
Palliative psychiatry for a patient with treatment-refractory schizophrenia and severe chronic malignant catatonia: case report
Highlight box
Key findings
• Palliative psychiatry is an innovative approach to caring for patients with severe and persistent mental illness (SPMI). In acutely life-threatening situations such as treatment-resistant catatonia, this can take on the form of end-of-life care.
What is known and what is new?
• Some patients with schizophrenia experience little to no effect of clozapine and electroconvulsive therapy. This is the first report describing end-of-life care of a patient with chronic malignant catatonia.
What is the implication, and what should change now?
• Palliative psychiatry has the potential to relief suffering both from treatment-resistant SPMI and treatments with poor benefit/burden ratios. More research is needed on the scope, transdiagnostic potential, and utility of palliative psychiatry to inform the eventual development of guidelines.
Introduction
Psychiatry and palliative care specialists have seen increasing cooperation over the past two decades. However, this has typically been limited to the area of psycho-oncology and psychiatry in palliative care (1). Relatively new are proposals of palliative approaches to caring for persons with severe and persistent mental illness (SPMI) in the absence of a somatic disease (i.e., palliative psychiatry). They are motivated by the acknowledgment that the typical goal of care in psychiatry, clinical remission, is not realistic for some patients carrying diagnoses such as severe and persistent schizophrenia, anorexia nervosa, substance use disorders, or bipolar disorder (1,2).
Palliative psychiatry is an emerging subdiscipline of psychiatry which accepts that SPMI can be irremediable and that a shift towards more palliative goals might be beneficial (1,3). Palliative goals of care center around maximizing quality of life in the present, such as preserving patient dignity and autonomy as much as possible and minimizing physical and psychological suffering. This includes abstaining from treatments with poor benefit/burden ratio that are misaligned with patient goals and preferences and giving less weight to long-term consequences.
Importantly, like palliative care in general, palliative psychiatry should be provided based on need rather than prognosis. It is thus not limited to persons at the end of life (EOL) and compatible with treatment aiming at clinical remission or improved psychosocial functioning (3). An example for palliative psychiatry in this broad sense is Oyster care (4).
When SPMI is life-threatening, taking a palliative approach to care can mean providing EOL care. EOL care is the part of palliative care that is addressed to patients at the EOL, which is often defined as a life expectancy of 6 months or less (5). Setting the dementias aside, EOL care for persons dying from mental disorders has been almost exclusively debated in the context of anorexia nervosa so far (1,3). However, other SPMI can become life-threatening too, such as when leading to catatonia. To promote discussion of palliative psychiatry and its target population, we present this case in accordance with the CARE reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-23-586/rc) (6).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). It was not possible to obtain written informed consent from the patient for publication of this case report because he passed away. Instead, explicit verbal informed consent to publish the case has been obtained by his mother as his legal representative.
“Mr. B” was a 49-year-old single male with schizophrenia, which was diagnosed at age 32, with six prior hospitalizations and one past suicide attempt, who was previously stable on olanzapine monotherapy for many years. He had one prior episode of catatonia in the year leading up to his presentation to New York-Presbyterian/Weill Cornell Medicine, which was treated with six right unilateral electroconvulsive therapy (ECT) sessions. He was discharged home. He had no prior documented history of neuroleptic malignant syndrome (NMS). He lived with his mother and grandparents in public housing and followed with an outpatient psychiatrist. During the weeks prior to presentation in our emergency department, Mr. B was increasingly disorganized, bizarre, and paranoid, without a clear precipitant. His medications at the time were lorazepam 1 mg every 8 hours and olanzapine 10 mg at bedtime. From our psychiatric emergency department, Mr. B was transferred to an outside hospital where he continued to exhibit psychotic behavior and attacked a staff member. A few days after this event, he was started on clozapine after which Mr. B developed a fever to 38.3 ℃ along with tachycardia, lethargy, and hypotension (see Figure 1 for an overview of symptoms and treatments). His creatinine kinase (CK) was elevated to 1,823 U/L and he was transferred back to New York-Presbyterian/Weill Cornell Medicine and admitted to the medical service due to concern for NMS secondary to clozapine [often, it is hard to diagnostically differentiate between NMS and malignant/pernicious catatonia because of the overlap in clinical presentation, treatment, and likely genetics (7)]. Infectious workup, head imaging, and routine electroencephalogram (EEG) were unremarkable. All antipsychotics were stopped while on the medical service, and lorazepam was increased. Though his CK normalized 10 days after admission to medicine, Mr. B remained withdrawn, paranoid toward staff, appeared internally preoccupied, and was therefore transferred to our inpatient psychiatric unit for continued management.
