
Clinical outcomes of antithrombin III supplementation in an overt disseminated intravascular coagulation: a longitudinal single-institutional experience and retrospective analysis
Highlight box
Key findings
• Antithrombin III administration may be effective tools for disseminated intravascular coagulation (DIC) treatment, especially in the cases of DIC, which is a frequent complication of septic shock, sepsis, and other critical disease entities and which is associated with a high level of mortality.
What is known and what is new?
• Despite the theoretical benefits of antithrombin supplementation, the optimal antithrombin activity for heparin efficacy and the benefits of antithrombin supplementation in various disease entities are not yet fully understood.
• The total doses and periods of antithrombin III administration, which recommended by national guidelines, may be insufficient.
What is the implication, and what should change now?
• Prolongation of period and increase of total dose of antithrombin III supplement might be necessary.
• Antithrombin administration may be more positively considered, especially in the cases of DIC, which is a frequent complication of septic shock, sepsis, and other critical disease entities and which is associated with a high level of mortality.
• In accordance with clinical situation, disease entity, age, serum antithrombin level and others, the precise calculation formula, which are reflecting the initial loading dose, maintenance dose and administration period, may be essential.
Introduction
Antithrombin functions as an important regulator of blood coagulation by serving as the dominant inhibitor of thrombin, factor IXa, and factor Xa in plasma, although it also inactivates other serine proteases in the intrinsic coagulation pathway, such as factors XIa and XIIa, as well as some non-coagulation serine proteases, such as kallikrein, the complement enzyme C1 and plasmin. It plays a leading major factor on coagulation pathway, therefore it must be administered to treat serious clinical conditions such as disseminated intravascular coagulation (DIC). Recent studies have indicated that the recovery of antithrombin activity to within the normal range (>70%) is necessary to achieve favorable outcomes (1-3). recent retrospective nationwide database study from Japan demonstrated that antithrombin administration may be associated with reduced 28-day mortality in patients with severe pneumonia and sepsis-associated DIC (2). The Japan Septic Disseminated Intravascular Coagulation study group also reported that anticoagulant therapy showed a survival benefit [adjusted hazard ratio (HR), 0.601; 95% confidence interval (CI): 0.451–0.800] in patients with sepsis-induced coagulopathy and/or very severe disease (3). However, despite these favorable reports regarding the effect of antithrombin therapy on patients’ outcomes, there are still several conflicting reports on the utility of antithrombin supplementation in the critical conditions such as DIC and DIC associated with trauma or sepsis. The optimal antithrombin activity for heparin efficacy and the benefits of antithrombin supplementation in various disease entities are not yet fully understood, there are no disease-specific guidelines for the appropriate target antithrombin activity during supplementation, and there are also no age-specific guidelines, especially for neonatal patients. In an observational study 2019, Kim et al. reported high-dose antithrombin supplementation significantly improved 28-day mortality in septic shock patients with DIC (4), and Akahoshi et al. addressed targeted antithrombin activity should be at least 70%, and ideally 80%, and sufficient antithrombin doses to maintain this activity should be required to achieve better outcomes for DIC patients in Japan (5). In most recent systematic review, meta-analysis and trial sequential analysis, the improvement of antithrombin level in perioperative cardiopulmonary bypass surgery showed no significant effect on blood conservation, contrary to expectations this might increase in hospital mortality and the incidence of acute kidney injury (6). The optimal dosing requirement may depend on the impaired action of heparin or the increased volume of distribution and clearance of heparin across individuals and/or specific disease entities. Therefore, it is unclear whether lower antithrombin activity alone accounts for increased heparin dosing requirements. Unfractionated heparin exerts its anticoagulatory action by enhancing the inhibitory effect of antithrombin. Several conditions, including acute respiratory distress syndrome, sepsis, and DIC, decrease antithrombin activity and functionality. An immature coagulation system in a critically ill patient might require high dose of antithrombin supplementation, as this might increase heparin efficacy to achieve optimal therapeutic anti-Xa levels (7). DIC, which is a frequent complication of septic shock, sepsis, and other critical disease entities, is affiliated with a high level of mortality. In these extremely critical situations, a debilitated physiological anticoagulant mechanism results in weakened antithrombin activity and consequentially leads to excessive microthrombus formation, microcirculatory dysfunction, and end-organ failure. We present this article in accordance with the TREND reporting checklist (available at https://apm.amegroups.com/article/view/10.21037/apm-23-535/rc).
Methods
A retrospective study design involves the DIC and antithrombin III program. In a single medical center, single investigator and single arm on the DIC program was first proposed from January 2010, and active DIC treatment was consistently carried out on adult patients, aged ≥18 years, suffering from critical conditions such as DIC and DIC associated with trauma or sepsis between January 1, 2010 and October 31, 2021. All the patients in this investigation were managed in the intensive care unit (ICU), and both the sequential organ failure assessment (SOFA) and simplified acute physiology score II (SAPS II) were calculated at the time of ICU admission. In the non-hemorrhaging patient with DIC, prophylactic anticoagulation with low doses of unfractionated heparin (UFH) or low molecular weight heparins (LMWH) was strongly the initiated. To minimize and avoid selection bias, the inclusion criteria were as follows: critically sick adult patients both who suffered from critical conditions such as DIC and DIC associated with trauma or sepsis and who conducted with the supplementation of antithrombin III on critical adult patients on DIC/DIC associated with trauma and/or sepsis, strictly complying with the administration guidelines by the National Health Insurance Service (NHIS) and the Ministry of Food and Drug Safety (MFDS). The exclusion criteria were as follows: (I) who not meet The Korean Society of Thrombosis and Hemostasis (KSTH) DIC diagnostic criteria. The KSTH defined the DIC, which satisfied more than three diagnostic criteria of four items as follows: (i) fibrin degradation product level >10 µL/mL or D-dimer level >320 mg/dL, (ii) platelet count <100,000/µL, (iii) fibrinogen <150 mg/dL, (iv) prothrombin time >3 seconds to normal range (11–12.5 seconds) or activated partial thromboplastin time >5 seconds to normal range (30–40 seconds); (II) total administration periods of antithrombin III <2 days; (III) total administration amounts of antithrombin III <8 vials; (IV) who expired within three days since initiation of antithrombin III administration; (V) who not meet the diagnostic criteria on sepsis and septic shock; (VI) who were aged under 18 years. The total administration period of antithrombin III was limited to within 3 days in most cases, and the total administration dose was below 7,000 international unit (IU), (loading dose, 1,000 IU in 1 hour: maintenance dose, 500 IU every 6 hours for 3 days) following the MFDS guidelines (8).
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Konkuk University Chungju Hospital (No. KUCH 2022-05-003) and individual consent for this retrospective analysis was waived.
Statistical analysis
Statistical Analyses were performed using the IBM SPSS software (version 21; IBM Corp., Armonk, NY, USA) and the MedCalc for Windows version 22.016, 64-bit (MedCalc software, Ostend, Belgium). All data were collected and analyzed using Microsoft Excel spread sheet (Microsoft, Redmond, WA, USA). Continuous variables were tested for normality using the Kolmogorov-Smirnov test. Continuous variables showing normality were analyzed using Student’s t-test and are expressed as the arithmetic mean ± standard deviation, and those not showing normality were interpreted using the Mann-Whitney U test and are expressed as the median with 25–75th interquartile range. Categorical variables are displayed as frequency distributions and were calculated with Fisher’s exact test or Pearson’s Chi-square test. To avoid type 1 errors, Bonferroni post hoc correction (B-corrected) was performed to data that were initially deemed statistically significant by multiplying the number of variables by the P value. Cox proportional hazards model was used to identity independent predictors of successful survival. Overall survival was estimated according to the Kaplan-Meier method. Independent predictors of overall survival were also determined by using the Cox proportional hazards model. Statistical significance was set at P<0.05. To confirm independent factors associated with mortality of patient, we applied univariate and multivariate stepwise logistic regression models. Multiple logistic regression analysis using backwards stepwise regression was performed. Variables with a level of significance defined as P<0.20 for univariate logistic regression analysis, as well as clinically important variables, were analyzed as independent predictors for the multivariate models. The data are expressed as odds ratios (OR) with standard error (SE), 95% CI and relevant P values. To evaluate the predictive power of the logistic regression model, receiver operating characteristic (ROC) curves were manipulated, and we calculated the area under the curve (AUC). The Hosmer-Lemeshow goodness-of-fit test was used to compare the numbers of observed and predicted deaths in risk groups for the entire range of death probabilities. Discrimination was assessed using the area under the ROC curves. Cumulative survival curves as a function of time were generated by the Kaplan-Meier approach and were compared between the groups using the log rank test.
