
Prognostic impact of limited resection vs. inadequate adjuvant therapy in patients with pathologic stage II or III non-small cell lung cancer: results from the Korean Association of Lung Cancer Registry
Highlight box
Key findings
• Patients in group A (standard surgery with adjuvant therapy) had the best survival rates. Group C (adjuvant therapy after limited surgery) had worse outcomes than group B (omitted adjuvant therapy with standard surgery). Surgical treatment is recommended for those unable to complete both surgeries and adjuvant therapy to improve non-small cell lung cancer (NSCLC) outcomes.
What is known and what is new?
• Current guidelines recommend personalized lung cancer treatments, but the impact of treatment deviations on outcomes is unclear.
• This manuscript adds valuable insights into the consequences of deviations from standard lung cancer treatment guidelines, helping clinicians make informed decisions for their patients.
What is the implication, and what should change now?
• The implications stress the need for evidence-based, guideline-concordant care for lung cancer patients. Actions include guideline revision, patient education, multidisciplinary care promotion, and research to enhance prognosis and quality of life for stage II and III NSCLC individuals.
Introduction
Lung cancer is a major cause of cancer-related deaths, leading to 1.6 million deaths worldwide (1), and a large proportion of cancer deaths each year in South Korea specifically (2,3). Although medical knowledge and surgical technology have advanced, the long-term survival of patients with lung cancer remains poor. Its high mortality rate is because most patients are already in an advanced stage at the time of diagnosis with a poor response to chemotherapy.
According to the current guidelines published by the National Comprehensive Cancer Network (NCCN; 2023.03) and the American Society of Clinical Oncology, complete surgical resection and mediastinal lymph node dissection provide the best treatment for lung cancer and the opportunity to improve long-term survival in patients with stage I or II (T1–2, N1) non-small cell lung cancer (NSCLC). Multimodality therapy is recommended for patients with stage III NSCLC, whereas systemic therapies such as neoadjuvant/adjuvant chemotherapy and targeted immunotherapies, are recommended for patients with stage IIIB and IV NSCLC.
Because lung cancer is a heterogeneous disease, cancer treatment should be personalized according to individual performance status (PS), degree of disease, histology, and tumor biology. Primary lung cancer is generally classified into three categories: early or resectable, local progressive, and metastatic lung cancer (1). Even where complete therapeutic surgical resection is undergone, the general survival rate of lung cancer is low (4), with the 5-year survival rate for all stages being only around 22% (5).
Since ‘radical lobectomy’ was reported by Cahan in 1960 (6), the standard surgery for lung cancer has been lobectomy, in which one lobe or more is excised followed by dissection of mediastinal lymph nodes.
In the 2000s, several randomized controlled trials demonstrated the efficacy of adjuvant chemotherapy after complete surgical resection. Currently, this regimen is considered the standard treatment for pathological stages II and IIIA NSCLC (7-9).
Locally advanced lung cancer requires multimodal treatment (chemotherapy, surgery, radiation therapy, immunotherapy, and biological therapy). Selected patients with stage IIIA disease are eligible for surgical resection; however, the high risk of complications during and after surgery should be considered. Therefore, this option is only typically proposed for experienced surgical centers (10,11).
Thus, for patients with stage II and III lung cancer, various multi-modality treatments are required. However, depending on the individual condition, if they undergo limited instead of standard surgery or if they do not receive standard adjuvant therapy, even though they have undergone standard surgery, there will be a significantly poorer prognosis. Many previous papers have reported on the prognosis of adjuvant therapy or limited surgery compared with standard therapy (7,9). However, few have compared the prognoses of treatments that fail to adhere to standard guidelines. Therefore, the purpose of this study was to investigate the outcome of inappropriate lung cancer treatment in patients with stage II and III NSCLC by comparing the treatment results of patients who underwent limited surgery with those who did not receive proper adjuvant treatment. We present this article in accordance with the STROBE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-23-526/rc).
