
Efficacy of oral nutritional supplement in cancer patients receiving chemotherapy: a systematic review and meta-analysis of randomized controlled trials
Highlight box
Key findings
• The positive impact of oral nutritional support (ONS) on the change in weight was particularly noticeable in elderly patients, those with low baseline body weight, females, non-Asian patients. ONS has the positive impact on Patient-Generated Subjective Global Assessment scores and significant improved quality of life (QoL).
What is known and what is new?
• The meta-analysis in 2018 suggested that ONS achieved a significant positive effect on change body weight. This study directly compared standard of care with or without ONS in patients receiving chemotherapy and included six trials that recently published.
• Our study was the first meta-analysis that evaluate effect of ONS on nutritional status.
What is the implication, and what should change now?
• ONS may be beneficial for cancer patients receiving chemotherapy, including increase body weight, improve nutritional status, and enhance QoL, particularly for those at high risk of malnutrition, which was elderly patients, those with low baseline body weight, and females.
IntroductionOther Section
Cancer is a leading cause of mortality and morbidity worldwide (1). Studies have shown that malnutrition affects up to 51.6% of cancer patients, and cancer-associated malnutrition has been associated with a negative impact on survival, quality of life (QoL), and cancer treatment tolerance (2-4). Pretreatment weight loss was common in cancer patients, accounting for 34%, and was associated with poor overall survival (4). Furthermore, in patients with gastrointestinal (GI), lung, and ovarian cancers undergoing chemotherapy, weight stabilization correlated with a significant improvement in survival (5-7). Maintaining body weight during chemotherapy may serve as a surrogate outcome for cancer treatment.
As a result, global recommendations emphasize the importance of nutritional screening and early intervention for the prevention of malnutrition related to cancer (8-11), but unfortunately, the best intervention for cancer-associated malnutrition has not yet been identified. Oral nutritional supplement (ONS) provide energy, protein, macronutrients, and micronutrients for patients (8,9,11,12). According to the ESPEN practical guidelines on clinical nutrition in cancer, all cancer patients should receive dietary counseling for adequate energy and substrate requirements, regardless of baseline nutrition status, cancer staging, or a history of previous weight loss (9). The additional use of ONS was advised to help achieve nutritional goals as an adjunct to dietary counseling (9,10). Weight stabilization during chemotherapy was associated with survival outcomes (5-7). Therefore, cancer patients receiving chemotherapy were the target population for evaluating the efficacy of ONS in terms of body weight changes.
While ONS has been proposed as a modality for preventing or alleviating cancer-associated malnutrition, clinical trials of ONS with or without dietary counseling (DC) have obtained contradictory results. Some trials have reported the benefits of reducing weight loss by using ONS, but others have claimed that it did not improve patients’ nutritional status, QoL, or survival outcomes (13,14).
Meanwhile, a meta-analysis conducted in 2018 demonstrated that ONS achieved statistically significant improvements in cancer patients’ body weight (15); however, this meta-analysis was limited by its inclusion of control groups that also received ONS. More recent randomized controlled trials (RCTs) have been conducted since then, and our meta-analysis was designed to evaluate the efficacy of ONS compared with DC alone in terms of body weight, nutritional status, and QoL in cancer patients receiving chemotherapy. We present this article in accordance with the PRISMA reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-23-558/rc).
MethodsOther Section
Study design
This study was a systematic review and meta-analysis of ONS among cancer patients receiving chemotherapy. It was registered in the International Prospective Register of Systematics Reviews (PROSPERO), number CRD42023400471.
Literature search and study selection
A literature search was performed to cover all available studies, including unpublished and ongoing, proceeding up to 16 Feb 2023. The data were identified using: (I) search engine and database: PubMed, including the MEDLINE database, and OVID; (II) references of previous systematic reviews. Phase II and III RCTs were selected and were eligible if they met the following criteria: studies of adult cancer patients receiving chemotherapy; compared ONS with calories containing formula and standard of care (nutritional counseling); and reported at least one outcome of interest, such as body weight, Patient-Generated Subjective Global Assessment (PG-SGA) score, global domain QoL score, or fatigue domain of QoL score. The exclusion criteria were those studies which had insufficient data for pooling after three contact attempts with authors at two-week intervals, or studies which were not published in English and could not be translated. The search terms were constructed based on population, intervention, comparator, and outcome (PICO) format including population (adult cancers patient receiving chemotherapy), intervention (ONS) and comparator (nutritional counseling). Outcome domain was omitted for broader search results, and no publication dates or language restrictions were applied. The full search items are displayed in Tables S1,S2.
