
Genomic testing and targeted therapy of non-small cell lung cancer in China: a nationwide survey of physicians and clinical pathologists
Highlight box
Key findings
• Both physicians’ recommendations and patients’ receiving targeted therapies were inadequate; there was a higher proportion of targeted therapy in non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor mutation or anaplastic lymphoma kinase rearrangement than those with ROS proto-oncogene 1 fusion.
What is known and what is new?
• Genomic diagnostic testing is necessary to guide optimal treatment for NSCLC patients.
• The perception of genomic testing and targeted therapy for NSCLC were assessed.
What is the implication, and what should change now?
• Genomic testing and targeted therapy require improvement in physician awareness.
• Detection capabilities at non-tertiary hospitals should be enhanced.
Introduction
Lung cancer (LC) is one of the leading causes of cancer-related mortality worldwide, with more than 2,200,000 new cases and approximately 1,800,000 deaths in 2020 (1). It is still a major public health concern in China, accounting for approximately 20% of all new cancer cases and almost 30% of all deaths annually (2). Non-small cell lung cancer (NSCLC) represents most of LC (2,3), and about 70% of patients are diagnosed with locally advanced or metastatic disease (3). Despite recent advances in prevention and treatment, incidence and mortality rates of LC in China are still very high, this remains a huge burden for the country and society (4).
Upon the discovery of several oncogenic driver alterations, treatment of NSCLC has shifted to targeted therapy. First, activating mutations of epidermal growth factor receptor (EGFR) were reportedly targeted by tyrosine kinase inhibitors (TKIs), doubling the survival of NSCLC patients. After that, the introduction of crizotinib significantly extended the survival time of NSCLC patients with anaplastic lymphoma kinase (ALK) rearrangement (5). ROS proto-oncogene 1 (ROS-1) fusions proved to be responsible to TKIs, and crizotinib in particular showed satisfactory results in lowering mortality rate (6). American Society of Clinical Oncology for 2018 added stand-alone ROS-1 and B-Raf proto-oncogene, serine/threonine kinase (BRAF) testing in all patients with advanced lung adenocarcinoma, along with rearranged during transfection (RET), human epidermal growth factor receptor-2 (HER2), Kirsten rat sarcoma viral oncogene homologue (KRAS), and mesenchymal-epithelial transition (MET) testing as part of larger panels. Following the identification of multiple oncogenic driver alterations, as delineated in the National Comprehensive Cancer Network (NCCN) guidelines, the treatment paradigm for NSCLC has increasingly shifted towards targeted therapies (7). Alterations with currently approved targeted therapies include EGFR mutations, ALK rearrangements, ROS-1 rearrangements, BRAF mutations, and neurotrophic tyrosine receptor kinase (NTRK) gene fusions.
However, despite genomic diagnostic testing being necessary to guide optimal treatment, the proportion of NSCLC patients whose treatment was selected based on genomic testing is still unknown in many countries or needs further improvement (8). While previous studies, such as the one by Li et al. (9) have detailed the historical progression and distribution of genomic testing for LC in China, there has been limited reporting on the contemporary rate of genomic testing for specific alterations in NSCLC, the differential perceptions of pathologists and physicians regarding these tests, and the direct impact of test results on clinical treatment decisions. Our study addresses this gap by conducting a comprehensive questionnaire survey aimed at evaluating the current understanding of NSCLC genomic testing among pathology and clinical departments, assessing the detection capabilities of hospitals for NSCLC genomic alterations, exploring clinical recommendations for such testing, and examining the specifics of targeted therapy administration in NSCLC patients. We present this article in accordance with the SURGE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-23-509/rc).
Methods
Study design
This cross-sectional study recruited clinical pathologists and physicians in non-tertiary and tertiary hospitals across 135 cities in China from May to September 2020. Doctors from pathology, oncology, respiratory, and thoracic surgery departments with more than 5 years of experience in NSCLC genomic testing/diagnosis (pathology department) or with more than 5 years of experience in treatment of NSCLC cases (clinical departments) were included. Physicians whose workload did not meet the predetermined criteria (such as treated more than ten NSCLC patients every month and prescribed at least one TKI in the last 6 months) or who failed to complete the questionnaire were excluded from the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Since this is a survey, no ethical approval is required. All of the participants had signed an informed consent form.
Questionnaire
Three questionnaires were designed to collect the information from pathologists and physicians.