Results
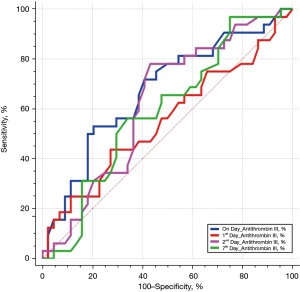
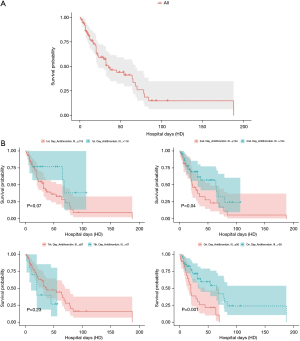
We identified 76 eligible for investigation according to the above-mentioned criteria in our institution between January 1, 2010 and October 31, 2021. The clinical characteristics of the study patients and detailed demographic are summarized in Table 1 and Table S1. ROC 76 patients (male/female, 59/17), 44 were identified to the non-survivor group (male/female, 32/12) and 32 patients were recognized as the survivor group (male/female, 27/5). The baseline parameters in the non-survivor and survivor groups were comparable with no significant differences in age (66.5±18.1 vs. 66.0±16.2 years, P=0.90), sex (male/female) (32/12 vs. 27/5, P=0.35), hospital length of stay (days) (31.1±34.5 vs. 31.2±26.1, P=0.99), SOFA (7.3±2.5 vs. 6.6±2.0, P=0.22), SAPS II (46.0±8.8 vs. 43.5±9.2, P=0.23), cause for DIC (P=0.95), and underlying disease (P=0.38). There was no definite statistical significant difference between the non-survivor and survivor groups in laboratory findings as the elapse of days (on day, 1st day, 2nd and 7th day) by antithrombin III administration; D-dimer; coagulation battery including thrombin time, fibrinogen degradation products (FDP), prothrombin time (PT), activated partial thromboplastin time (aPTT), international normalized ratio (INR), fibrinogen; electrolyte battery including Na, K, Cl; artery blood gas analysis including pH, pO2, pCO2, cHCO3, base excess, SO2; chemical battery including glucose, iCa, lactate, aspartate aminotransferase (AST; GOT), alanine aminotransferase (ALT; GPT), total bilirubin, direct bilirubin, total protein, albumin, blood urea nitrogen (BUN), creatinine, phosphate, amylase; complete blood cell count with differential count including WBC, RBC, hemoglobin, hematocrit, platelet, neutrophil, lymphocyte, monocyte, eosinophil, basophil; inflammation marker such as C-reactive protein (CRP), procalcitonin, presepsin; cardiac marker such as troponin-I, myoglobin, CK-MB (creatine kinase MB isoenzyme). The clinical laboratory levels of antithrombin III (%) on the day just before the administration significantly lower in the non-survivor groups than in the survivor groups (50.1%±13.6% vs. 57.6%±12.5%, P=0.01). The hemoglobin level in the 2nd day and 7th day after antithrombin III administration was significantly different between the non-survivor and survivor groups (9.9±1.9 vs. 11.0±2.0 g/dL, P=0.01, and 9.4±1.8 vs. 10.5±1.6 g/dL, P=0.006). One-way analysis of variance (ANOVA) showed significant changes between levels of antithrombin III as the elapse of days (on day, 1st day, 2nd and 7th day) by antithrombin III administration (53.21%±13.6%; SE, 1.55; 95% CI of difference, 50.11% to 56.32% vs. 89.65%±29.1%; SE, 3.33; 95% CI of difference, 83.01% to 96.30% vs. 109.18%±22.7%; SE, 2.60; 95% CI of difference, 103.99% to 114.37% vs. 66.32%± 19.7%; SE, 2.25; 95% CI of difference, 61.82% to 70.83%; P<0.001), and repeated measures ANOVA demonstrated significant difference in antithrombin III levels between the non-survivor and survivor groups (P=0.02) (Tables 2,3; Figures 1,2). Multiple logistic regression analysis by stepwise backward regression demonstrated significant OR on antithrombin III (%) on the day just before the administration and SAPS II for the difference between the non-survivor and survivor groups (P=0.04; OR, 1.0447; SE, 0.022; 95% CI of difference, 1.0003 to 1.0911 vs. P=0.03; OR, 0.9370; SE, 0.031; 95% CI of difference, 0.8817 to 0.9958) (Table 4; Figure 3). To evaluate the predictive power of the logistic regression model, ROC curves were generated to obtain classification AUCs. The AUCs for the antithrombin III levels as the elapse of days (on day, 1st day, 2nd and 7th day) were assessed by using multiple regression models (Tables 5,6). The antithrombin III levels on the day of administration (AUC =0.672) manifested significantly better prediction of mortality than the antithrombin III levels on 1st day (AUC =0.552), 2nd day (AUC =0.624), and 7th day (AUC =0.593) (Figure 4). Kaplan-Meier curves for the cumulative survival probability as hospital days showed the statistically significant cut-off levels of antithrombin III between the non-survivor and survivor groups in on day and 2nd day antithrombin III administration, as the elapse of days (on day, 1st day, 2nd and 7th day) by antithrombin III administration (cut-off levels 50.0, P=0.01; vs. cut-off levels 118.0, P=0.07; vs. cut-off levels 104.0, P=0.04; vs. cut-off levels 87.0, P=0.23; respectively) (Figure 5A,5B). These results demonstrate that the antithrombin III levels on the day of administration might be the best prediction tools for in-hospital mortality, and SAPS II, representing the state of patient clinical situation, could be a useful parameter for survival.