Studies were identified by two independent reviewers (S.S., P.P.) from abstract and title information, and the full articles of selected studies were subsequently reviewed based on eligible criteria. Disagreement was resolved by discussions between the reviewers’ teams.
Data extraction
Data were extracted from the full texts by two independent reviewers (S.S., P.P.) using the data extraction form, which consisted of six parts: general information about the study (author, year of publication); study characteristics (phase, center, region of study); general characteristics of participants (number of participants, age, gender, type of cancer, stage IV disease); interventions [calories, protein, eicosatetraenoic acid (EPA), duration of treatment]; outcomes (body weight, PG-SGA score, global and fatigue domain QoL score); and the data for pooling.
Data pooling
The primary outcome was the final body weight, defined as body weight at the end of the study, reported in kilograms (kg). Change in weight was subsequently assessed to correct for baseline weight imbalances among all included studies. This outcome was defined as the difference between the final and baseline body weights, reported in kg.
The secondary outcomes included nutritional status score and QoL score. The final PG-SGA score, which used to evaluate patients’ nutritional status, was defined as the PG-SGA score at the end of study. This score ranged from 0 (no problems) to 36 (worst problems). The final global QoL score was the global QoL score reported by patients at the end of the study, with a range from 0 (worst QoL) to 100 (best QoL). Similarly, the final fatigue QoL score was the fatigue QoL score at the end of the study, also a patient-reported outcome, ranging from 0 (least fatigue) to 100 (worst fatigue).
Risk of bias assessment
The risk of bias was assessed by two independent reviewers (S.S., P.P.) who were blinded to each other’s evaluations, which were made using the Revised Cochrane risk-of-bias tool for randomized trials (RoB2) (16). The tool consisted of 5 domains: the randomization process; deviations from the intended interventions; missing outcome data; measurement of the outcomes; and selection of the reported results. The overall risk of bias was judged to be overall “Low”, “Some concerns”, or “High” risk of bias in accordance with RoB2 criteria.
Statistical analysis
All the outcomes of interest were continuous outcomes and were reported as mean, standard deviation (SD) and number of patients. All data were prepared using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions, version 6.3. The outcomes of interest were pooled and analyzed using the mean difference (MD) with a corresponding 95% confidence interval (CI) and then summarized in forest plots. Heterogeneity was assessed by Cochrane’s Q statistics or I2 test (17). If the results showed no significance in P value of the Q test (P>0.1 and I2 statistic <25%), a fixed-effect model was employed using inverse variance method. Otherwise, if there was heterogeneity between studies (P value of Q test ≤0.1 or I2 statistic ≥25%), pooling of the effect size was applied by using a random-effect model (DerSimonian and Laird procedure) (18).
Subgroup analysis of body weight outcome was further assessed based on differences of patient and intervention characteristics among the included studies. Sensitivity analysis of nutritional status and QoL outcomes were subsequently explored in response to the imbalance in baseline scores. Imbalance of baseline score was defined as a baseline difference of greater than 20% or when there was a statistically significant difference between the baseline scores of the intervention and control groups.
A funnel plot and Egger’s test were used to evaluate publication bias. A funnel plot with symmetrical distribution suggested no publication bias, and Egger’s test was used to confirm asymmetry, with a P value of less than 0.05 suggesting the presence of publication bias (19). A P value <0.05 was generally considered statistically significant with the exception of Cochrane’s Q test which used P value <0.1. All analyses were performed using STATA, version 17.0.
ResultsOther Section
Study selection
A total of 705 articles were identified from database searches, 257 from PubMed, and 448 from OVID. After the removal of duplicated articles, a total of 465 articles were selected for screening based on inclusion and exclusion criteria. Out of these, 28 articles met the criteria for full-text review. After conducting a thorough full-text review, six articles were selected for data pooling. A further 4 articles were identified from cross-references, so that ten eligible RCTs were finally included in this study. The selection process is illustrated in Figure 1.