The quantitative questionnaire of the pathology department included three domains: (I) the testing qualification of the pathology department and the detection capability of the pathology department; (II) the status quo of NSCLC genomic testing in the pathology department; (III) the evaluation/suggestions about the genomic testing in NSCLC of the pathology department.
Each quantitative questionnaire for surgeons and physicians consisted of five parts: (I) the general situation of the patients with NSCLC treated by the doctor; (II) the detection ability of the hospital’s genomic testing in NSCLC; (III) the status quo of genomic testing of NSCLC patients; (IV) and the evaluation of and suggestions about the genomic testing of NSCLC patients; (V) as well as targeted treatment in patients with advanced NSCLC.
Before the formal survey, an associate chief physician of the department of respiratory department and a chief physician of the pathology department took part in the preliminary survey to evaluate the questionnaire, and they believed that the content and setting of the questionnaire could be understood and met the needs of this investigation.
In this study, the classification of hospitals was based on the tier system of cities in China. First-tier cities, including Beijing, Shanghai, and Guangzhou, represent the most medically advanced and economically prosperous regions. Second-tier cities, such as provincial capitals like Hangzhou, Chengdu, and Wuhan, offer considerable but varied medical resources. Third-tier cities encompass all other areas, typically with less developed healthcare infrastructure.
Statistical analysis
Data analysis was performed using SPSS 25.0 (IBM, Armonk, NY, USA). Descriptive statistics was used. Categorical variables were represented by n (%).
Results
General characteristics of participants
A total of 15,687 questionnaires were issued to surgeons and physicians, and 496 were collected; 8,000 questionnaires were issued to the pathology departments, and 198 were collected. As some doctors did not pass the screening or did not complete the questionnaire, a total of 94 questionnaires were excluded, and 600 doctors were finally included in the study, of which 150 (25%) were clinical pathologists and 450 (75%) were physicians (225 oncologists, 159 respiratory physicians, and 66 thoracic surgeons) (Table 1). Tertiary hospitals were located in the first-, second- and third-tier cities, while the non-tertiary hospitals were located in the third-tier cities (Table 1).
Table 1
| Clinical departments | Total (n=600) | Tertiary hospital, n (%) | Non-tertiary hospital, third-tier cities (n=129), n (%) | ||
|---|---|---|---|---|---|
| First-tier cities (n=84) | Second-tier cities (n=131) | Third-tier cities (n=256) | |||
| Oncology department | 225 | 27 (12.0) | 45 (20.0) | 108 (48.0) | 45 (20.0) |
| Respiratory department | 159 | 19 (11.9) | 32 (20.1) | 76 (47.8) | 32 (20.1) |
| Thoracic surgery department | 66 | 8 (12.1) | 14 (21.2) | 32 (48.5) | 12 (18.2) |
| Pathology department | 150 | 30 (20.0) | 40 (26.7) | 40 (26.7) | 40 (26.7) |
Each clinical pathologist performed genomic testing on 73 specimens of NSCLC patients per month. More than 90% of hospitals in first-tier cities had at least one certification, while more than 23% of hospitals in third-tier cities did not have any qualifications or certificates.
Each physician treated an average of 41 patients with NSCLC per month. Most (70%) of the patients had NSCLC. In the thoracic surgery department, majority of patients (48%) had stage I LC, while 80% and 75% of patients had stage III and IV LC in the oncology and respiratory department, respectively.
Demands of genomic testing from physicians
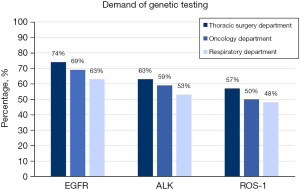
Among the demands which clinical pathologists received, the EGFR testing was the most frequent testing regardless if it was from the thoracic surgery department (74%), oncology department (69%) or respiratory department (63%), followed by ALK testing (63% vs. 59% vs. 53%) and ROS-1 testing (57% vs. 50% vs. 48%) (Figure 1).
Perception of clinical pathologists and physicians on the detection capability of the hospital
There were 2–4 clinical pathologists responsible for genomic testing in each pathology department and most of the pathologists were qualified to issue pathology reports. 92.5%, 90.0% and 80.0% of the clinical pathologists in non-tertiary hospitals believed their departments were certified by the authoritative organization for EGFR, ALK and ROS-1 testing, while the percentages were 85.5%, 88.2%, and 78.2% in tertiary hospitals, respectively.