Methods
Study populations and methods
Data were collected from the Korean Association of Lung Cancer Registry (KALC-R) between January 2014 and December 2016 and were retrospectively analyzed. In 2014–2016, the KALC registered 8,110 patients with newly diagnosed lung cancer at 19 institutions. From them, those with NSCLC were selected. To investigate the results of inappropriate lung cancer treatment, patients with stage II or III NSCLC were then selected and divided into three groups according to treatment method. Inappropriate lung cancer treatment was defined as a failure to adhere to standard guidelines.
Patients in group A underwent standard surgery and adjuvant therapy (chemotherapy or radiation therapy), those in group B were not provided adjuvant chemotherapy (less than 4 chemotherapy sessions) or radiation therapy after standard surgery, and those in group C underwent limited resection (wedge resection and, segmentectomy) because of poor condition or refusal of standard surgery for any reason.
Standard surgery was defined as lobectomy or more than one lobar resection with mediastinal lymph node dissection, limited resection as sub-lobar resection with curable intent, and standard adjuvant chemotherapy as a treatment that included more than four cycles of chemotherapy.
A standardized protocol was used to collect data regarding patient age, sex, body mass index (BMI), smoking history, symptoms, PS, clinical stage, treatment modality, surgical method, histopathological tumor type, pathological stage, and 5-year survival rate. Patients were followed up until December 2019. Tumor histology was performed according to the World Health Organization classification system. All patients were staged according to the 7th edition of the tumor-node-metastasis (TNM) staging system published in 2010. Patients with missing data were excluded. After analyzing 721 patients with stage II or III NSCLC, the effectiveness of inadequate adjuvant treatment was compared with that of limited surgery.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was reviewed and approved by the Institutional Review Board of the National Cancer Center (NCC2018-0193). A waiver of consent was approved by the ethics board owing to the minimal risk posed by the study and the infeasibility of obtaining consent in a retrospective manner.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) or median [interquartile range (IQR)]. Categorical variables were expressed as percentages. To compare two independent groups, categorical variables were analyzed using Fisher’s exact test, and continuous variables were analyzed using the independent t-test or Wilcoxon rank-sum test for categorical data before and after PS matching depending on normality satisfaction. To minimize the effects of potential confounders on selection bias, propensity scores were generated using multiple logistic regressions to estimate the prognostic impact of inadequate adjuvant therapy (group B) and limited resection (group C) on lung cancer treatment. Covariates included in the propensity score matching (PSM) were age, sex, BMI, PS, clinical stage, and pathological stage. PSM (1:3 match) was performed to adjust for differences in baseline clinical characteristics, yielding 156 participants (117 in group B; 39 in group C).
The survival rate was estimated using the Kaplan-Meier method. A log-rank test was performed using the group comparison method for categorical data before and after PS matching. All P values were two-tailed, and statistical significance was set at P<0.05. Statistical analyses were performed using R (https://cran.r-project.org) version 4.1.3, with additional packages for survival analysis.
Results
Patient characteristics
From 2014 to 2016, the KALC registered 721 patients newly diagnosed with stage II or III NSCLC. Patients diagnosed with stage II or III NSCLC were assigned to one of the three groups. Baseline characteristics and demographic data of the enrolled patients are shown in Table 1.