Characteristics of included studies
The detailed characteristics of the ten eligible RCTs (14,20-28) are described in Table 1. These comprised nine phase II and one phase III studies and included 1,101 patients. Only one was a multi-center study (14), and most were conducted in Asia (24-28). Eight out of ten reported the age of patients, the mean of which was 59.87 years (range, 47.00 to 68.20 years). The mean percentage of female patients was 46.88% (range, 24.20% to 100%). All except one RCT (28) recorded baseline body weight, with a mean of 66.13 kg (range, 57.28 to 74.84 kg). Five included GI tract cancer patients (14,21,24,27,28), three included head and neck cancer cases (23,25,26), two included non-small cell lung cancer patients (14,22), and 1 selected only individuals with breast cancer (20). The mean percentage of stage IV disease from nine RCTs was 52.52% (range, 6.60% to 100%) (14,20-24,26-28).
Table 1
| Author, year | Phase | Center | Region | Cancer type, % | Treatment | N | Stage IV, % | Age, years | Female, % | Baseline weight, kg | ONS | Final weight, kg | Weight change, kg | Final PG-SGA score | Final global QoL | Final fatigue QoL | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GI | HN | NSCLC | Calories, kcal/day | Protein, g/day | EPAs, g/day | Duration, weeks | |||||||||||||||
| Elkort RJ, 1981 (20) | II | Single center | USA | 0.0 | 0.0 | 0.0 | ONS | 12 | 21.4 | NR | 100.0 | 74.8±16.33 | 500 | 16.25 | 0 | 52 | 76.38±10.62 | 1.18 | NR | NR | NR |
| DC | 14 | 25.0 | NR | 100.0 | 72.6±16.19 | – | 73.71±9.72 | 0.21 | NR | NR | NR | ||||||||||
| Trabal J, 2010 (21) | II | Single center | Europe | 100.0 | 0.0 | 0.0 | ONS | 7 | 100.0 | 61.5±15.8 | 44.0 | 69.9±15.90 | 590 | 16 | 2 | 12 | NR | NR | NR | 64.30±11.19 | 32.19 |
| DC | 6 | 100.0 | 68.2±15.6 | 29.0 | 72.2±11.70 | – | NR | NR | NR | 74.06±12.36 | 27.16 | ||||||||||
| Baldwin C, 2011 (14) | II | Multicenter | Europe | 73.0 | 0.0 | 24.0 | ONS | 86 | 100.0 | 65.4±13.9 | 75.0 | 69.4±13.30 | 588 | 11.9 | 0 | 6 | 70.87±10.70 | 0.21 | NR | NR | NR |
| DC | 96 | 100.0 | 67.2±11.7 | 73.0 | 69.6±8.28 | – | 70.14±10.09 | −0.35 | NR | NR | NR | ||||||||||
| Sánchez-Lara K, 2014 (22) | II | Single center | South America | 0.0 | 0.0 | 1.0 | ONS | 46 | 80.4 | 58.8±14 | 54.5 | 60.4±11.00 | 590 | 33 | 2.2 | 8 | 60.10±11.00 | −0.33±3.00 | NR | 65.40±23.00 | 32.30±24.00 |
| DC | 46 | 76.0 | 61.0±12.4 | 50.0 | 64.7±13.00 | – | 62.60±14.00 | −2.20±3.00 | NR | 56.50±26.00 | 34.70±20.00 | ||||||||||
| Cereda E, 2018 (23) | II | Single center | Europe | 0.0 | 100.0 | 0.0 | ONS | 78 | 26.9 | 66.5±14.5 | 30.8 | 68.9±14.00 | 500 | 23 | 0 | 12 | 68.30±14.50 | −0.60 | NR | 77.70±24.40 | NR |
| DC | 81 | 30.9 | 63.8±12.7 | 25.9 | 69.2±14.80 | – | 64.60±14.10 | −4.60 | NR | 70.70±29.10 | NR | ||||||||||
| Kim SH, 2019 (24) | II | Single center | Asia | 100.0 | 0.0 | 0.0 | ONS | 15 | 66.7 | 64.5±2.6 | 53.3 | 57.3±2.20 | 400 | 19 | 0 | 8 | 61.38±3.20 | −0.23±1.00 | 5.60±0.80 | 65.60±4.06 | 20.70±4.58 |