Table 1
| Parameters | Non-survivor (N=44) | Survivor (N=32) | Total (N=76) | P |
|---|---|---|---|---|
| Age (years) | 66.5±18.1 | 66.0±16.2 | 66.2±17.2 | 0.90 |
| Sex | 0.35 | |||
| Male | 32 (72.7) | 27 (84.4) | 59 (77.6) | |
| Female | 12 (27.3) | 5 (15.6) | 17 (22.4) | |
| Hospital days | 31.1±34.5 | 31.2±26.1 | 31.2±31.1 | 0.99 |
| Cause for DIC | 0.95 | |||
| Sepsis | 10 (22.7) | 8 (25.0) | 18 (23.7) | |
| Trauma | 10 (22.7) | 6 (18.8) | 16 (21.1) | |
| Respiratory failure | 10 (22.7) | 8 (25.0) | 18 (23.7) | |
| Surgery/rhabdomyolysis | 9 (20.5) | 5 (15.6) | 14 (18.4) | |
| Others | 5 (11.4) | 5 (15.6) | 10 (13.2) | |
| Underlying disease | 0.38 | |||
| Arterial hypertension | 13 (29.5) | 4 (12.5) | 17 (22.4) | |
| Diabetes | 8 (18.2) | 9 (28.1) | 17 (22.4) | |
| COPD | 9 (20.5) | 9 (28.1) | 18 (23.7) | |
| Cardiac comorbidities | 4 (9.1) | 5 (15.6) | 9 (18.4) | |
| Dyslipidemia | 5 (11.4) | 5 (15.6) | 10 (11.3) | |
| SOFA | 7.3±2.5 | 6.6±2.0 | 7.0±2.3 | 0.22 |
| SAPS II | 46.0±8.8 | 43.5±9.2 | 44.9±9.0 | 0.23 |
| Transfusion | 11 (14.5) | 5 (6.6) | 16 (21.1) | 0.39 |
| Major bleeding | 1 (1.3) | 2 (3.6) | 3 (3.9) | 0.56 |
| D-dimer (ng/mL) | ||||
| On day | 8,346.0±14,478.4 | 6,256.3±7,517.1 | 7,466.1±12,025.8 | 0.41 |
| 1st day | 8,814.5±12,669.4 | 8,528.4±7,775.7 | 8,694.0±10,818.4 | 0.90 |
| 2nd day | 11,909.0±15,900.1 | 13,621.6±14,762.2 | 12,639.7±15,346.2 | 0.63 |
| 7th day | 5,196.3±4,128.1 | 4,987.6±4,271.3 | 5,108.4±4,162.0 | 0.83 |
| Thrombin time (sec) | ||||
| On day | 34.9±59.8 | 21.7±9.2 | 29.4±46.2 | 0.15 |
| 1st day | 22.4±13.9 | 20.8±6.8 | 21.8±11.4 | 0.50 |
| 2nd day | 26.7±42.9 | 30.5±51.0 | 28.3±46.2 | 0.72 |
| 7th day | 84.5±24.2 | 83.5±18.8 | 84.1±21.9 | 0.83 |
| Antithrombin III (%) | ||||
| On day | 50.1±13.6 | 57.6±12.5 | 53.2±13.6 | 0.01* |
| 1st day | 87.2±27.3 | 93.0±31.5 | 89.7±29.1 | 0.40 |
| 2nd day | 105.5±24.5 | 114.2±19.3 | 109.2±22.7 | 0.10 |
| 7th day | 63.6±21.7 | 70.1±16.2 | 66.3±19.7 | 0.15 |
| FDP (microgram/mL) | ||||
| On day | 109.2±111.2 | 108.8±103.6 | 109.0±107.4 | 0.98 |
| 1st day | 82.7±88.3 | 88.1±54.7 | 85.0±75.6 | 0.74 |
| 2nd day | 35.5±50.3 | 44.8±26.5 | 39.4±42.0 | 0.30 |
| 7th day | 21.5±18.4 | 26.0±26.9 | 23.4±22.3 | 0.42 |
| PT (sec) | ||||
| On day | 16.1±4.1 | 15.1±3.4 | 15.7±3.8 | 0.27 |
| 1st day | 15.9±6.0 | 15.6±5.6 | 15.8±5.8 | 0.80 |
| 2nd day | 16.3±8.4 | 14.2±2.7 | 15.4±6.7 | 0.11 |
| 7th day | 14.2±1.9 | 14.1±1.9 | 14.1±1.9 | 0.81 |
| aPTT (sec) | ||||
| On day | 47.3±18.5 | 52.0±68.2 | 49.3±46.1 | 0.70 |
| 1st day | 48.1±32.9 | 36.0±13.5 | 43.0±27.1 | 0.03* |
| 2nd day | 40.9±13.3 | 37.3±14.6 | 39.4±13.9 | 0.27 |
| 7th day | 39.8±12.1 | 36.5±12.8 | 38.4±12.4 | 0.25 |
| INR | ||||
| On day | 1.52±0.4 | 1.44±0.3 | 1.50±0.3 | 0.34 |
| 1st day | 1.58±0.5 | 1.05±0.5 | 1.54±0.5 | 0.95 |
| 2nd day | 1.88±2.2 | 1.32±0.2 | 1.61±1.7 | 0.16 |
| 7th day | 1.26±0.2 | 1.27±0.2 | 1.26±0.2 | 0.60 |
| Fibrinogen (mg/dL) | ||||
| On day | 374.7±243.9 | 410.0±176.7 | 389.6±217.5 | 0.48 |
| 1st day | 415.4±275.4 | 477.9±250.3 | 441.7±265.2 | 0.31 |
| 2nd day | 429.7±225.5 | 409.3±231.1 | 421.1±226.6 | 0.70 |
| 7th day | 393.9±201.2 | 375.2±204.9 | 386.1±201.6 | 0.69 |
| Na (mmol/L) | ||||
| On day | 139.0±7.0 | 138.6±6.0 | 138.9±6.6 | 0.79 |
| 1st day | 3.6±0.6 | 3.9±1.0 | 3.7±0.8 | 0.12 |
| 2nd day | 140.6±8.1 | 141.5±7.3 | 141.0±7.7 | 0.62 |
| 7th day | 139.5±8.8 | 138.8±8.5 | 139.2±8.6 | 0.75 |
| K (mmol/L) | ||||
| On day | 3.7±0.5 | 3.9±0.8 | 3.8±0.7 | 0.37 |
| 1st day | 3.6±0.6 | 3.9±1.0 | 3.7±0.8 | 0.12 |
| 2nd day | 3.7±0.6 | 3.9±0.9 | 3.8±0.8 | 0.34 |
| 7th day | 4.1±1.0 | 3.9±0.6 | 4.0±0.8 | 0.41 |
| Cl (mmol/L) | ||||
| On day | 103.9±7.8 | 96.6±25.4 | 100.9±17.7 | 0.12 |
| 1st day | 103.9±8.0 | 103.7±5.5 | 103.8±7.0 | 0.87 |
| 2nd day | 102.8±8.8 | 105.0±7.5 | 103.7±8.3 | 0.24 |
| 7th day | 104.3±8.5 | 101.5±19.8 | 103.2±14.3 | 0.45 |
| pH (ABGA) | ||||
| On day | 7.34±0.2 | 7.45±0.2 | 7.42±0.2 | 0.26 |
| 1st day | 7.31±0.2 | 7.42±0.1 | 7.40±0.2 | 0.54 |
| 2nd day | 7.31±0.2 | 7.44±0.2 | 7.38±0.2 | 0.16 |
| 7th day | 7.30±0.2 | 7.40±0.1 | 7.40±0.2 | 0.29 |
| pO2 (ABGA) (mmHg) | ||||
| On day | 108.3±61.4 | 108.9±70.4 | 108.5±64.9 | 0.96 |
| 1st day | 94.5±49.6 | 102.2±51.2 | 97.8±50.1 | 0.51 |
| 2nd day | 110.8±42.5 | 102.5±38.3 | 107.3±40.8 | 0.38 |