Table 1
| Characteristics | Overall (n=721) | Group A (n=239) | Group B (n=437) | Group C (n=45) | P value |
|---|---|---|---|---|---|
| Sex (male) | 533 (73.9) | 177 (74.1) | 320 (73.2) | 36 (80.0) | 0.614 |
| Age (years) | 64.60±9.45 | 62.13±9.61 | 65.70±9.08 | 66.93±9.64 | <0.001 |
| BMI (kg/m2) | 23.82±3.25 | 23.98±3.36 | 23.68±3.19 | 24.27±3.20 | 0.324 |
| Symptom at diagnosis | 297 (41.2) | 107 (44.8) | 180 (41.2) | 10 (22.2) | 0.015 |
| ECOG | 0.685 | ||||
| 0 | 374 (62.4) | 121 (59.3) | 230 (64.6) | 23 (59.0) | |
| 1 | 203 (33.9) | 72 (35.3) | 116 (32.6) | 15 (38.5) | |
| 2 | 17 (2.8) | 9 (4.4) | 7 (2.0) | 1 (2.6) | |
| 3 | 3 (0.5) | 1 (0.5) | 2 (0.6) | 0 (0.0) | |
| 4 | 2 (0.3) | 1 (0.5) | 1 (0.3) | 0 (0.0) | |
| FEV1 (L) | 2.45±0.62 | 2.51±0.65 | 2.42±0.60 | 2.33±0.62 | 0.127 |
| DLCO (%) | 86.14±22.10 | 87.20±23.29 | 86.76±21.37 | 73.71±19.18 | <0.001 |
| Histopathologic type | |||||
| Squamous cell carcinoma | 269 (37.3) | 80 (33.5) | 178 (40.7) | 11 (24.4) | 0.032 |
| Adenocarcinoma | 410 (56.9) | 143 (59.8) | 236 (54.0) | 31 (68.9) | 0.083 |
| Large cell carcinoma | 12 (1.7) | 5 (2.1) | 7 (1.6) | 0 (0.0) | 0.891 |
| NSCLC NOS | 46 (6.4) | 14 (5.9) | 31 (7.1) | 1 (2.2) | 0.501 |
| Carcinoid tumor | 1 (0.1) | 0 (0.0) | 1 (0.2) | 0 (0.0) | >0.99 |
| Miscellaneous | 42 (5.8) | 14 (5.9) | 25 (5.7) | 3 (6.7) | 0.890 |
| Post-surgical TNM stage | |||||
| c stage | NA | ||||
| I | 215 (30.5) | 51 (21.8) | 143 (33.6) | 21 (46.7) | |
| II | 268 (38.0) | 78 (33.3) | 179 (42.0) | 11 (24.4) | |
| III | 205 (29.1) | 96 (41.0) | 98 (23.0) | 11 (24.4) | |
| IV | 17 (2.4) | 9 (3.8) | 6 (1.4) | 2 (4.4) | |
| p stage | NA | ||||
| IIa | 275 (38.1) | 55 (23.0) | 209 (47.8) | 11 (24.4) | |
| IIb | 138 (19.1) | 36 (15.1) | 92 (21.1) | 10 (22.2) | |
| IIIa | 301 (41.7) | 145 (60.7) | 134 (30.7) | 22 (48.9) | |
| IIIb | 7 (1.0) | 3 (1.3) | 2 (0.5) | 2 (4.4) | |
| Extent of surgery | |||||
| Lobectomy | 572 (79.3) | 200 (83.7) | 372 (85.1) | 0 (0.0) | <0.001 |
| Bilobectomy | 41 (5.7) | 12 (5.0) | 29 (6.6) | 0 (0.0) | 0.161 |
| Segmentectomy | 30 (4.2) | 2 (0.8) | 9 (2.1) | 19 (42.2) | <0.001 |
| Wedge resection | 109 (15.1) | 23 (9.6) | 55 (12.6) | 31 (68.9) | <0.001 |
| Sleeve lobectomy | 24 (3.3) | 11 (4.6) | 13 (3.0) | 0 (0.0) | 0.264 |
| Pneumonectomy | 44 (6.1) | 18 (7.5) | 26 (5.9) | 0 (0.0) | 0.137 |
| Pleura bx. | 6 (0.8) | 0 (0.0) | 5 (1.1) | 1 (2.2) | 0.110 |
| LN bx. | 24 (3.3) | 3 (1.3) | 18 (4.1) | 3 (6.7) | 0.038 |
| Miscellaneous | 11 (1.5) | 2 (0.8) | 9 (2.1) | 0 (0.0) | 0.499 |
Data are presented as n (%) or mean ± SD. Group A: standard surgery + adjuvant treatment; group B: standard surgery only, refers to the group of patients who received incomplete adjuvant therapy after standard surgery; group C: limited surgery + adjuvant treatment. Statistical significance was set at P<0.05. NSCLC, non-small cell lung cancer; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; FEV1, forced expiratory volume in 1 second; DLCO, diffusing capacity for carbon monoxide; NOS, not otherwise specified; TNM, tumor-node-metastasis; NA, not applicable; bx., biopsy; LN, lymph node; SD, standard deviation.