As shown in Table 2, pathology departments that can conduct polymerase chain reaction (PCR)/reverse transcription PCR (RT-PCR), fluorescence in situ hybridization (FISH), immunohistochemistry (IHC), Ventana-IHC and next-generation sequencing (NGS) testing accounted for 96.7%, 93.3%, 98.0%, 62.2%, and 50.3%, respectively. Physicians believed that the most common method of genomic testing in the pathology department was PCR/RT-PCR, followed by IHC, FISH, Ventana-IHC, and NGS testing. And 76.7% of pathology departments can perform liquid biopsy. While 57.0% of physicians believed that the pathology department of the hospital can perform liquid biopsy.
Table 2
| Variables | Clinical pathologists (%) | Physicians (%) | |||||
|---|---|---|---|---|---|---|---|
| Total (n=150) | Non-tertiary hospital (n=40) | Tertiary hospital (n=110) | Total (n=450) | Non-tertiary hospital (n=89) | Tertiary hospital (n=361) | ||
| The proportion of testing methods available in the pathology department of the hospital | |||||||
| PCR/RT-PCR | 96.7 | 97.5 | 96.4 | 85.0 | 74.0 | 87.0 | |
| FISH | 93.3 | 90.0 | 94.5 | 72.0 | 51.0 | 77.0 | |
| IHC | 98.0 | 92.5 | 100.0 | 75.0 | 49.0 | 82.0 | |
| Ventana-IHC | 62.2 | 37.9 | 73.2 | 44.0 | 23.0 | 50.0 | |
| NGS | 50.3 | 36.2 | 56.7 | 34.0 | 22.0 | 36.0 | |
| Liquid biopsy | 76.7 | 82.5 | 74.5 | 57.0 | 50.0 | 59.0 | |
| The proportion of genetic testing available in the pathology department of the hospital | |||||||
| EGFR | 95.3 | 100.0 | 93.6 | 81.9 | 63.3 | 86.5 | |
| ALK | 94.7 | 92.5 | 95.5 | 75.5 | 52.2 | 81.3 | |
| ROS-1 | 84.7 | 82.5 | 85.5 | 65.6 | 40.0 | 71.9 | |
| Ratio of specimen sent out for testing | 21.0 | 26.0 | 20.0 | 49.7 | 61.0 | 47.0 | |
| Positive rate of genetic test | |||||||
| EGFR | 51.3 | 61.4 | 47.5 | 49.6 | 48.0 | 50.0 | |
| ALK | 8.4 | 9.4 | 8.2 | 8.0 | 7.9 | 8.4 | |
| ROS-1 | 5.0 | 5.5 | 4.9 | 3.7 | 3.0 | 4.5 | |
PCR/RT-PCR, polymerase chain reaction/reverse transcription polymerase chain reaction; FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; NGS, next-generation sequencing; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; ROS-1, ROS proto-oncogene 1.
Clinical pathologists believed that the information of previous genomic test and previous targeted therapy were provided in 79.3% and 76.7% of the testing application forms. The imperfect filling of testing application information was more prominent in non-tertiary hospitals; 95.3%, 94.7%, and 84.7% of pathology departments were capable of EGFR, ALK, and ROS-1 testing, for which the most commonly used testing methods were all nine-link multi-gene testing kits. And 81.9%, 75.5%, and 65.6% of physicians believed that the pathology department of the hospital was capable of performing EGFR, ALK, and ROS-1 testing (Table 2).
Specimens sent out for testing
The clinical pathologists reported the percentage of sending out specimens for testing from the pathology departments is 21%, while the report proportion of non-tertiary hospitals (26%) was higher than that of tertiary hospitals (20%) (Table 2). The main reason that the clinical pathologists reported were that the pathology department of the hospital cannot perform the testing method or technique (85.7%), followed by re-testing (38.8%), patient chose to be sent out of the hospital (36.7%) and preferential price (8.2%). The distribution of these reasons was different between tertiary and non-tertiary hospitals.
The physicians believed the percentage of sending out specimens for testing is 49.7%, and the report proportion of non-tertiary hospitals (61.0%) was higher than that of tertiary hospitals (47.0%) (Table 2). Feedback obtained from the clinicians we interviewed revealed that the primary reasons for sending out specimens included that the pathology department of the hospital cannot perform the testing method or technique (78.9%), patient chose to be sent out of the hospital (26.4%), preferential price (17.7%) and re-testing (12.2%).