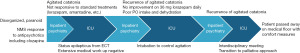
Initially, the patient was restarted on olanzapine 5 mg at night and continued on lorazepam 1.5 mg four times a day. Numerous attempts were made to decrease lorazepam, however with each attempt the patient developed symptoms of catatonia, including mutism, immobility, catalepsy and stereotypy, odd cycling of the legs, staring off into space, and often falling off his bed or running into the wall. Bush-Francis Catatonia Rating Scales completed at this stage of treatment resulted in a score of 36, indicating a severe presentation. In response to these episodes, Mr. B was frequently given lorazepam 2 mg intramuscularly (IM), which typically quelled his motor symptoms. Olanzapine was titrated to a dose of 20 mg nightly and lorazepam often exceeded 20 mg daily, with up to 10 mg daily given emergently and IM. During this time, however, Mr. B’s behavior remained disorganized and unpredictable, with episodes of thrashing and screaming, and prolonged air cycling of the legs. For the psychiatrists, nurses and other team members involved in his care, Mr. B’s episodes were frightening and highly unusual. Given his history of assaulting a staff member in a prior admission, these episodes also carried a threat of unpredictable violence, and Mr. B was under the constant care of a staff member, and often an additional security detail.
Due to the limited response to medication in context of worsening catatonia, a trial of bilateral ECT was initiated. On the patient’s fifth session, he went into status epilepticus, which continued for several minutes until broken by intravenous (IV) midazolam. The patient was sedated, intubated, and transferred to the neuro intensive care unit (ICU), placed on video EEG and was levetiracetam loaded. During this period, all neurological and medical work up for encephalopathy were negative including lumbar puncture, computed tomography/magnetic resonance imaging (CT/MRI) brain with and without contrast, full body and brain PET/CT, meningitis/encephalitis panels as well as serum and cerebrospinal fluid (CSF) paraneoplastic panels. Mr. B was extubated several days later as no ictal discharges or movement episodes were recorded on video EEG; however, he required re-intubation and sedation with propofol due to concerns of the patient’s inability to protect his upper airway. He was switched from lorazepam to IV diazepam, titrated to 12.5 mg four times a day, and IV valproic acid (800 mg three times a day). He was found to have a deep vein thrombosis in his left internal jugular and left superficial cephalic vein and was put on enoxaparin. By this point in his hospital course, Mr. B was well known to most of our psychiatric staff, as well as medical, neurological and intensive care teams. His refractory symptoms produced a great deal of confusion and grief for those attempting to provide the most compassionate and least restrictive care possible. The primary psychiatric team was also in close contact with Mr. B’s mother and father, providing support to them as well as explaining Mr. B’s symptoms and the treatments being offered.
A few days later, he was again extubated, and a nasogastric tube was placed. He was started on amantadine, low-dose chlorpromazine, and continued on diazepam, valproic acid, and after several weeks was medically cleared for transfer back to our inpatient psychiatric unit, now 3 months post initial admission. On his return to the inpatient unit, Mr. B was at his most interactive and discussed his goals during a family meeting with his parents, “to get back to how I used to be, playing basketball, watching football, and living at home.” The psychiatric team who had cared for Mr. B for most of his hospital course experienced a confused sense of relief. It was unclear why Mr. B had gotten better, and we posited that perhaps a period of sedation had allowed his brain to recover, or that we had found the correct combination of medications for him. Our team began discharge planning and setting up aftercare for Mr. B.