The median patient age was 65 years (IQR, 29–88 years), men accounted for 73.9%, and the mean BMI was 23.82±3.25 kg/m2. The majority (96.3%) of them showed good PS [0–1]. At the time of diagnosis, 41.2% were symptomatic, with symptoms that included cough (28.7%), sputum (14.7%), chest pain (10.5%), dyspnea (8.2%), hemoptysis (5.5%), weight loss (5.5%), and hoarseness (0.4%). Never smokers accounted for 30.1%, and smoking duration was 35.78±12.73 pack-year. Forced expiratory volume in 1 second (FEV1; i.e., spirometry) was 2.45±0.62 L and diffusing capacity for carbon monoxide (DLCO) was 86.14%±22.10%.
Preoperative diagnostic tools included bronchoscopy (32.5%), endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA, 13.0%), percutaneous needle aspirations (46.2%), surgical biopsy (95.1%), and lymph node biopsy (2.6%). Histopathologically, adenocarcinoma (56.9%) was the leading subtype in all patients; others included squamous cell carcinoma (37.3%) and large cell carcinoma (1.7%). Pre-operative clinical stages were I (30.5%), II (38.0%), III (29.1%), and IV (2.4%). Among 721 patients with NSCLC, 38.1% had pathological stage IIa, 19.1% had stage IIb, 41.7% had stage IIIa, and 1.0% had stage IIIb of the disease. The most common type of surgery was lobectomy (79.3%); other types included wedge resection (15.1%), pneumonectomy (6.1%), bi-lobectomy (5.7%), segmentectomy (4.2%), and sleeve lobectomy (3.3%).
Of the 721 patients with NSCLC, 239, 437, and 45 were assigned to groups A, B, and C, respectively. Age (62.13±9.61 vs. 65.70±9.08 vs. 66.93±9.64 years, P<0.001), duration of smoking (33.33±12.71 vs. 36.97±12.73 vs. 37.41±11.36 pack-year, P=0.013), and percentage of symptomatic patients at the time of diagnosis (44.8% vs. 41.2% vs. 22.2%, P=0.015) were significantly different among the three groups. Mean DLCO was significantly lower in group C (87.20%±23.29% in group A vs. 86.76%±21.37% in group B vs. 73.71%±19.18% in group C, P<0.001). EBUS-TBNA was a significant diagnostic tool (19.7% vs. 10.1% vs. 6.7%, P=0.001). In groups A and B, there were a few cases in which lobectomy and wedge resection or segmentectomy were performed together owing to tumor location. In 4.1% of all cases, complete resection was planned, but only a biopsy was performed.
Of all patients with NSCLC with stage II or III, 44.4% of patients underwent surgery and 35.2% of patients received adjuvant therapy after surgery with initial diagnosis. Regarding the initial treatment except for surgery, 10.3% and 1.7% received chemotherapy and radiation therapy, respectively. In contrast, 5.5% of patients received concomitant chemoradiation therapy.
Prognostic factor and survival analysis
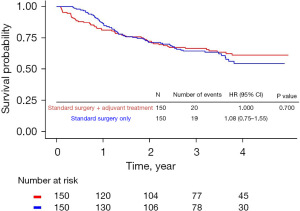
A total of 721 patients with stage II or III NSCLC were followed up for a median of 36.60 months (IQR, 23.23–44.57 months). After 1:1 PS matching for groups A and B, the overall survival (OS) rates were 61.1% in group A and 54.5% in group B (Figure 1).
To determine the prognostic factors and 5-year survival rates of the group that underwent limited surgery and the group that received non-complete adjuvant therapy after standard surgery, we compared groups B and C. The baseline characteristics and demographic data after 1:3 PS matching for groups B and C are shown in Table 2. Univariate Cox analysis showed that old age (over 65 years old) [hazard ratio (HR): 2.21; 95% confidence interval (CI): 1.20–4.06; P<0.010], ex-smoker (HR: 2.56; 95% CI: 1.28–5.13; P=0.008), pathological stage IIb (HR: 2.66; 95% CI: 1.22–5.82; P=0.014), and pathological stage IIIa (HR: 2.37; 95% CI: 1.11–5.08; P=0.026) were significant predictors of mortality.
