms, skin disease, and weight loss: necrolytic migratory erythema and a glucagonoma. Lancet 2020;395:985. [Crossref] [PubMed]
- Hanna SM. Carcinoid syndrome associated with psychosis. Postgrad Med J 1965;41:566-7. [Crossref] [PubMed]
- Kohen I, Arbouet S. Neuroendocrine carcinoid cancer associated with psychosis. Psychiatry (Edgmont) 2008;5:29-30. [PubMed]
- Trivedi S. Psychiatric symptoms in carcinoid syndrome. J Indian Med Assoc 1984;82:292-4. [PubMed]
- Isenberg-Grzeda E, MacGregor M, Bergel A, et al. Antidepressants appear safe in patients with carcinoid tumor: Results of a retrospective review. Eur J Surg Oncol 2018;44:744-9. [Crossref] [PubMed]