Around one week later, Mr. B again became unexpectedly confused and within days progressed to a mute and catatonic state. Again, his CK rose to 1,000 U/L and chlorpromazine was stopped and switched to quetiapine. After a single dose of 25 mg, he became febrile to 38.6 ℃ and quetiapine was discontinued. His prior severe agitated catatonia episodes as described returned in full force, with Bush-Francis scores exceeding 30. We stopped diazepam and opted for a trial of high-dose lorazepam, as this had not yet been formally attempted. Over the following weeks Mr. B was titrated to a dose of 36 mg of standing lorazepam daily, with only minor improvements in catatonic symptoms, with Mr. B remaining mute, withdrawn, with staring, grimacing and frequent stereotyped and repetitive movements. He remained on an around the clock safety watch with a staff member.
After about 10 days of 36 mg of lorazepam, amantadine 600 mg, and valproic acid at a therapeutic level, Mr. B suddenly switched from mute and unresponsive to extremely agitated and disinhibited, manic appearing, often shouting loudly, screaming that he and his mother were being attacked. He would sprint furiously around the unit, refusing to drink water or eat, writhing on the floor, throwing himself against walls, and flailing his limbs in a disturbing manner. Because he was already on such high doses of lorazepam and had such difficulty tolerating antipsychotic medications, the only remaining way to safeguard Mr. B and the other patients in the unit was to use physical restraints. With Mr. B’s prior improvement and pending discharge just weeks prior to this new emergence of symptoms, those who had been closely involved in his care became demoralized and particularly concerned about the use of restraints, and worried that despite the most genuine efforts to improve his condition, Mr. B was being harmed rather than helped.
In this state, he accepted very little oral food or hydration, and accordingly, his kidney function rapidly declined. Attempts at keeping lines for IV fluids in place were unsuccessful, as Mr. B quickly pulled them out or caused access to be lost due to his frantic movements even when restrained. Amantadine was tapered off quickly with the thought that it might have been worsening his psychosis at such doses. Lithium and aripiprazole were added, the team considering the possibility that there was an underappreciated bipolar diathesis presenting itself. His kidney function continued to decline and his liver function tests also showed signs of damage. His CK was 5,978 U/L. Given his ongoing psychological and physical disturbances and inability to provide adequate oral or IV hydration, coordination with the ICU team began. After lengthy discussions with senior faculty members, including the chiefs of inpatient psychiatry and intensive care at New York-Presbyterian/Weill Cornell Medicine, it was decided that the safest and least restrictive measure was to sedate and intubate Mr. B to gain IV access and properly address his looming end organ damage due to poor hydration and toxic effects of muscle breakdown. This option was discussed with the patient’s parents, who were upset and distraught over their son’s progressively worsening behavior and condition, and agreed with this plan. The patient was intubated in our inpatient psychiatric unit under the supervision of anesthesia and the ICU teams and again transferred to the ICU. While sedated and intubated, bilateral ECT was attempted again since the first course was not completed, but a seizure was unable to be induced, thought likely due to his level of sedation. Lorazepam was titrated back to a dose of 2 mg every 8 hours. Mr. B eventually self-extubated in the context of weaning propofol and fentanyl. Though his agitated catatonic episodes did not immediately resume, Mr. B remained largely unable to take part in conversations, spoke very little and made poor eye contact, though he seemed to recognize some of the psychiatric team who had been taking care of him for the previous 6 months. His medical condition had improved sufficiently as to permit his return to the psychiatric inpatient unit. Given his difficulty tolerating most neuroleptics, he was started on pimavanserin and, over the next month, Mr. B began to make more eye contact, was slightly more capable of conversing with staff and team members, and did not appear as guarded and withdrawn. Once again, the team taking care of Mr. B began to feel a cautious sense of optimism that he may have turned the corner and perhaps could be discharged home in the coming weeks. Though Mr. B had multiple negative anti-NMDA panels, the New York-Presbyterian/Weill Cornell Medicine neurology chair reviewed Mr. B’s case and suggested a 3-day course of empiric IV immunoglobulins. These were given to Mr. B with no notable change in symptoms.