| 7th day | 100.1±41.4 | 97.2±31.4 | 98.9±37.3 | 0.73 |
| pCO2 (ABGA) (mmHg) | ||||
| On day | 47.0±16.9 | 42.1±13.4 | 44.9±15.6 | 0.17 |
| 1st day | 48.1±18.1 | 45.7±16.1 | 47.1±17.2 | 0.55 |
| 2nd day | 40.7±11.7 | 43.1±13.2 | 41.7±12.3 | 0.41 |
| 7th day | 42.9±10.5 | 39.5±9.6 | 41.5±10.2 | 0.15 |
| cHCO3 (ABGA) (mmol/L) | ||||
| On day | 23.9±6.5 | 24.8±6.9 | 24.3±6.6 | 0.56 |
| 1st day | 25.1±8.0 | 25.4±7.0 | 25.3±7.5 | 0.86 |
| 2nd day | 25.7±8.1 | 26.1±7.5 | 25.9±7.8 | 0.83 |
| 7th day | 25.1±5.8 | 25.9±5.7 | 25.5±5.7 | 0.55 |
| Base excess (ABGA) (mmol/L) | ||||
| On day | −0.7±7.0 | 0.4±7.5 | −0.2±7.2 | 0.50 |
| 1st day | −0.7±8.7 | 0.6±7.3 | −0.2±8.1 | 0.49 |
| 2nd day | 1.4±8.2 | 1.9±7.3 | 1.6±7.8 | 0.74 |
| 7th day | 0.0±6.2 | 1.5±5.3 | 0.6±5.8 | 0.27 |
| SpO2 (ABGA) (%) | ||||
| On day | 93.1±9.8 | 93.9±8.7 | 93.4±9.3 | 0.70 |
| 1st day | 92.9±7.5 | 93.5±10.7 | 93.1±8.9 | 0.79 |
| 2nd day | 93.7±9.6 | 95.2±6.7 | 94.3±8.5 | 0.39 |
| 7th day | 92.6±.1 | 94.2±5.0 | 93.3±7.0 | 0.31 |
| Glucose (mg/dL) | ||||
| On day | 132.5±46.1 | 145.4±43.7 | 137.9±45.3 | 0.22 |
| 1st day | 158.1±63.9 | 156.8±63.9 | 157.5±63.5 | 0.93 |
| 2nd day | 149.9±44.8 | 158.6±56.2 | 153.6±49.7 | 0.45 |
| 7th day | 153.9±65.7 | 172.3±81.7 | 161.7±72.9 | 0.28 |
| iCa (mg/dL) | ||||
| On day | 1.9±1.5 | 1.6±1.3 | 1.8±1.4 | 0.32 |
| 1st day | 2.1±1.4 | 2.2±1.6 | 2.1±1.5 | 0.68 |
| 2nd day | 2.2±1.3 | 2.1±1.5 | 2.2±1.4 | 0.83 |
| 7th day | 2.8±1.6 | 2.5±1.6 | 2.7±1.6 | 0.45 |
| Lactate (mmol/L) | ||||
| On day | 2.4±3.1 | 2.4±3.0 | 2.4±3.1 | 0.97 |
| 1st day | 3.0±.9 | 2.4±2.7 | 2.7±3.4 | 0.37 |
| 2nd day | 2.0±2.2 | 2.0±1.4 | 2.0±1.9 | 0.95 |
| 7th day | 1.6±1.1 | 1.8±1.4 | 1.7±1.3 | 0.45 |
| WBC (×103/μL) | ||||
| On day | 13.9±7.8 | 17.4±32.2 | 15.4±21.6 | 0.55 |
| 1st day | 12.2±7.0 | 12.8±6.1 | 12.5 ±6.6 | 0.70 |
| 2nd day | 13.1±7.8 | 18.2±28.5 | 15.3±19.4 | 0.32 |
| 7th day | 15.8±8.8 | 16.0±8.4 | 15.9±8.5 | 0.92 |
| RBC (×106/μL) | ||||
| On day | 3.2±0.8 | 3.3±0.6 | 3.3±0.7 | 0.48 |
| 1st day | 3.3±0.6 | 3.4±0.7 | 3.3±0.6 | 0.39 |
| 2nd day | 3.1±0.5 | 3.3±0.7 | 3.2±0.6 | 0.20 |
| 7th day | 3.1±0.7 | 3.4±0.6 | 3.3±0.6 | 0.07 |
| Hemoglobin (g/dL) | ||||
| On day | 9.9±2.4 | 10.6±1.9 | 10.2±2.2 | 0.14 |
| 1st day | 10.2±1.8 | 0.8±2.2 | 10.4±2.0 | 0.20 |
| 2nd day | 9.9±1.9 | 11.0±2.0 | 10.3±2.0 | 0.01* |
| 7th day | 9.4±1.8 | 10.5±1.6 | 9.9±1.8 | 0.006* |
| Hematocrit (%) | ||||
| On day | 29.3±6.9 | 31.1±5.6 | 30.1±6.4 | 0.23 |
| 1st day | 29.8±4.7 | 31.5±6.6 | 30.5±5.6 | 0.23 |
| 2nd day | 29.5±5.4 | 32.2±6.2 | 30.6±5.9 | 0.04 |
| 7th day | 29.6±5.7 | 32.0±5.3 | 30.6±5.6 | 0.06 |
| Platelet (×103/μL) | ||||
| On day | 125.2±87.4 | 123.8±80.5 | 124.6±84.0 | 0.94 |
| 1st day | 125.1±104.3 | 121.0±98.0 | 123.4±101.1 | 0.86 |
| 2nd day | 120.2±85.5 | 122.7±78.6 | 121.2±82.1 | 0.89 |
| 7th day | 149.1±105.6 | 184.1±130.9 | 163.8±117.4 | 0.20 |
| Neutrophil (%) | ||||
| On day | 84.9±14.6 | 85.7±11.1 | 85.2±13.2 | 0.79 |
| 1st day | 85.1±14.8 | 87.8±7.8 | 86.3±12.4 | 0.30 |
| 2nd day | 84.3±16.4 | 87.8±5.9 | 85.8±13.1 | 0.19 |
| 7th day | 81.9±17.5 | 83.4±.9 | 82.5±14.7 | 0.64 |
| Lymphocyte (%) | ||||
| On day | 8.9±13.3 | 7.5±8.2 | 8.4±11.4 | 0.57 |
| 1st day | 9.5±15.2 | 6.8±6.8 | 8.4±12.4 | 0.29 |
| 2nd day | 9.3±13.9 | 5.4±3.1 | 7.7±10.9 | 0.08 |
| 7th day | 10.4±14.5 | 9.0±5.6 | 9.8±11.6 | 0.53 |
| Monocyte (%) | ||||
| On day | 5.6±3.5 | 5.8±3.9 | 5.7±3.6 | 0.76 |
| 1st day | 4.9±3.1 | 4.7±2.9 | 4.8±3.0 | 0.75 |
| 2nd day | 5.0±3.8 | 5.4±4.0 | 5.2±3.9 | 0.70 |
| 7th day | 5.1 ±4.1 | 5.3±3.1 | 5.2±3.7 | 0.86 |
| Eosinophil (%) | ||||
| On day | 0.4±1.0 | 0.6±1.6 | 0.5±1.3 | 0.56 |
| 1st day | 0.5±1.1 | 0.4±0.8 | 0.4±.0 | 0.78 |
| 2nd day | 1.1±2.3 | 0.7 ±1.1 | 0.9±1.9 | 0.35 |
| 7th day | 1.4±2.4 | 2.2±4.1 | 1.7±3.2 | 0.32 |
| Basophil (%) | ||||
| On day | 0.2±0.2 | 0.3±0.5 | 0.2±0.4 | 0.13 |
| 1st day | 0.3±0.6 | 0.3±0.4 | 0.3±0.5 | 0.66 |
| 2nd day | 0.2±0.2 | 0.3±0.4 | 0.3±0.3 | 0.32 |
| 7th day | 0.5±1.0 | 0.4±0.3 | 0.4±0.8 | 0.41 |
| AST (GOT) (IU/L) | ||||
| On day | 162.4±471.4 | 543.9±1,401.5 | 323.0±987.5 | 0.14 |
| 1st day | 333.5±944.4 | 686.0±1,873.2 | 481.9±1,411.5 | 0.33 |
| 2nd day | 214.8±757.7 | 202.0±502.3 | 209.4±658.4 | 0.93 |
| 7th day | 77.4±76.8 | 68.6±71.3 | 73.7±74.1 | 0.61 |
| ALT (GPT) (IU/L) | ||||
| On day | 96.4±257.9 | 239.6±591.8 | 156.7±433.5 | 0.20 |