| DC | 19 | 57.9 | 65.8±2.1 | 52.6 | 60.9±2.50 | – | 58.93±3.30 | −0.17±1.10 | 19.00±9.10 | 59.20±4.97 | 36.30±5.69 | ||||||||||
| Huang S, 2020 (25) | II | Single center | Asia | 0.0 | 100.0 | 0.0 | ONS | 58 | 0.0 | 49.1±9.2 | 24.2 | 65.8±10.92 | 400 | 16 | 0 | 12 | 60.54±9.98 | −5.24 2.29 | 12.00±1.75 | NR | NR |
| DC | 56 | 0.0 | 51.2±7.9 | 30.4 | 65.7±9.45 | – | 61.56±8.89 | −4.14 ±2.31 | 11.00±2.50 | NR | NR | ||||||||||
| Dou S, 2020 (26) | II | Single center | Asia | 0.0 | 100.0 | 0.0 | ONS | 23 | 47.8 | 48.0±11.3 | 24.2 | 67.2±12.25 | 294 | 35.7 | 0 | 6 | 61.38±12.27 | −5.81±2.45 | 16.60±6.33 | NR | NR |
| DC | 19 | 36.8 | 47.0±7.3 | 30.4 | 63.5±10.25 | – | 58.05±10.64 | −5.44±2.58 | 21.33±4.00 | NR | NR | ||||||||||
| Meng Q, 2021 (27) | III | Single center | Asia | 100.0 | 0.0 | 0.0 | ONS | 171 | 7.0 | 60.8±11.5 | 32.7 | 58.8±12.27 | 500 | 20.5 | 0 | 12 | 55.96±10.45 | −2.89±2.90 | NR | 75.00±10.50 | 11.00±8.25 |
| DC | 166 | 6.6 | 59.0±10.9 | 31.9 | 59.4±10.94 | – | 55.19±10.34 | −4.23±2.67 | NR | 73.00±12.50 | 22.00±11.00 | ||||||||||
| Huong LT, 2021 (28) | II | Single center | Asia | 100.0 | 0.0 | 0.0 | ONS | 52 | 84.9 | NR | 37.7 | NR | 500 | 20 | 0 | 8 | 51.60±7.80 | −1.76±2.17 | 8.90±6.00 | NR | NR |
| DC | 50 | 82.0 | NR | 38.0 | NR | – | 50.90±7.10 | −2.46±2.41 | 10.90±6.20 | NR | NR | ||||||||||
Cancer type, stage IV, and female were reported as percentages. Age, baseline weight, final weight, weight change, final PG-SGA score, final global QoL score, and final fatigue QoL were reported as mean ± standard deviation. GI, gastrointestinal; HN, head and neck cancer; NSCLC, non-small cell lung cancer; N, number of participants; ONS, oral nutritional supplement; DC, dietary counseling; EPA, eicosatetraenoic acid; PG-SGA, Patient-Generated Subjective Global Assessment; global QoL, European Organization for Research and Treatment of Cancer core quality of life score (EORTC QLQ-C30) in global domain; fatigue QoL, European Organization for Research and Treatment of Cancer core quality of life score (EORTC QLQ-C30) in fatigue domain; USA, United States; NR, not reported.
Interventions
All included RCTs prescribed ONS as an intervention of interest for cancer patients receiving chemotherapy. The mean caloric intake per day was 486.20 kilocalories (kcal) (range, 294 to 590 kcal) and the mean total protein consumed per day was 21.13 grams (gm) (range, 11.9 to 35.7 gm). Only two RCTs provided ONS with EPA formula (21,22). The median duration of treatment was 13.6 weeks (range, 6 to 52 weeks). In the control group, all the included RCTs provided dietary counseling as standard care for patients who were randomized into the control arm.
Risk of bias assessment
Nine out of ten RCTs had an overall low risk of bias, while one (28) was found to have a high risk due to its poor randomization process. The overall risk of bias of this systematic review and meta-analysis was low at 90%. The assessment of risk of bias using the RoB2 tool (16) is shown in Figure S1.