After this short period of relative stability on pimavanserin, valproic acid, and 2 mg lorazepam every 8 hours, his condition again began to deteriorate unexpectedly and without a clear precipitant, now 8 months after initial hospitalization. He again showed now-familiar signs of agitated catatonia. As previously describe, he had difficulty controlling periods of agitation that often again required 5-point restraints. Pimavanserin was discontinued and the patient was restarted on clozapine. The team hoped that perhaps clozapine would not cause a fever as before, and perhaps help reduce symptoms. However, Mr. B’s clinical presentation became so severe that he was receiving IM lorazepam as often as every hour, and for several days was in restraints for approximately 10 hours for persistent violent and agitated behavior. He appeared internally preoccupied and completely unable to acknowledge clinical staff now very well known to him. With decreased oral intake, CK increasing to 592 U/L, now with hematuria, the decision was made together with the ICU team to again transfer the patient back to the ICU for sedation. With Mr. B once again returning to the ICU, his mother, who was his official health care proxy, began to express significant concerns that her son was suffering significantly, and again agreed with medically sedating Mr. B to stabilize his condition.
After this intervention, several interdisciplinary meetings with psychiatry, palliative care, the hospital ethics committee, critical care, and the neurology teams were held over the ensuing days. The option of pursuing further treatments, ranging from retrial of ECT to reattempting medications were discussed. Of note, a brain biopsy was also discussed with an eye toward a possible underlying genetic condition, however this was not further considered as it was decided it would not change Mr. B’s treatment possibilities and would expose him to further morbidity. At this stage, the transition to comfort focused care was also discussed for the first time. All these possibilities were discussed in detail with Mr. B’s parents, including a shift to a palliative approach. The interdisciplinary team explained to his parents that comfort focused care would mean that the team would no longer aggressively try to treat or remediate Mr. B’s mental illness but would rather shift toward medical treatments that would promote his comfort and dignity. The team also explained that this approach may result in Mr. B’s condition either improving or worsening, and that such a palliative approach would neither hasten nor prevent his decline or death. Both parents expressed feeling that their son had suffered tremendously, both physically and psychologically, throughout his hospitalization without any durable improvement in his symptoms, and that further treatments would be unnecessarily painful and “prolong the inhumane”. They explicitly did not want placement of a nasogastric tube and expressed wishes to make the patient as comfortable as possible, with priority of removal of his restraints and minimizing needle sticks. His mother signed a Do Not Resuscitate/Do Not Intubate order. After several weeks, Mr. B was transferred out of the ICU to a general medical floor, where he was continued on standing diazepam, phenobarbital, valproic acid, and clonidine. During this phase of his hospital course, Mr. B appeared physically comfortable and did not require restraints. Several weeks later, and 10 months after his initial presentation to our emergency room, Mr. B died peacefully in our hospital medical unit. The cause of death was respiratory arrest in the context of aspiration pneumonia.
Discussion
While available literature about palliative psychiatry has not addressed psychotic disorders in-depth, there is a widespread recognition among clinicians who treat those patients that a small proportion of patients with a primary diagnosis of schizophrenia show little to no response to antipsychotics (clozapine resistance) or ECT (8-11). The above case highlights that sometimes, psychiatric, neurological, and behavioral symptoms continue unabated despite the most aggressive treatments available. After failure to tolerate or improve despite multiple antipsychotics and ECT trials, with the support of the critical care physicians and interdisciplinary team, Mr. B’s parents ultimately made the decision to prioritize his current quality of life over treating his catatonia and keeping him alive.
As Mr. B seemed most at ease and capable of social engagement with staff and peers after receiving IV sedation on ICU level of care, this medication was continued with the goal of relieving his suffering from catatonic symptoms (palliative sedation). Sedation also reduced the need for restraints, thus preventing iatrogenic suffering and promoting Mr. B’s autonomy and dignity. Treatments aiming at reducing this catatonia such as ECT were stopped as they were burdensome and of minimal clinical benefit. Treatments aiming at keeping him alive (such as nasogastric feeding or resuscitation) were forgone as he was unlikely to achieve a subjectively acceptable quality of life again. In sum, this constituted taking a palliative approach in the management of a mental disorder, more specifically, providing EOL care including palliative sedation for catatonic schizophrenia.