| 1st day | 195.9±652.8 | 299.4±772.4 | 239.5±702.6 | 0.53 |
| 2nd day | 76.9±123.3 | 154.8±373.8 | 109.7±260.7 | 0.26 |
| 7th day | 71.5±77.5 | 57.1±61.0 | 65.4±71.0 | 0.38 |
| Total bilirubin (mg/dL) | ||||
| On day | 2.3±2.9 | 1.8±1.0 | 2.1±2.3 | 0.31 |
| 1st day | 2.4±2.7 | 2.0±1.2 | 2.2±2.2 | 0.41 |
| 2nd day | 2.6±2.8 | 2.5±1.6 | 2.6±2.4 | 0.84 |
| 7th day | 4.8±5.8 | 3.8±3.4 | 4.4±4.9 | 0.33 |
| Direct bilirubin (mg/dL) | ||||
| On day | 0.8±1.5 | 0.6±0.3 | 0.7±1.2 | 0.27 |
| 1st day | 1.3±2.1 | 1.0±0.3 | 1.2±1.6 | 0.25 |
| 2nd day | 0.9±1.6 | 0.6±0.4 | 0.8±1.3 | 0.33 |
| 7th day | 2.3±2.7 | 2.1±1.4 | 2.2±2.3 | 0.65 |
| Total protein (g/dL) | ||||
| On day | 5.4±1.0 | 5.4±1.0 | 5.4±1.0 | 0.95 |
| 1st day | 5.7±0.9 | 5.8±0.9 | 5.7±0.9 | 0.51 |
| 2nd day | 5.6±0.9 | 5.9±1.0 | 5.7±1.0 | 0.09 |
| 7th day | 5.7±0.8 | 5.7±0.9 | 5.7±0.9 | 0.85 |
| Albumin (g/dL) | ||||
| On day | 3.0±0.7 | 3.2±0.7 | 3.1±0.7 | 0.19 |
| 1st day | 3.2±0.6 | 3.5±0.6 | 3.3±0.6 | 0.05 |
| 2nd day | 3.3±0.7 | 3.6±0.7 | 3.4±0.7 | 0.11 |
| 7th day | 3.3±0.7 | 3.4±0.8 | 3.4±0.7 | 0.76 |
| BUN (mg/dL) | ||||
| On day | 30.1±17.0 | 28.0±16.1 | 29.2±16.6 | 0.60 |
| 1st day | 32.5±20.7 | 30.1±16.8 | 31.5±19.1 | 0.60 |
| 2nd day | 38.1±22.0 | 32.3±16.4 | 35.6±19.9 | 0.21 |
| 7th day | 41.9±25.5 | 28.1±14.3 | 36.1±22.5 | 0.004* |
| Creatinine (mg/dL) | ||||
| On day | 1.1±0.6 | 1.1±0.6 | 1.1±0.6 | 0.69 |
| 1st day | 1.1±0.5 | 1.0±0.6 | 1.1±0.6 | 0.55 |
| 2nd day | 1.2±0.6 | 1.0±0.5 | 1.1±0.6 | 0.09 |
| 7th day | 1.3±0.8 | 0.9±0.7 | 1.2±0.8 | 0.01 |
| Phosphate (mg/dL) | ||||
| On day | 3.7±1.9 | 3.3±2.0 | 3.6±1.9 | 0.34 |
| 1st day | 3.4±2.0 | 3.1±2.8 | 3.3±2.3 | 0.59 |
| 2nd day | 2.4±1.2 | 1.8±1.2 | 2.1±1.2 | 0.05 |
| 7th day | 3.5±1.3 | 3.2±0.8 | 3.3±1.1 | 0.30 |
| Amylase (U/L) | ||||
| On day | 140.6±124.7 | 139.9±151.8 | 140.3±135.8 | 0.98 |
| 1st day | 242.2±488.7 | 183.1±380.8 | 217.3±444.7 | 0.57 |
| 2nd day | 139.2±217.1 | 131.1±198.0 | 135.8±207.9 | 0.86 |
| 7th day | 181.5±142.4 | 161.6±115.2 | 173.1±131.2 | 0.51 |
| CRP (mg/dL) | ||||
| On day | 10.9±7.9 | 10.7±6.8 | 10.8±7.4 | 0.90 |
| 1st day | 10.9±6.8 | 11.5±6.1 | 11.1±6.5 | 0.69 |
| 2nd day | 11.7±6.6 | 11.3±6.9 | 11.5±6.7 | 0.77 |
| 7th day | 10.7±7.3 | 7.8±5.9 | 9.5±6.9 | 0.06 |
| Procalcitonin (ng/mL) | ||||
| On day | 14.7±36.6 | 11.1±26.8 | 13.1±32.6 | 0.63 |
| 1st day | 12.7±27.3 | 6.1±10.6 | 9.9±21.9 | 0.15 |
| 2nd day | 10.8±20.2 | 12.5±36.1 | 11.5±27.7 | 0.81 |
| 7th day | 6.4±19.8 | 2.6±4.1 | 4.8±15.4 | 0.22 |
| Presepsin (pg/mL) | ||||
| On day | 927.3±604.8 | 802.2±158.2 | 874.6±473.2 | 0.19 |
| 1st day | 1,697.9±804.3 | 1,493.4±554.1 | 1,611.8±712.8 | 0.19 |
| 2nd day | 1,196.6±571.2 | 1,039.3±270.6 | 1,130.4±472.7 | 0.11 |
| 7th day | 1,694.9±1021.7 | 1,386.1±396.2 | 1,564.9±828.8 | 0.07 |
| Troponin-I (ng/mL) | ||||
| On day | 0.6±1.2 | 0.6±1.2 | 0.6±1.2 | 0.81 |
| 1st day | 1.4±4.2 | 0.7±0.9 | 1.1±3.2 | 0.31 |
| 2nd day | 0.7±1.9 | 0.4±0.4 | 0.6±1.4 | 0.34 |
| 7th day | 0.5±0.5 | 0.9±2.3 | 0.7±1.5 | 0.32 |
| Myoglobin (ng/mL) | ||||
| On day | 856.9±1,472.9 | 1,770.4±2,934.7 | 1,241.6±2,238.2 | 0.11 |
| 1st day | 1,452.9±4,309.6 | 1,340.7±2,866.2 | 1,405.7±3,747.9 | 0.89 |
| 2nd day | 796.0±15,22.8 | 688.5±703.0 | 750.7±1,239.6 | 0.68 |
| 7th day | 914.1±726.0 | 1,183.7±2,098.1 | 1,027.6±1,462.7 | 0.49 |
| CK-MB (ng/mL) | ||||
| On day | 9.0±16.2 | 8.8±12.9 | 8.9±14.8 | 0.95 |
| 1st day | 14.9±43.3 | 7.1±8.0 | 11.6±33.4 | 0.24 |
| 2nd day | 5.6±10.6 | 4.0±2.3 | 4.9±8.2 | 0.34 |
| 7th day | 2.8±3.1 | 7.7±30.1 | 4.8±19.7 | 0.36 |
Values are expressed as n (%) or means ± standard deviation. *, P<0.05. DIC, disseminated intravascular coagulation; COPD, chronic obstructive pulmonary disease; IU, international unit; SOFA, sequential organ failure assessment; SAPS II, simplified acute physiology score II; FDP, fibrinogen degradation products; PT, prothrombin time; aPTT, activated partial thromboplastin time; INR, international normalized ratio; ABGA, artery blood gas analysis; SpO2, O2 saturation; iCa, ionized calcium; RBC, red blood cell; WBC, white blood cell; AST (GOT), aspartate aminotransferase (glutamic oxaloacetic transaminase); ALT (GPT), alanine aminotransferase (glutamic pyruvic transaminase); BUN, blood urea nitrogen; CRP, C-reactive protein; CK-MB, creatine kinase MB isoenzyme.