Table 2
| Characteristics | Overall (n=156) | Group B (n=117) | Group C (n=39) | P value |
|---|---|---|---|---|
| Sex (male) | 119 (76.3) | 89 (76.1) | 30 (76.9) | >0.99 |
| Age (years) | 67.10±8.98 | 67.13±8.63 | 67.00±10.10 | 0.944 |
| BMI (kg/m2) | 24.25±2.97 | 24.26±2.90 | 24.21±3.21 | 0.940 |
| Symptom at diagnosis | 61 (39.1) | 53 (45.3) | 8 (20.5) | 0.012 |
| ECOG | 0.872 | |||
| 0 | 79 (61.7) | 59 (62.8) | 20 (58.8) | |
| 1 | 44 (34.4) | 31 (33.0) | 13 (38.2) | |
| 2 | 5 (3.9) | 4 (4.3) | 1 (2.9) | |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| FEV1 (L) | 2.35±0.61 | 2.36±0.60 | 2.29±0.64 | 0.579 |
| DLCO (%) | 83.58±21.93 | 86.60±21.70 | 73.85±20.02 | 0.003 |
| Histopathologic type | ||||
| Squamous cell carcinoma | 55 (35.3) | 45 (38.5) | 10 (25.6) | 0.208 |
| Adenocarcinoma | 92 (59.0) | 65 (55.6) | 27 (69.2) | 0.188 |
| Large cell carcinoma | 3 (1.9) | 3 (2.6) | 0 (0.0) | 0.574 |
| NSCLC NOS | 14 (9.0) | 13 (11.1) | 1 (2.6) | 0.192 |
| Carcinoid tumor | 1 (0.6) | 1 (0.9) | 0 (0.0) | >0.99 |
| Miscellaneous | 4 (2.6) | 2 (1.7) | 2 (5.1) | 0.260 |
| Post-surgical TNM stage | ||||
| c stage | 0.508 | |||
| I | 69 (44.2) | 52 (44.4) | 17 (43.6) | |
| II | 54 (34.6) | 43 (36.8) | 11 (28.2) | |
| III | 25 (16.0) | 16 (13.7) | 9 (23.1) | |
| IV | 8 (5.1) | 6 (5.1) | 2 (5.1) | |
| p stage | 0.693 | |||
| IIa | 44 (28.2) | 33 (28.2) | 11 (28.2) | |
| IIb | 48 (30.8) | 38 (32.5) | 10 (25.6) | |
| IIIa | 64 (41.0) | 46 (39.3) | 18 (46.2) | |
| IIIb | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Extent of surgery | ||||
| Lobectomy | 103 (66.0) | 103 (88.0) | 0 (0.0) | <0.001 |
| Bilobectomy | 6 (3.8) | 6 (5.1) | 0 (0.0) | 0.338 |
| Segmentectomy | 22 (14.1) | 5 (4.3) | 17 (43.6) | <0.001 |
| Wedge resection | 38 (24.4) | 12 (10.3) | 26 (66.7) | <0.001 |
| Sleeve lobectomy | 3 (1.9) | 3 (2.6) | 0 (0.0) | 0.574 |
| Pneumonectomy | 6 (3.8) | 6 (5.1) | 0 (0.0) | 0.338 |
| Pleura bx. | 2 (1.3) | 1 (0.9) | 1 (2.6) | 0.439 |
| LN bx. | 10 (6.4) | 7 (6.0) | 3 (7.7) | 0.712 |
| Miscellaneous | 4 (2.6) | 4 (3.4) | 0 (0.0) | 0.573 |
Data are presented as n (%) or mean ± SD. Group B: standard surgery only, refers to the group of patients who received incomplete adjuvant therapy after standard surgery; group C: limited surgery + adjuvant treatment. Statistical significance was set at P<0.05. PS, performance status; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; FEV1, forced expiratory volume in 1 second; DLCO, diffusing capacity for carbon monoxide; NSCLC, non-small cell lung cancer; NOS, not otherwise specified; TNM, tumor-node-metastasis; bx., biopsy; LN, lymph node; SD, standard deviation.