Primary outcomes
Final body weight
Nine of the RCTs, comprising 1,088 participants, reported final body weight (14,20,22-28), and ONS in patients receiving chemotherapy was found to not significantly improve final body weight when compared to DC. Pooled MD for final body weight was −0.07 kg (95% CI: −0.99 to 0.84, P=0.88) with no evidence of heterogeneity [I2=0.00%, Q test: Chi-square 6.88, degree of freedom (DF) 8, P=0.55]. Results of a meta-analysis of final body weight outcomes are shown in Figure 2. Prespecified subgroup analysis revealed no statistically significant difference among all subgroups, as shown in Figure S2.

Change in weight
A baseline body weight imbalance was observed among the nine RCTs which reported final body weight. Therefore, the change in weight was taken as the primary outcome to minimize the effect of this imbalance. Nine studies with 1,088 participants were used to determine the change in weight for pooling (14,20,22-28). ONS in patients receiving chemotherapy increased the change in weight but without statistical significance. Pooled MD of weight change was 0.90 kg (95% CI: −0.48 to 2.28, P=0.20). There was evidence of heterogeneity between studies (I2=91.87%, Q test: Chi-square 98.38, DF 8, P<0.01). The results of a meta-analysis of the change in weight are shown in Figure 3.
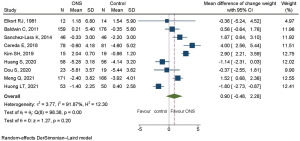
Subgroup analysis identified some factors associated with significant increases in weight after ONS treatment (Figure 4). These factors were age 60 years or more, with pooled MD of 2.47 kg (95% CI: 0.73 to 4.21, P=0.01); female sex no less than 50%, with pooled MD of 2.37 kg (95% CI: 1.33 to 3.42, P<0.01); baseline body weight lower than 65 kg, with pooled MD of 2.14 kg (95% CI: 1.20 to 3.09, P<0.01); and non-Asian ethnicity, with pooled MD of 1.87 kg (95% CI: 0.12 to 3.62, P=0.04). The results of subgroup analysis of these factors are displayed in Figure S3A-S3D. A moderate reduction in heterogeneity was observed in groups with no less than 50% female patients and baseline body weight less than 65 kg, but no reduction in heterogeneity was found in patients aged 60 years or more and of non-Asian ethnicity.
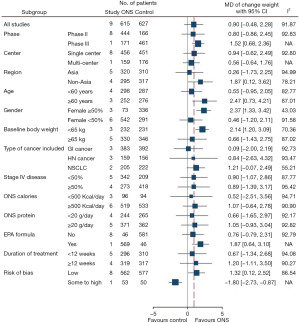
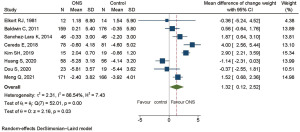
Risk of bias was of high concern in one study because of its randomization process (28). After excluding this study in sensitivity analysis, weight change showed a statistically significant improvement, with pooled MD of 1.32 kg (95% CI: 0.12 to 2.52, P=0.03). The results are shown in Figure 5.
Secondary outcomes
PG-SGA score
Four RCTs, comprising 292 participants, reported PG-SGA score (24-26,28). ONS in patients receiving chemotherapy achieved an improvement in PG-SGA score, with pooled MD of −2.13 points, but this was not statistically significant (95% CI: −5.07 to 0.82, P=0.16) when compared to DC. There was heterogeneity between studies (I2=91.00%, Q test: Chi-square 33.34, DF 3, P<0.01) as shown in Figure 6. None of these 4 RCTs had an imbalance of baseline nutrition score; therefore, sensitivity analysis of the PG-SGA score was not performed.

Global domain in QoL
Five RCTs, comprising 635 participants, reported global QoL score according to the EORTC QLQ-C30 questionnaire (21-24,27). The global domain QoL revealed a statistically significant improvement after ONS treatment, with pooled MD of 4.01 points (95% CI: 0.08 to 7.94, P=0.05). There was heterogeneity between studies (I2=62.00%, Q test: Chi-square 10.53, DF 4, P=0.03), as shown in Figure 7. One RCT had baseline global domain QoL imbalance (21) while another did not report it (27). Sensitivity analysis was performed by excluding these two RCTs (21,27) and revealed that the benefit of ONS in terms of global domain QoL was more notable, with a statistically significant improvement of pooled MD of 6.66 points (95% CI: 3.86 to 9.46, P<0.01). No heterogeneity was observed after removing RCTs with baseline imbalance (I2=00.00%, Q test: Chi-square 0.22, DF 2, P=0.89), as shown in Figure S4.