Table 2
| Day of antithrombin III administration | Mean | SE | 95% CI |
|---|---|---|---|
| On day antithrombin III (%) | 53.21 | 1.55 | 50.11 to 56.32 |
| 1st day antithrombin III (%) | 89.65 | 3.33 | 83.01 to 96.30 |
| 2nd day antithrombin III (%) | 109.18 | 2.60 | 103.99 to 114.37 |
| 7th day antithrombin III (%) | 66.32 | 2.25 | 61.82 to 70.83 |
ANOVA, analysis of variance; SE, standard error; CI, confidence interval.
Table 3
| Statistical factors | Source of variation | Sum of squares | DF | Mean square | F | P |
|---|---|---|---|---|---|---|
| Test of between-subjects effects | Non-survival vs. survival | 3,753.380 | 1 | 3,753.380 | 5.26 | 0.02 |
| Residual | 52,762.676 | 74 | 713.009 | |||
| Test of within-subjects effects | ||||||
| Factor | Sphericity assumed | 137,332.471 | 3 | 45,777.490 | 114.91 | <0.001 |
| Greenhouse-Geisser | 137,332.471 | 2.570 | 53,446.116 | 114.91 | <0.001 | |
| Huynh-Feldt | 137,332.471 | 2.670 | 51,436.204 | 114.91 | <0.001 | |
| Group × factor interaction | Sphericity assumed | 90.884 | 3 | 30.295 | 0.076 | 0.97 |
| Greenhouse-Geisser | 90.884 | 2.570 | 35.370 | 0.076 | 0.95 | |
| Huynh-Feldt | 90.884 | 2.670 | 34.039 | 0.076 | 0.96 | |
| Residual | Sphericity assumed | 88,437.323 | 222 | 398.366 | – | – |
| Greenhouse-Geisser | 88,437.323 | 190.147 | 465.100 | – | – | |
| Huynh-Feldt | 88,437.323 | 197.577 | 447.610 | – | – |
ANOVA, analysis of variance; DF, degrees of freedom; F, F-distribution or F-ratio.
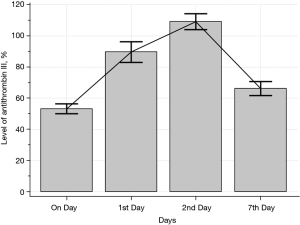
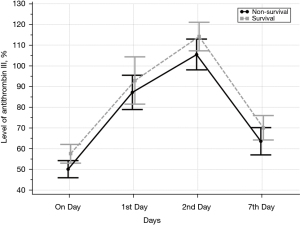
Table 4
| Variable | Coefficient | SE | Wald | OR | 95% CI | P |
|---|---|---|---|---|---|---|
| On day antithrombin III (%) | 0.043739 | 0.022 | 3.8980 | 1.0447 | 1.0003 to 1.0911 | 0.04 |
| 2nd day hemoglobin | 0.27496 | 0.141 | 3.7611 | 1.3165 | 0.9971 to 1.7382 | 0.05 |
| SAPS II | −0.065079 | 0.031 | 4.3940 | 0.9370 | 0.8817 to 0.9958 | 0.03 |
OR, odds ratio; SAPS II, simplified acute physiology score II; SE, standard error; CI, confidence interval.
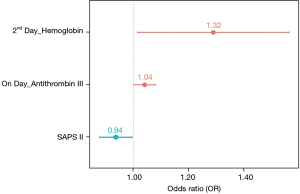
Table 5
| Antithrombin III (%) | AUC | SEa | 95% CIb | z statistic | P | Youden index J | Associated criterion | Sensitivity, % | Specificity, % |
|---|---|---|---|---|---|---|---|---|---|
| On day | 0.672 | 0.0638 | 0.555 to 0.776 | 2.698 | 0.007 | 0.3267 | >61.3 | 53.13 | 79.55 |
| 1st day | 0.552 | 0.0691 | 0.433 to 0.666 | 0.750 | 0.45 | 0.1648 | >99 | 43.75 | 72.73 |
| 2nd day | 0.624 | 0.0651 | 0.506 to 0.733 | 1.909 | 0.05 | 0.3494 | >104 | 78.12 | 56.82 |
| 7th day | 0.593 | 0.0660 | 0.474 to 0.704 | 1.409 | 0.15 | 0.2216 | >99 | 56.25 | 65.91 |
a, DeLong et al., 1988 (9); b, Binomial exact; AUC, area under the curve; DIC, disseminated intravascular coagulation; SE, standard error; CI, confidence interval.
Table 6
| Antithrombin III (%) | Difference between areas | Standard error | 95% CI | z statistic | P |
|---|---|---|---|---|---|
| On to 1st day | 0.12 | 0.0779 | −0.0323 to 0.273 | 1.546 | 0.12 |
| On to 2nd day | 0.0479 | 0.0876 | −0.124 to 0.220 | 0.547 | 0.58 |
| On to 7th day | 0.0792 | 0.0933 | −0.104 to 0.262 | 0.849 | 0.39 |
| 1st to 2nd day | 0.0724 | 0.0705 | −0.0657 to 0.211 | 1.028 | 0.30 |
| 1st to 7th day | 0.0412 | 0.0892 | −0.134 to 0.216 | 0.462 | 0.64 |
| On to 1st day | 0.0313 | 0.0986 | −0.162 to 0.225 | 0.317 | 0.75 |
ROC, receiver operating characteristic; CI, confidence interval.
Discussion
Antithrombin is a small plasma glycoprotein synthesized in the liver that belongs to the serpin family of serine protease inhibitors and inactivates several enzymes in the coagulation pathway. It has a 58,200-Dalton single-chain structure, which consists of 432 amino acids, contains three disulfide bonds and four possible glycosylation sites, and has a biological half-life of 55–70 hours. This molecule also contains four carbohydrate side chains that make up around 15% of the molecular mass. Antithrombin was discovered in 1905 by Morawitz, a German internist and physiologist who made great achievements in the study of blood coagulation and transfusion. The designations antithrombin-I through antithrombin-IV originated in early studies on antithrombin reactions to prothrombin activation carried out in the 1950s by Seegers et al. (10). Antithrombin-I promotes the absorption of thrombin onto fibrin after thrombin has activated fibrinogen. Antithrombin-II is a cofactor in plasma that together with heparin interferes with interactions between thrombin and fibrinogen. Antithrombin-III is a substance in plasma that inactivates thrombin, and antithrombin-IV is an antithrombin that becomes activated during and shortly after blood coagulation. Only antithrombin-III and possibly antithrombin-I are medically significant. For this reason, antithrombin-III is generally referred to solely as antithrombin. Antithrombin shows several noteworthy characteristics in normal plasma at a concentration of about 150 mg/L. Alpha (α)-antithrombin, which is the predominant form of antithrombin found in blood plasma, has an oligosaccharide moiety occupying each of its four glycosylation sites. A single glycosylation site is consistently unoccupied in beta (β)-antithrombin, the minor form of antithrombin. Antithrombin inactivates several enzymes of the coagulation cascade, in particular factor IIa (thrombin) and factor Xa (activated Stuart-Prower factor). The activity of antithrombin is increased by many orders of magnitude by anticoagulant drugs such as heparin, which enhances the binding of antithrombin to factor IIa and factor Xa (11).