Multi-variate analysis (Cox proportional hazard regression analysis) was performed to determine prognostic factors for OS and recurrence. Results showed that limited resection (group C) (OS: HR, 2.15; 95% CI: 1.24–3.71; P=0.006; and recurrence: HR, 4.17; 95% CI: 2.12–8.23; P<0.001) was a significant poor prognostic factor. In stage II patients, limited resection (OS: HR, 2.88; 95% CI: 1.38–6.00; P=0.005; and recurrence: HR, 5.87; 95% CI: 2.52–13.69; P<0.001) was a significant poor prognostic factor for both survival and recurrence. However, in stage III patients, limited resection (OS: HR, 1.36; 95% CI: 0.57–3.23; P=0.486; and recurrence: HR, 4.24; 95% CI: 1.23–14.62; P=0.022) was a significant prognostic factor for only recurrence.
After 1:3 PS matching, the 5-year survival rate of patients with stage II or III was 68.0% in group B and 26.7% in group C, and the 5-year disease-free survival (DFS) rates were 59.1% in group B and 16.2% in group C. In the Kaplan-Meier survival curve according to the groups determined by the 7th edition of TNM, limited surgery demonstrated worse survival probability and DFS compared to inadequate adjuvant therapy (Figure 2A,2B). In stage II, the OS rate was 72.3% in group B and 36.7% in group C, and DFS rates were 81.0% in group B and 25.2% in group C (Figure 3A,3B). In stage III, the OS rate was 60.9% in group B and 22.7% in group C, and the DFS rate was 86.0% in group B and 60.1% in group C (Figure 4A,4B).

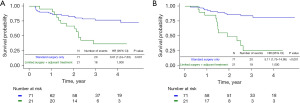
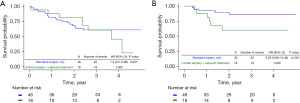
Discussion
Advanced lung cancer can be best treated using various modalities. If complete surgical resection is possible, subsequently performing chemotherapy or radiation therapy as an adjuvant treatment is considered the best-known treatment. However, if it is impossible to apply all treatments because of the patient’s condition, it is difficult to determine which treatment should be prioritized. The development of treatment technologies such as targeted immunotherapy and stereotactic body radiation therapy (SBRT) may become another treatment option with guaranteed performance comparable to that of surgical treatment for patients with advanced lung cancer who cannot undergo surgery.
While previous studies have reported the therapeutic effect of limited resection in early lung cancer (12,13), few have investigated the effect of limited resection on stages II or III NSCLC.
In stage I or II NSCLC, sub-lobar resection is specifically limited to high-risk patients or those with nodules measuring <2 cm, with lobectomy is considered the gold standard treatment for lung cancer (14).
This study compared the results of patients with lung cancer in Korea and showed that the treatment completion rate for patients with stage II or III lung cancer was 33%. However, for patients with stage II or III NSCLC, lung cancer surgery or chemotherapy may not be feasible due to various complex reasons such as physical ability, economic situation, or co-morbidity. The KALC-R was established in 2013 to produce unbiased and reliable demographic data. Accordingly, the data have been collected annually (15). According to the Korea National Cancer Innovation Database, the 5-year relative survival rate for all patients with lung cancer, regardless of stage, was 34.7% between 2014 and 2018 (2).
In our study, the OS rates (Figure 1) in groups A and B were similar after initial recovery from standard surgery. However, survival rates and prognoses differed depending on whether or not the patients received adjuvant therapy. Therefore, adjuvant therapy may affect the prognosis of patients with lung cancer.
After PS matching, both the OS and DFS rates were worse in group C than in groups B and C. These results indicate that patients receiving adjuvant therapy after limited resection had significantly lower 5-year survival and DFS rates than those receiving inadequate adjuvant therapy after standard lobectomy. This indicates that limited surgery has a worse effect on survival and recurrence of lung cancer than inadequate adjuvant therapy. Standard surgery provides the best treatment results for lung cancer; therefore, it is necessary to perform standard surgeries actively, and patients who have undergone limited resection under compromised conditions should be managed with more active and careful treatment to improve survival and prevent a recurrence.