Recommendation and results of genomic testing
For newly diagnosed NSCLC patients, EGFR mutation, ALK and ROS-1 rearrangement, testing was recommended by 83.4%, 72.9% and 68.3% of physicians, respectively (Figure 2A). For patients who had not received any TKI therapy, who had received one line, and who had received ≥2 lines of TKI therapy, the recommended percentage of genomic testing by physicians declined gradually, being 81.0%, 56.0% and 45.0%, respectively (Figure 2B). The recommended percentage of physicians, the percentage of patients’ genomic testing were all higher in tertiary hospitals (Figure 2B).
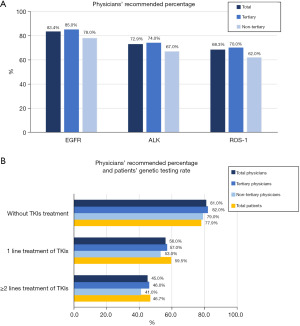
The main reasons why physicians did not recommend genomic testing in newly diagnosed and previously untreated TKIs patients were: “test results are already available” (55.8%) and “non-adenocarcinoma, test not recommended” (51.8%). According to the physicians’ view, the main reasons for the newly diagnosed patients not receiving the testing were: “cannot afford the cost of testing” (75.4%), “no treatment plan with TKIs” (38.9%) and “no understanding of genomic testing” (38.6%).
The positive rates of the genomic test that reported by clinical pathologists and physicians were similar.
Clinical pathologists and physicians’ view on genomic testing
Both clinical pathologists and physicians believed that genomic testing could support clinical decision-making [score: 6.4 vs. 6.5 (the total score was 1–7, 1 meant “not supported at all”, and 7 meant “fully supportable”)]. The information to be refined was mainly previous genomic test results and history of targeted drug use, which was more prominent in non-tertiary hospitals. According to the feedback of the physicians, the factors having most priority when choosing genomic testing institutions were “reliability of test report”, “laboratory quality control” and “experience of the clinical pathologists”. Physicians’ satisfaction with out-of-hospital institutions was higher than that of in-hospital departments in all aspects, especially in the selection of detection methods and detection targets.
Targeted therapy for patients with advanced NSCLC
Among the newly diagnosed patients with EGFR mutation, 77% received TKI therapy as first-line treatment, of which 49% were treated with gefitinib. Among NSCLC patients (EGFR+) who were newly diagnosed and treated with TKI, 31% received TKI combined with other drugs (41%—EGFR inhibitors combined with antiangiogenic drugs, 37%—EGFR inhibitors combined with chemotherapeutic drugs). The percentage of patients receiving TKI therapy was higher in tertiary hospitals (37%).
Furthermore, among patients with ALK rearrangement, 71% received TKI therapy during first-line treatment, of which 64% were treated with crizotinib. Among patients with ROS-1 fusion, 65% received TKI therapy during first-line treatment, of which 88% patients were treated with crizotinib.
Discussion
Personalized therapy of NSCLC is based on the genomic testing, which is necessary to guide optimal treatment. In this study, three questionnaires were used to survey the understanding of pathology and clinical departments on NSCLC genomic testing and the hospitals’ detection capability of NSCLC. We found that clinical pathologists and physicians had similar views on the genomic testing, and support genomic testing for clinical decision making. Patients with EGFR mutation or ALK rearrangement received more targeted therapy than those with ROS-1 fusion.
Results showed that the requirements of target testing in clinical departments in China were EGFR > ALK > ROS-1, which is in line with other reports, and those needed were essentially met by testing capabilities of pathology departments. Nevertheless, clinical pathologists and physicians had different view on the detection capability, with pathology departments being capable of EGFR, ALK, and ROS-1 testing in 95.3%, 94.7%, and 84.7%, respectively, but only 81.9%, 75.5%, and 65.6% of physicians believing that the pathology department of the hospital was capable of above three testing. The laboratories have to meet the need of comprehensive genomic testing using only limited amount of tumor tissue, mostly fixed in formalin (10). In our study, when considering long-term costs and effects, liquid biopsy was reported as the most effective and most costly strategy, while tissue biopsy was the least effective and least costly. This is primarily attributed to the longer turnaround time for tissue biopsy results, with liquid biopsy NGS returning results faster, as indicated by Raez et al. (11). This delay in obtaining tissue biopsy results can be crucial, particularly when physicians aim to receive results within a shorter timeframe to expedite treatment decisions. According to our survey, 76.7% of pathology departments can perform liquid biopsy, but only 57.0% of physicians believed that the pathology department of the hospital can perform liquid biopsy. The primary factor contributing to the observed disparities in perception regarding the detection capabilities of pathology departments between clinicians and pathologists is attributed to insufficient communication between these two groups. In the future, more attention should be paid to enhance the clinical interaction, improve the communication effect, and provide more suitable treatment plans for patients.