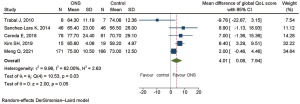
Fatigue domain in QoL
Four RCTs, comprising 476 participants, reported fatigue QoL score using the EORTC QLQ-C30 questionnaire (21,22,24,27). There was a significant improvement in fatigue domain QoL score, with pooled MD of −7.63 points (95% CI: −13.87 to −1.39, P=0.02). There was heterogeneity between studies (I2=86.02%, Q test: Chi-square 21.46, DF 3, P<0.01), as shown in Figure 8. One of these RCTs had baseline fatigue domain QoL imbalance (21), and one did not report it (27). Sensitivity analysis was performed by excluding these two RCTs, and found that ONS achieved greater improvement in fatigue domain QoL, with pooled MD of −9.68 points (95% CI: −22.55 to 3.19, P=0.14); however, this was not statistically significant. There was no reduction in heterogeneity after removal of RCTs with baseline imbalance (I2=85.95%, Q test: Chi-square 7.12, DF 1, P=0.01), as shown in Figure S5.

Publication bias
Publication bias assessment of primary outcome was evaluated by a funnel plot and Egger’s test. A funnel plot for overall pooling suggested there was symmetry, and Egger’s test showed no significant evidence of asymmetry [coefficient 0.97, standard error (SE) 0.74, P=0.25]. Thus, there was no evidence of publication bias, as depicted in Figure S6.
DiscussionOther Section
This systemic review and meta-analysis of ONS results compared to those of DC alone in cancer patients receiving chemotherapy did not reveal any improvement in final body weight. The change in weight was assessed to minimize imbalances in baseline body weight, and ONS achieved positive results in terms of this factor, notably in older patients, females, those with low baseline body weight and non-Asian patients. After adjustment for high risk of bias, ONS obtained a statistically significant increase in weight change. There was some disagreement between our results and those of a prior meta-analysis conducted in 2018 (15), which suggested that ONS achieved a significant positive effect on change body weight. There were several differences between these two meta-analyses. First, this study included only RCTs that directly compared standard of care with or without ONS whereas in the previous meta-analysis, ONS was allowed to be the control arm. Secondly, all patients in this meta-analysis received chemotherapy, whereas it was not a requisite in the prior study. This meta-analysis also included six RCTs that were published after 2016. As final body weight and the change in weight may not be very different in routine clinical practice, both meta-analyses confirmed ONS’s positive impact on body weight.
Our study demonstrated that ONS had a particularly positive effect in elderly, female and low baseline body weight patients, which was probably because these patients were at a higher risk of malnutrition, as elderly patients tend to have a smaller appetite and consume fewer calories (29). Interestingly, non-Asian patients tended to benefit from ONS more than Asians. A previous population-based study showed that the mean BMI levels of Asian men and women were 21.4 and 21.8 kg/m2, respectively compared to 32.2 and 34.8 kg/m2 in American and European men and women (30). Additionally, Americans and Europeans consumed an average of 300 more kilocalories per person per day compared to their counterparts in other regions (31). It is well established that cancer patients receiving chemotherapy often experience a loss of appetite due to a variety of factors (32-34); taking this into account, ONS may more effectively fill the energy gap in non-Asian cancer patients receiving chemotherapy than in Asian patients.
This study was the first meta-analysis to assess nutritional status using a PG-SGA. Although ONS enhanced PG-SGA status, the improvement was not statistically significant, probably because calorie intake was not the only factor affecting nutritional status in cancer patients. Other factors that may influence the effectiveness of ONS include type of cancer, patient co-morbidities, treatment compliance, the effectiveness of dietary counseling, and patient preferences. Three meta-analyses, including our research, have demonstrated that ONS significantly improves global QoL score (35), and in our study, fatigue domain QoL scores also tended to improve as a result of ONS.