This functions as an important regulator of blood coagulation by serving as the major inhibitor of thrombin, factor IXa, and factor Xa in plasma, although it also inactivates other serine proteases in the intrinsic coagulation pathway, such as factors XIa and XIIa, as well as some non-coagulation serine proteases, such as plasmin, kallikrein, and the complement enzyme C1. Since these proteases are all inactivated much more slowly than thrombin, thrombin is the most important enzyme in the blood coagulation cascade. It clots blood by converting factor I (fibrinogen) into clot-forming fibrin monomers and activates factor XIII (fibrin-stabilizing factor), thereby strengthening the blood clot by strong cross-linking. Thrombin also activates platelets and cofactors—factor V (labile factor or proaccelerin), and factor VIII (antihemophilic factor)—to accelerate its own generation and provide a quick response to injury. Thrombin generation is fully completed by clotting the blood content within minutes in adults; therefore, thrombin activity must be closely controlled to prevent abnormal fibrin deposition in the vasculature. In this respect, the inhibitory regulation of thrombin is of paramount importance, and it is primarily achieved by two principally different mechanisms. The first mechanism of the inhibitory regulation of thrombin activity is that when the thrombin binds to the membrane protein thrombomodulin, which is mainly present on the surface of intact vascular endothelium, it loses all of its procoagulant properties. In response, thrombomodulin dramatically accelerates the rate of activation of protein C, and activated protein C finally degrades factors Va and VIIIa, effectively impeding further thrombin generation. The other regulatory mechanism of thrombin activity during blood clotting is provided by a group of circulating enzyme inhibitors, including antithrombin. Within this group, antithrombin is the major inhibitor, accounting for approximately more than 80% of the thrombin inhibitory activity in plasma. Antithrombin inhibits thrombin activity through the formation of a stable 1:1 complex between the active domain of the serine protease and the reactive site of antithrombin, which proteases initially recognize as a substrate. When the bond is cleaved at the reactive site in antithrombin, a conformational change occurs in the inhibitor that traps the protease. Although protease-antithrombin interactions are slow, they are dramatically enhanced in the presence of glycosaminoglycans (GAGs), a category of sulfated polysaccharides. It is believed that vascular GAGs, the best-known of which is heparan sulfate, bind both antithrombin and thrombin and thereby catalyze the antithrombin-thrombin reaction. This permits the selective enhancement of antithrombin actions at blood-cell interfaces, where coagulation enzymes are generated. Commercial heparin, which is an important antithrombotic drug, is a mixture of GAGs extracted mainly from bovine or porcine intestinal mucosa. Both heparin and heparan sulfate catalyze the actions of antithrombin by inducing a conformational change in the antithrombin molecule at its reactive site. Thrombin binds to heparin in a non-specific manner and slides along the chain until it encounters the bound antithrombin. Heparin has a much lower affinity to the thrombin-antithrombin complex than to free antithrombin (12).
Despite the theoretical benefits of antithrombin supplementation, due to its role as an important physiological anticoagulant that affects nearly all of the intrinsic, extrinsic, and common coagulation pathways, as well as exerting anti-inflammatory effects, current clinical trial results do not fully support the common use of antithrombin in patients with DIC. The established recommendations are based on data from the Phase III KyberSept clinical trial, which demonstrated that high-dose antithrombin (30,000 IU in total over 4 days) therapy had no effect on 28-day all-cause mortality in adult patients and was associated with an increased risk of hemorrhage when administered with heparin (13). However, a meta-analysis of randomized controlled trials on the efficacy and safety of antithrombin therapy in three specific patient groups with sepsis showed beneficial effects on mortality (risk ratio 0.72; 95% CI: 0.62–0.85) in the patients with sepsis-induced DIC (14). The Food and Drug Administration approved additional indications for antithrombin, and antithrombin has been suggested for the treatment of patients with DIC associated with trauma or sepsis. However, the 2009 British guidelines for the diagnosis and management of DIC did not recommend antithrombin in patients with DIC without further prospective evidence in randomized controlled trials (15). A 2016 Cochrane review of antithrombin administration in critically ill patients likewise concluded that there is insufficient evidence to support its use in any category of critically ill patients, including those with sepsis and DIC (16). In 1989, Hayakawa et al. (17) reported that despite antithrombin supplementation therapy, in-hospital mortality was significantly reduced only in patients with very low antithrombin activity (≤43%; adjusted HR, 0.603; 95% CI: 0.368–0.988; P=0.04) and concluded that antithrombin supplementation therapy in patients with sepsis-induced DIC and very low antithrombin activity might improve survival without increasing the risk of bleeding. However, a recent systematic review showed that antithrombin supplementation may be associated with reduced in-hospital all-cause mortality in patients with sepsis-induced DIC (18). These ambiguities and limitations regarding the efficacy of antithrombin supplementation have resulted in inconsistencies in clinical practice.
As discussed above, there is no clear consensus regarding antithrombin administration in the cases of antithrombin deficiency by DIC. Furthermore, since antithrombin supplementation involves the investment of costly and valuable medical resources, it must be performed only in extremely restricted clinical situations. In such situations, antithrombin supplementation must be fully individualized—especially concerning the loading dose, maintenance dose, and dosing intervals—based on the confirmed diagnosis, clinical condition, patient’s weight, amount of deficiency, physician’s judgment, desired level of antithrombin activity, and actual plasma levels achieved as verified by appropriate laboratory tests. Previous research has reported that 1 U/kg of antithrombin supplementation might raise the level of antithrombin by 1.4%. The desired antithrombin level after the first dose supplement should be about 120% of normal (normal level range is 0.1 to 0.2 g/L), and antithrombin levels must be maintained at normal or at least above 80% of normal for 2 to 8 days depending on individual patient factors. Although different views exist regarding the maintenance dose, an antithrombin maintenance dose is usually recommended once a day, and concomitant administration of heparin is also usually indicated. In adults, antithrombin loading and maintenance doses are calculated using body weight, baseline antithrombin activity, and a targeted antithrombin activity of 80% to 120% (19). The supplementation of antithrombin in the initial loading dose can be calculated using the following formula (assuming a plasma volume of 40 mL/kg): dosage units = [desired antithrombin level (%) – baseline antithrombin level (%)] × body weight (kg)/1.4 (%). A maintenance dose of approximately 60% of the loading dose every 24 hours is the average amount required to maintain plasma levels between 80% and 120%. Plasma levels should be measured pre-infusion, 20 minutes’ post-infusion (peak), 12 hours’ post-infusion, and preceding the next infusion (trough). In 1989, Schwartz et al. reported that antithrombin supplementation in asymptomatic adults with hereditary antithrombin deficiency increased antithrombin activity by 1.4% per U/kg of antithrombin concentration, with a 50% decrease in activity at 22 hours after administration (20).
My country (South Korea) has a universal health care system largely financed by the government-operated National Health Insurance Service (NHIS); therefore, NHIS guidelines should be applied to all domains of medical practice. The Ministry of Food and Drug Safety (MFDS) issued an approval for antithrombin medication with very strict guidelines, as follows: platelet count <100,000/µL; prothrombin time >3 seconds to normal range (11–12.5 seconds) or activated partial thromboplastin time >5 seconds to normal range (30–40 seconds); fibrin degradation product level >10 µL/mL or D-dimer level >320 mg/dL; fibrinogen <150 mg/dL. In addition, they robustly request clear objective evidence on the role of antithrombin deficiency in DIC, which was defined in 2016 by the Korean Society of Thrombosis and Hemostasis as follows: antithrombin examination numerical values, latex agglutination immunoassay or enzyme-linked immunosorbent assay, antithrombin levels below 20 mg/dL in adults or below 18 mg/dL in neonates; and antithrombin activity below 70% of normal in adults or below 60% of normal in neonates. The total administration period of antithrombin must be carefully limited to within 2 or 3 days in most cases, with a maximum of 5 days, and the total administration dose must be below 7,000 IU (loading dose, 1,000 IU in 1 hour: maintenance dose, 500 IU every 6 hours for 3 days) following the MFDS guidelines (8). However, these standardized recommendations for antithrombin supplement may be not enough for critical ill patients suffering DIC, and this finding is prominently observed in our study. For the treatment of DIC, most of medications are available in my country, South Korea, including antithrombin III concentrate, activated protein C and synthesized protease inhibitors, such as gabexate mesilate and nafamostat mesilate, etc. However, recombinant human soluble thrombomodulin, that binds thrombin which serves to augment the conversion of protein C to activated protein C and inhibits inflammation and organ injury caused by damage-associated molecular patterns, is not yet available in South Korea (21,22).