There is also difference in the detection capability between non-tertiary and tertiary hospitals, reflected in the proportion of specimens sent out from pathology departments of non-tertiary hospitals (26.0%) being higher than that of tertiary hospitals (20.0%). Regarding pathology departments, the lack of qualified staff and equipment has still not completely solved. Twenty-three percent of the pathology departments in the third-tier cities failed to pass any qualification review.
A proposed solution in the literature to address the low rate of genomic testing is the automatic initiation of genomic testing by pathologists immediately following the histological diagnosis of advanced NSCLC with adenocarcinoma (12). Updated genomic testing guidelines from the College of American Pathologists in 2018 recommended reasonable redirection of decision-making to pathologists, provided that such testing is an institutional decision and includes close communication with clinical departments, such as a standard practice with other solid tumors (13). However, in China, patient consent is required for molecular/genomic testing, and the decision is often made without consulting the pathology department, led mostly by patients’ view on procedure necessity and/or economic burden of testing. Moreover, results of our survey showed that pathology departments were capable of doing the most of genomic testing. The most common reason from pathologists involved in the survey to request the testing in external institutions was to confirm the already obtained test results (85.7% in non-tertiary hospitals vs. 31.0% in tertiary hospitals) or the testing price (28.6% in non-tertiary hospitals vs. 4.8% in tertiary hospitals), which reflects the difference in understanding not only between pathology and clinical departments, but also between tertiary and non-tertiary hospitals. In the face of such situation, it may be necessary to cooperate with a third-party testing company, adopting the medical consortium model. Development of that model, focused on the key points of diagnosis and treatment, should consider different levels of hospitals, common target detection capabilities and detection methods, and take into account various ways, forms and channels of data exchange, in order to solve the practical problems in NSCLC detection.
To address the significant percentage of physicians lacking understanding in genomic testing, we advocate for the widespread dissemination of educational materials on NSCLC genomic testing to patients and their families. This approach aims not only to enhance the awareness of patients and their families but also to potentially improve compliance with testing recommendations, thereby bridging the knowledge gap and facilitating more informed clinical decisions. Another finding of this study suggests that the recommendation rate of genomic testing in patients with NSCLC needs to be improved, especially in cases of rare alterations (such as ROS-1); the recommendation rates of genomic testing in patients who received first-line and second-line therapy were low, so it is necessary to further strengthen the detection awareness and precision of diagnosis and treatment. In addition, the acceptance rate of genomic testing by patients also needs to be improved, for which it is necessary to popularize relevant knowledge to familiarize patients with genomic testing. Our survey showed that even when a sensitive alteration was detected, some patients still did not follow the guidelines to receive reasonable medication and standardized treatment. Therefore, it is necessary to strengthen the appropriate application of targeted drugs. More education is needed for both doctors and patients, especially about ALK and ROS-1 fusions, in order to ensure that every NSCLC patient can receive timely and standardized targeted treatment.
The study found that both physicians’ recommendations and patients’ receiving targeted therapies were inadequate, even though there was a higher proportion of targeted therapy in patients with EGFR mutation or ALK rearrangement than those with ROS-1 fusion. The percentage of targeted therapy in tertiary hospitals was higher than non-tertiary hospitals. Targeted therapy is insufficient for patients in different treatment lines, especially in first line treatment. All of these were caused by the lack of patient’s recognition, insufficient testing capacity of the hospital and physician’s incomprehension of application of medicine. The solutions are enhancing education for physicians, pathologists and patients, and collaborating with medical consortium. To complement educational efforts and enhanced collaboration between clinicians and pathologists, we also recognize the importance of patient involvement and advocacy. Actively engaging patients in sharing their experiences with genomic testing and targeted therapy can significantly bolster patient confidence and involvement in their own care. Such patient-centric approaches can be pivotal in enhancing the overall effectiveness of NSCLC treatment strategies.