The strengths of this study included its inclusion of recent RCTs and a focus on the sole effectiveness of ONS in cancer patients receiving chemotherapy. Moreover, this meta-analysis examined a range of outcomes, including weight, nutritional status, and QoL and also explored subgroups of patients with particular interests. Its limitation was the lack of oncologic outcomes, including progression-free survival, overall survival, or treatment-related side effects. This study was also unable to determine the benefit of EPA formula because only two RCTs evaluated this factor. There was limited data on baseline pre-cachexia and cachexia from the selected RCTs. Only four RCTs reported baseline nutrition status of patients, making it unable to perform a subgroup analysis based on baseline nutrition scores. Therefore, the benefit of ONS for specific malnourished patients receiving chemotherapy could not be fully assessed in this study.
ConclusionsOther Section
This systematic review and meta-analysis suggested that ONS may increase weight change in cancer patients receiving chemotherapy. The positive impact of ONS on the change in weight was particularly noticeable in elderly patients, those with low baseline body weight, females, and non-Asian patients. This study also identified ONS’s positive impact on PG-SGA scores as well as its role in significantly improving patients’ QoL. Based on these findings, ONS may be beneficial for cancer patients receiving chemotherapy, particularly those at high risk of malnutrition.
AcknowledgmentsOther Section
Funding: The funding of this manuscript was from
FootnoteOther Section
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-23-558/rc
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-23-558/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-23-558/coif). All authors report the funding from Rajavithi Hospital, College of Medicine, Rangsit University. S.S. reports honorarium for lecture from Eisai. P.T. reports honorarium for lectures/educational events from AstraZeneca, BMS, MSD, Celltrion, Novartis, Roche, Eisai, Taiho, Thai Osuka, ZP therapeutics, Fresenius Kabi and Pfizer; travel grant from Celltrion, ZP therapeutics, Fresenius Kabi and Eisai for attending international cancer meeting. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 1980;69:491-7. [Crossref] [PubMed]
- Langius JA, Bakker S, Rietveld DH, et al. Critical weight loss is a major prognostic indicator for disease-specific survival in patients with head and neck cancer receiving radiotherapy. Br J Cancer 2013;109:1093-9. [Crossref] [PubMed]
- Gannavarapu BS, Lau SKM, Carter K, et al. Prevalence and Survival Impact of Pretreatment Cancer-Associated Weight Loss: A Tool for Guiding Early Palliative Care. J Oncol Pract 2018;14:e238-50. [Crossref] [PubMed]
- Andreyev HJ, Norman AR, Oates J, et al. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer 1998;34:503-9. [Crossref] [PubMed]
- Ross PJ, Ashley S, Norton A, et al. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer 2004;90:1905-11. [Crossref] [PubMed]
- Hess LM, Barakat R, Tian C, et al. Weight change during chemotherapy as a potential prognostic factor for stage III epithelial ovarian carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 2007;107:260-5. [Crossref] [PubMed]
- August DA, Huhmann MBAmerican Society for Parenteral and Enteral Nutrition. (A.S.P.E.N. A.S.P.E.N. clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN J Parenter Enteral Nutr 2009;33:472-500. [Crossref] [PubMed]
- Muscaritoli M, Arends J, Bachmann P, et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin Nutr 2021;40:2898-913. [Crossref] [PubMed]
- Arends J, Strasser F, Gonella S, et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines ESMO Open 2021;6:100092. [Crossref] [PubMed]
- Liposits G, Orrevall Y, Kaasa S, et al. Nutrition in Cancer Care: A Brief, Practical Guide With a Focus on Clinical Practice. JCO Oncol Pract 2021;17:e992-8. [Crossref]
- Hubbard GP, Elia M, Holdoway A, et al. A systematic review of compliance to oral nutritional supplements. Clin Nutr 2012;31:293-312. [Crossref] [PubMed]