This study had several limitations. First, it had a retrospective design. To compare the treatment outcomes of limited resection and inadequate adjuvant therapy, the variables of the patient groups were not controlled. Second, this study had a selection bias. As mentioned earlier, the patients who underwent sub-lobar resection were significantly older. Therefore, it is reasonable to assume that high-risk and older patients may have preferentially undergone limited resection. Elderly and high-risk patients in the limited resection group may have been unfit to receive adjuvant therapy, which might have adversely affected OS. Group A, which received standard treatment, was not compared with the group that received incomplete treatment. This study aimed to emphasize the importance of surgery in patients who unavoidably received incomplete lung cancer treatment.
Third, although large-scale nationwide data were used, socioeconomic status and education level were not included in our data, making it impossible to analyze the effects of these factors on the prognosis and treatment of lung cancer. Due to the large-scale statistical data, detailed individual information could not be obtained, and the patients were only categorized into those who completed four cycles of chemotherapy. Fourth, this study did not include or compare the latest technologies used in lung cancer treatment.
Despite these limitations, this was the first nationwide study in Korea to focus on limited resection and inadequate adjuvant therapy for patients with stage II or III lung cancer.
Conclusions
Treatments such as targeted therapy, SBRT, and immunotherapy can be considered for patients with stage II or III NSCLC who cannot receive standard surgery or adjuvant treatment. However, these treatment alternatives may not be easily accessible due to excessive medical costs in Korea. Therefore, this study suggests that the effectiveness of surgery for patients with advanced lung cancer who cannot undergo surgery for reasons other than medical conditions should be emphasized and that a multidisciplinary approach is needed for patients with general conditions that make surgery impossible.
Acknowledgments
The data used in this study were provided by the Korean Association for Lung Cancer and the Ministry of Health and Welfare, Korea Central Cancer Registry; https://www.ncc.re.kr/main.ncc?uri=english/sub04_ControlPrograms02. The statistics for this study were conducted with support from the Department of Biostatistics of the Biomedical Research Institute, Pusan National University Hospital.
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-23-526/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-23-526/dss
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-23-526/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-23-526/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was reviewed and approved by the Institutional Review Board of the National Cancer Center (NCC2018-0193), which waived the requirement for informed consent due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gridelli C, Rossi A, Carbone DP, et al. Non-small-cell lung cancer. Nat Rev Dis Primers 2015;1:15009. [Crossref] [PubMed]
- Kang MJ, Won YJ, Lee JJ, et al. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2019. Cancer Res Treat 2022;54:330-44. [Crossref] [PubMed]
- Park S, Choi CM, Hwang SS, et al. Lung Cancer in Korea. J Thorac Oncol 2021;16:1988-93. [Crossref] [PubMed]
- Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997;111:1710-7. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Cahan WG. Radical lobectomy. J Thorac Cardiovasc Surg 1960;39:555-72. [Crossref] [PubMed]
- Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351-60. [Crossref] [PubMed]
- Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 2005;352:2589-97. [Crossref] [PubMed]
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7:719-27. [Crossref] [PubMed]
- Petrella F, Toffalorio F, Brizzola S, et al. Stem cell transplantation effectively occludes bronchopleural fistula in an animal model. Ann Thorac Surg 2014;97:480-3. [Crossref] [PubMed]
- Pelosi G, Petrella F, Sandri MT, et al. A primary pure yolk sac tumor of the lung exhibiting CDX-2 immunoreactivity and increased serum levels of alkaline phosphatase intestinal isoenzyme. Int J Surg Pathol 2006;14:247-51. [Crossref] [PubMed]
- Divisi D, De Vico A, Zaccagna G, et al. Lobectomy versus sublobar resection in patients with non-small cell lung cancer: a systematic review. J Thorac Dis 2020;12:3357-62. [Crossref] [PubMed]
- Toste PA, Lee JM. Limited resection versus lobectomy in early-stage non-small cell lung cancer. J Thorac Dis 2016;8:E1511-3. [Crossref] [PubMed]
- Asamura H, Aokage K, Yotsukura M. Wedge Resection Versus Anatomic Resection: Extent of Surgical Resection for Stage I and II Lung Cancer. Am Soc Clin Oncol Educ Book 2017;37:426-33. [Crossref] [PubMed]
- Kim YC, Won YJ. The Development of the Korean Lung Cancer Registry (KALC-R). Tuberc Respir Dis (Seoul) 2019;82:91-3. [Crossref] [PubMed]