This study has some limitations. Based on the study design and nature, the responses to the questionnaire reflect the subjective impression of physicians’ clinical practice, which might be biased. The number of the participants of non-tertiary hospitals in our study was fewer than tertiary hospitals. A large sample study would help assess the situation of non-tertiary hospitals in the future.
Conclusions
In conclusion, while there is a need for overall enhancement in the genomic testing landscape for NSCLC patients in China, it is particularly crucial for biomarkers like ROS-1 fusion, along with others specified in the NCCN guidelines such as HER2, BRAF, KRAS, RET, NTRK, NRG1, and MET. Physicians may underestimate the detection capabilities of the pathology departments and the detection capabilities of non-tertiary hospitals in third-tier cities need to be enhanced. The improvement of the detection capabilities of pathology departments and the physicians’ awareness of genomic testing are needed for enhancing the rate of genomic testing and improving the prognosis in NSCLC patients in China.
Acknowledgments
We thank Vice President Junyang Cao of Pfizer Medical for his support and help in research and article writing. Medical writing support was provided by Zhang Lu at Shanghai MedSci Healthcare Co. Ltd.
Funding: The medical writing of this study was funded by
Footnote
Reporting Checklist: The authors have completed the SURGE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-23-509/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-23-509/dss
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-23-509/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-23-509/coif). X.M., M.W. and C.Q. are employees of Pfizer Investment Ltd. All authors report that the medical writing of this study was funded by Pfizer. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Since this is a survey, no ethical approval is required. All of the participants had signed an informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fois SS, Paliogiannis P, Zinellu A, et al. Molecular Epidemiology of the Main Druggable Genetic Alterations in Non-Small Cell Lung Cancer. Int J Mol Sci 2021;22:612. [Crossref] [PubMed]
- Zheng RS, Zhang SW, Sun KX, et al. Cancer statistics in China, 2016. Zhonghua Zhong Liu Za Zhi 2023;45:212-20. [PubMed]
- Zeng H, Ran X, An L, et al. Disparities in stage at diagnosis for five common cancers in China: a multicentre, hospital-based, observational study. Lancet Public Health 2021;6:e877-87. [Crossref] [PubMed]
- Wu F, Wang L, Zhou C. Lung cancer in China: current and prospect. Curr Opin Oncol 2021;33:40-6. [Crossref] [PubMed]
- Solomon BJ, Kim DW, Wu YL, et al. Final Overall Survival Analysis From a Study Comparing First-Line Crizotinib Versus Chemotherapy in ALK-Mutation-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:2251-8. [Crossref] [PubMed]
- Moro-Sibilot D, Cozic N, Pérol M, et al. Crizotinib in c-MET- or ROS1-positive NSCLC: results of the AcSé phase II trial. Ann Oncol 2019;30:1985-91. [Crossref] [PubMed]
- Zhou L, Xu G, Chen T, et al. Anlotinib plus camrelizumab achieved long-term survival in a patient with metastatic esophageal neuroendocrine carcinoma. Cancer Rep (Hoboken) 2023;6:e1855. [Crossref] [PubMed]
- Ryska A, Berzinec P, Brcic L, et al. NSCLC molecular testing in Central and Eastern European countries. BMC Cancer 2018;18:269. [Crossref] [PubMed]
- Li W, Lyu Y, Wang S, et al. Trends in Molecular Testing of Lung Cancer in Mainland People's Republic of China Over the Decade 2010 to 2019. JTO Clin Res Rep 2021;2:100163. [Crossref] [PubMed]
- Watanabe N, Umezu T, Kyushiki M, et al. Optimal DNA quality preservation process for comprehensive genomic testing of pediatric clinical autopsy formalin-fixed, paraffin-embedded tissues. Pol J Pathol 2022;73:255-63. [Crossref] [PubMed]
- Raez LE, Brice K, Dumais K, et al. Liquid Biopsy Versus Tissue Biopsy to Determine Front Line Therapy in Metastatic Non-Small Cell Lung Cancer (NSCLC). Clin Lung Cancer 2023;24:120-9. [Crossref] [PubMed]
- Gregg JP, Li T, Yoneda KY. Molecular testing strategies in non-small cell lung cancer: optimizing the diagnostic journey. Transl Lung Cancer Res 2019;8:286-301. [Crossref] [PubMed]
- Wolff AC, Hammond MEH, Allison KH, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med 2018;142:1364-82. [Crossref] [PubMed]