- Odelli C, Burgess D, Bateman L, et al. Nutrition support improves patient outcomes, treatment tolerance and admission characteristics in oesophageal cancer. Clin Oncol (R Coll Radiol) 2005;17:639-45. [Crossref] [PubMed]
- Baldwin C, Spiro A, McGough C, et al. Simple nutritional intervention in patients with advanced cancers of the gastrointestinal tract, non-small cell lung cancers or mesothelioma and weight loss receiving chemotherapy: a randomised controlled trial. J Hum Nutr Diet 2011;24:431-40. [Crossref] [PubMed]
- de van der Schueren MAE. Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: current evidence and guidance for design of future trials. Ann Oncol 2018;29:1141-53. [Crossref] [PubMed]
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials 2015;45:139-45. [Crossref] [PubMed]
- Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics 2018;74:785-94. [Crossref] [PubMed]
- Elkort RJ, Baker FL, Vitale JJ, et al. Long-term nutritional support as an adjunct to chemotherapy for breast cancer. JPEN J Parenter Enteral Nutr 1981;5:385-90. [Crossref] [PubMed]
- Trabal J, Leyes P, Forga M, et al. Potential usefulness of an EPA-enriched nutritional supplement on chemotherapy tolerability in cancer patients without overt malnutrition. Nutr Hosp 2010;25:736-40. [PubMed]
- Sánchez-Lara K, Turcott JG, Juárez-Hernández E, et al. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: randomised trial. Clin Nutr 2014;33:1017-23. [Crossref] [PubMed]
- Cereda E, Cappello S, Colombo S, et al. Nutritional counseling with or without systematic use of oral nutritional supplements in head and neck cancer patients undergoing radiotherapy. Radiother Oncol 2018;126:81-8. [Crossref] [PubMed]
- Kim SH, Lee SM, Jeung HC, et al. The Effect of Nutrition Intervention with Oral Nutritional Supplements on Pancreatic and Bile Duct Cancer Patients Undergoing Chemotherapy. Nutrients 2019;11:1145. [Crossref] [PubMed]
- Huang S, Piao Y, Cao C, et al. A prospective randomized controlled trial on the value of prophylactic oral nutritional supplementation in locally advanced nasopharyngeal carcinoma patients receiving chemo-radiotherapy. Oral Oncol 2020;111:105025. [Crossref] [PubMed]
- Dou S, Ding H, Jiang W, et al. Effect of oral supplements on the nutritional status of nasopharyngeal carcinoma patients undergoing concurrent chemotherapy: A randomized controlled Phase II trial. J Cancer Res Ther 2020;16:1678-85. [Crossref] [PubMed]
- Meng Q, Tan S, Jiang Y, et al. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: A randomized clinical trial. Clin Nutr 2021;40:40-6. [Crossref] [PubMed]
- Huong LT, Phuong DT, Anh DK, et al. Nutritional Intervention Improves Nutrition Outcomes in Stomach and Colon Cancer Patients Receiving Chemotherapy: Finding from a Quasi-Experiment in Vietnam. Healthcare (Basel) 2021;9:843. [Crossref] [PubMed]
- Giezenaar C, Chapman I, Luscombe-Marsh N, et al. Ageing Is Associated with Decreases in Appetite and Energy Intake--A Meta-Analysis in Healthy Adults. Nutrients 2016;8:28. [Crossref] [PubMed]
- Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016;387:1377-96. [Crossref] [PubMed]
- Nations FaAOoU. The broad picture: Historical developments and present situation. Food and Agriculture Organization of United Nations. 2020. Available online: https://www.fao.org/3/y4252e/y4252e04.htm#Ch2. Accessed April 17, 2023.
- Marinho EDC, Custódio IDD, Ferreira IB, et al. Impact of chemotherapy on perceptions related to food intake in women with breast cancer: A prospective study. PLoS One 2017;12:e0187573. [Crossref] [PubMed]
- Peixoto da Silva S, Santos JMO, Costa E. Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia and asthenia. J Cachexia Sarcopenia Muscle 2020;11:619-35. [Crossref] [PubMed]
- Hariyanto TI, Kurniawan A. Appetite problem in cancer patients: Pathophysiology, diagnosis, and treatment. Cancer Treat Res Commun 2021;27:100336. [Crossref] [PubMed]
- Baldwin C, Spiro A, Ahern R, et al. Oral nutritional interventions in malnourished patients with cancer: a systematic review and meta-analysis. J Natl Cancer Inst 2012;104:371-85. [Crossref] [PubMed]