This case touches on many ethical questions, for example concerning coerced treatment, substituted, and EOL decision-making. While we cannot expand on all of them, we would like to note that it was the hospital’s intensivists who eventually suggested a shift to comfort-focused care, not the psychiatrists who knew the patient best and were most privy to the details of his many treatment failures and had the clearest view of his suffering. In the authors’ view, this illustrates that psychiatrists—while uniquely accustomed to discussing the possibility of dying by suicide—tend to be less comfortable discussing death and dying in general as well as EOL matters (12). Therefore, implementing palliative approaches will require a major cultural shift in psychiatry (2).
Conclusions
Palliative psychiatry is an innovative approach with the potential to relief and prevent suffering from treatment-resistant SPMI and treatment attempts with poor benefit/burden ratio, particularly in but not limited to chronically hospitalized patients such as Mr. B. This includes but is not limited to end-of-life care. More research is needed on the scope, transdiagnostic potential, and utility of palliative psychiatry to inform the eventual development of guidelines.
Acknowledgments
The case described was presented and discussed by J.E., C.J., and M.T. at the Annual Meeting of the American Psychiatric Association in New Orleans 2022.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Palliative Medicine for the series “Ethics and Psychiatry Meet Palliative Medicine”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-23-586/rc
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-23-586/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-23-586/coif). The series “Ethics and Psychiatry Meet Palliative Medicine” was commissioned by the editorial office without any funding or sponsorship. M.T. served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). It was not possible to obtain written informed consent from the patient for publication of this case report because he passed away. Instead, explicit verbal informed consent to publish the case has been obtained by his mother as the legal representative.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Trachsel M, Irwin SA, Biller-Andorno N, et al. Palliative psychiatry for severe persistent mental illness as a new approach to psychiatry? Definition, scope, benefits, and risks. BMC Psychiatry 2016;16:260. [Crossref] [PubMed]
- Westermair AL, Buchman DZ, Levitt S, et al. Palliative Psychiatry for Severe and Enduring Anorexia Nervosa Includes but Goes beyond Harm Reduction. Am J Bioeth 2021;21:60-2. [Crossref] [PubMed]
- Westermair AL, Buchman DZ, Levitt S, et al. Palliative psychiatry in a narrow and in a broad sense: A concept clarification. Aust N Z J Psychiatry 2022;56:1535-41. [Crossref] [PubMed]
- Decorte I, Verfaillie F, Moureau L, et al. Oyster Care: An Innovative Palliative Approach towards SPMI Patients. Front Psychiatry 2020;11:509. [Crossref] [PubMed]
- Schüttengruber G, Großschädl F, Lohrmann C. A Consensus Definition of End of Life from an International and Interdisciplinary Perspective: A Delphi Panel Study. J Palliat Med 2022;25:1677-85. [Crossref] [PubMed]
- Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. J Med Case Rep 2013;7:223. [Crossref] [PubMed]
- Hirjak D, Sartorius A, Kubera KM, et al. Antipsychotic-induced catatonia and neuroleptic malignant syndrome: the dark side of the moon. Mol Psychiatry 2021;26:6112-4. [Crossref] [PubMed]
- Hasan A, Falkai P, Wobrock T, et al. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry 2012;13:318-78. [Crossref] [PubMed]
- Miyamoto S, Jarskog LF, Fleischhacker WW. Schizophrenia: when clozapine fails. Curr Opin Psychiatry 2015;28:243-8. [Crossref] [PubMed]
- Baldinger-Melich P, Fugger G, Kraus C, et al. Treatment-resistant catatonia—a case report. Clin Neuropsychiatry 2016;13:24-7.
- Rosenbaum DMS, Robertson D, Law S. Psychiatric Futility and Palliative Care for a Patient With Clozapine-resistant Schizophrenia. J Psychiatr Pract 2022;28:344-8. [Crossref] [PubMed]
- McKellar D, Ng F, Chur-Hansen A. Is death our business? Philosophical conflicts over the end-of-life in old age psychiatry. Aging Ment Health 2016;20:583-93. [Crossref] [PubMed]