This study has several limitations. Firstly, this study was conducted at a single institution and single investigator, which limited the generalizability of the study results, so multicenter study must be essential. Secondly, as an observational study in a single institution, there might be potential residual confounding. Thirdly, our study population was too small to draw statistical significance and the cohort was heterogeneous showing a narrow variety of cause for DIC. Fourthly, since our study focused only on DIC for the indication of an antithrombin supplementation, it is difficult to generalize our results to other indications for antithrombin, such as in patients with hereditary antithrombin deficiency for treatment and prevention of thromboembolism and prevention of peri-operative and peri-partum thromboembolism. Fifthly, since this study concentrated only on universal adult patients with DIC, it is difficult to generalize our results to other age groups, such as neonate, children, adolescent, advanced age. Therefore, multicenter, multinational, randomized, controlled trials and prospective cohort study are needed to evaluate whether antithrombin supplementation predict more positive clinical outcomes in patients with DIC for the proper management of these patients.
Conclusions
Our study suggests that the antithrombin administration might be effective treatment tools for DIC control, and may be more positively considered, especially in the cases of DIC. Up to date, there might be no clear consensus regarding antithrombin administration in the cases of antithrombin deficiency, moreover since antithrombin supplementation involves the investment of costly and valuable medical resources, it must be carefully performed only in extremely restricted clinical situations. Our study suggests that the antithrombin administration may be more positively considered, especially in the cases of DIC, which is a frequent complication of septic shock, sepsis, and other critical disease entities and which is associated with a high level of mortality. Furthermore, our study also suggests that the total doses and periods of antithrombin administration, which recommended by health care system guidelines, mainly operated by the government-operated National Health Insurance Service, may be insufficient, therefore further consideration for prolongation of period and increase of total dose of antithrombin supplement might be necessary. In accordance with clinical situation, disease entity, age, serum antithrombin level and others, the precise calculation formula, which are reflecting the initial loading dose, maintenance dose and administration period, may be essential.
Acknowledgments
Funding: This work was supported by
Footnote
Reporting Checklist: The authors have completed the TREND Reporting Checklist. Available at https://apm.amegroups.com/article/view/10.21037/apm-23-535/rc
Data Sharing Statement: Available at https://apm.amegroups.com/article/view/10.21037/apm-23-535/dss
Peer Review File: Available at https://apm.amegroups.com/article/view/10.21037/apm-23-535/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://apm.amegroups.com/article/view/10.21037/apm-23-535/coif). All authors report that this work was supported by SK Plasma. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of Konkuk University Chungju Hospital (No. KUCH 2022-05-003) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Iba T, Thachil J. Present and future of anticoagulant therapy using antithrombin and thrombomodulin for sepsis-associated disseminated intravascular coagulation: a perspective from Japan. Int J Hematol 2016;103:253-61. [Crossref] [PubMed]
- Tagami T, Matsui H, Horiguchi H, et al. Antithrombin and mortality in severe pneumonia patients with sepsis-associated disseminated intravascular coagulation: an observational nationwide study. J Thromb Haemost 2014;12:1470-9. [Crossref] [PubMed]
- Yamakawa K, Umemura Y, Hayakawa M, et al. Benefit profile of anticoagulant therapy in sepsis: a nationwide multicentre registry in Japan. Crit Care 2016;20:229. [Crossref] [PubMed]
- Kim YJ, Ko BS, Park SY, et al. Effect of High-dose Antithrombin Supplementation in Patients with Septic Shock and Disseminated Intravascular Coagulation. Sci Rep 2019;9:16626. [Crossref] [PubMed]
- Akahoshi T, Kaku N, Shono Y, et al. Impact of Antithrombin Activity Levels Following Recombinant Antithrombin Gamma Therapy in Patients with Sepsis-Induced Disseminated Intravascular Coagulation. Clin Appl Thromb Hemost 2022;28:10760296221135790. [Crossref] [PubMed]
- Li T, Bo F, Meng X, et al. The effect of perioperative antithrombin supplementation on blood conservation and postoperative complications after cardiopulmonary bypass surgery: A systematic review, meta-analysis and trial sequential analysis. Heliyon 2023;9:e22266. [Crossref] [PubMed]
- Lechner K, Kyrle PA. Antithrombin III concentrates—are they clinically useful? Thromb Haemost 1995;73:340-8. [Crossref] [PubMed]
- Ha SO, Park SH, Hong SB, et al. Performance Evaluation of Five Different Disseminated Intravascular Coagulation (DIC) Diagnostic Criteria for Predicting Mortality in Patients with Complicated Sepsis. J Korean Med Sci 2016;31:1838-45. [Crossref] [PubMed]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837-45. [Crossref] [PubMed]
- Seegers WH, Johnson JF, Fell C. An antithrombin reaction to prothrombin activation. Am J Physiol 1954;176:97-103. [Crossref] [PubMed]
- Finley A, Greenberg C. Review article: heparin sensitivity and resistance: management during cardiopulmonary bypass. Anesth Analg 2013;116:1210-22. [Crossref] [PubMed]
- Monagle P, Chan AKC, Goldenberg NA, et al. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e737S-801S.
- Warren BL, Eid A, Singer P, et al. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA. 2001;286:1869-78. Correction appears in JAMA 2002;287:192.
- Umemura Y, Yamakawa K, Ogura H, et al. Efficacy and safety of anticoagulant therapy in three specific populations with sepsis: a meta-analysis of randomized controlled trials. J Thromb Haemost 2016;14:518-30. [Crossref] [PubMed]
- Levi M, Toh CH, Thachil J, et al. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol 2009;145:24-33. [Crossref] [PubMed]
- Allingstrup M, Wetterslev J, Ravn FB, et al. Antithrombin III for critically ill patients. Cochrane Database Syst Rev 2016;2:CD005370. [PubMed]
- Hayakawa M, Yamakawa K, Kudo D, et al. Optimal Antithrombin Activity Threshold for Initiating Antithrombin Supplementation in Patients With Sepsis-Induced Disseminated Intravascular Coagulation: A Multicenter Retrospective Observational Study. Clin Appl Thromb Hemost 2018;24:874-83. [Crossref] [PubMed]
- Hayakawa M, Kudo D, Saito S, et al. Antithrombin Supplementation and Mortality in Sepsis-Induced Disseminated Intravascular Coagulation: A Multicenter Retrospective Observational Study. Shock 2016;46:623-31. [Crossref] [PubMed]
- Fourrier F, Chopin C, Huart JJ, et al. Double-blind, placebo-controlled trial of antithrombin III concentrates in septic shock with disseminated intravascular coagulation. Chest 1993;104:882-8. [Crossref] [PubMed]
- Schwartz RS, Bauer KA, Rosenberg RD, et al. Clinical experience with antithrombin III concentrate in treatment of congenital and acquired deficiency of antithrombin: The Antithrombin III Study Group. Am J Med 1989;87:53S-60S. [Crossref] [PubMed]
- Iba T, Asakura H. Comparison between British and Japanese guidelines for the diagnosis and treatment of disseminated intravascular coagulation. Br J Haematol 2010;149:461-2. [Crossref] [PubMed]
- Ushio N, Wada T, Ono Y, et al. Sepsis-induced disseminated intravascular coagulation: an international estrangement of disease concept. Acute Med Surg 2023;10:e00843. [Crossref] [PubMed]


