Efficacy of single fraction conventional radiation therapy for painful uncomplicated bone metastases: a systematic review and meta-analysis
Introduction
Bone is a common metastatic site accounting for cancer-related pain (1,2). Radiation therapy (RT) is a well-accepted treatment for painful uncomplicated bone metastases (3). Many studies have documented the effect of single fraction (SF) and multiple fraction (MF) regiments, with the majority of them concluding that the SFRT was equally as effective as MFRT for pain relief (4-20). These findings have been reflected in the guidelines from Choosing Wisely Canada and United States, the national Choosing Wisely campaign and the American Society for Therapeutic Radiology and Oncology—they all recommend SFRT for uncomplicated bone metastases (21-23). A recent study by Conway et al demonstrated that SFRT yields similar improvement to MFRT in patient-reported outcomes for pain, function and symptom frustration in both the complicated and uncomplicated setting of bone metastases (1).
The optimal conventional external beam SFRT dose for maximum pain relief remains unknown. In trials that directly compared 8 and 4 Gy, the larger-dose arm produced statistically superior pain responses (24). Across all trials included, trial doses of 8 Gy or more consistently produced superior response rates when compared to doses less than 8 Gy. Taking into account that 8 Gy has been by far the most commonly administered dose; the final recommendation from a past review was the adoption of 8 Gy as the standard dose to be compared against in future studies due to its reproducible pain response rates (24).
The past review included studies up until September 2012 (24). Since then, several papers have been published documenting the outcome of SFRT (25,26). The aim of this systematic review was to include recently-published papers that detailed SFRT outcomes and to conduct a meta-analysis to portray pain response rates by dose.
Methods
Search strategy
A literature search was conducted in Ovid MEDLINE(R) (1946 to June 2016 week 3), Embase Classic & Embase (1947 to 2016 week 26) and Cochrane Central Register of Controlled Trials (May 2016). Keywords and subject headings such as “bone metastasis”, “radiotherapy” and “single fraction” were employed. The search was limited to English-language papers and excluded reviews and re-irradiation studies (Figure 1). Titles and abstracts of search results were screened to determine eligibility for full-text article review.
Eligibility for full-text articles review
References were included if they reported outcomes of conventional external beam radiotherapy in a population where SFRT was administered for the first time, in either a prospective or retrospective setting. Articles not clearly identifying patient populations, study designs or dose fractions were conservatively included for review. Studies were excluded if they were duplicates, combined radiotherapy with other concurrent local or systematic treatments, or employed hemi-body-, radiopharmaceutical- or stereotactic radiation therapy.
Articles selected for synthesis
Full-text articles were included in this review if they reported pain response. Reference lists of articles were also reviewed, and full-text articles of relevant papers were obtained and similarly analyzed. Discrepancies for final selections were resolved by authors via consensus.
Data abstraction
The primary endpoints were pain response. When possible, reported pain response was categorized into partial, complete and overall pain response as reported in each study. Pain response assessments closest to 1–2 months following SFRT were recorded, as this is a common time to evaluate response and also a clinically important time frame for assessment of re-treatment (24,27,28).
Partial response (PR) rates were recorded as defined by authors in their studies, and complete response (CR) was generally defined as absence of pain following SFRT; defined criteria for CR and PR, were noted when reported. Overall response (OR) was defined as an improvement in pain after radiotherapy, and usually a summation of PR and CR. When studies did not separately document PR and CR, the response rate was documented as OR. PR, CR and OR were both documented under the analyses of both Intention-To-Treat (ITT) and Evaluable Patients (EP). Response rates when documented using percentages were converted to ratios; when multiple ratios yielded the same percentage, the number with lower patient response was noted. When conflicting number of EP were presented (8), the larger-value of EP was taken into account. Under circumstances where EP was not documented, ITT was recorded as EP.
The secondary endpoints were the rates of re-treatment, spinal cord compression, pathological fracture and acute toxicities such as pain flare, nausea, vomiting and diarrhea. Average duration of pain flare was recorded. Additional information extracted from articles included the type of study, key eligibility criteria, dose, pain assessment tool, and time to pain response.
Results
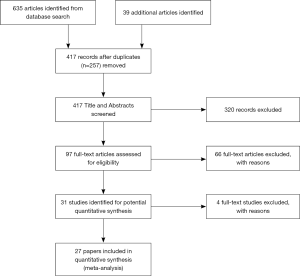
A total of 635 articles were identified from the database search, and with an additional 39 articles included from reference lists, 674 papers were reduced to 417 records after duplicates (n=257) were removed. Ninety two full-text articles were assessed for eligibility, with 31 identified for potential quantitative synthesis (Figure 2). Ultimately 27 studies that reported the appropriate endpoints were included in this review (Figure 2). Twenty-three (4-9,16,25,29-43) and 3 (25,44,45) studies reported about pain response and pain flare, respectively, while one paper (46) documented both. When compared with the last review (24), four published before 2012 (35,39,41,43) and five additional papers published after 2012 (16,25,32,37,40) have been included in the current review. Studies included in the prior review that was written in languages other than English were not included, to be consistent with the search strategy with language-limitation.
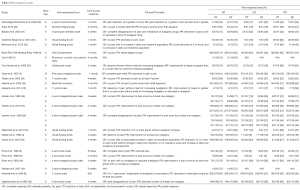
There were four studies reporting on 4 Gy from 1988–2015, 3 studies on 6 Gy from 1995–2002, 23 studies on 8 Gy from 1986–2015 and 1 study on 10 Gy published in 1997. Of the 24 studies that documented pain response, 1 (16) was a retrospective study, 1 (39) was an observational study, and the remaining 22 (4-9,25,29-38,40-43,46) were prospective studies. Only three studies (36-38) compared head-to-head different SFRT doses, while other studies reviewed SFRT vs. MFRT (4-9,16,25,29-34,38,41,46) or just SFRT alone (34,39,40,42). Key eligibility criteria varied slightly in each study; in general, enrolled patients were consenting adults with proven malignancy and pain due to metastatic disease (Table 1).

Full table
Studies differed in their employed assessment tool for pain response—one relied on physician consult and a patient diary (7), while others used numerical point scales (5,6,8,16,29-31,34,36,37,39,41-43,46), Brief Pain Inventory (35,37) or Visual Analog Scale (4,25,32,39,40). The majority of studies measured pain response within 1 month (4-6,9,16,25,29,31-34,36-40,43,46), with a few studies noting response after 6 weeks (42), 2 months (41), 3 months (35) or 6 months (7). CR and PR was reported in all but three studies (7,42), with study-specific criteria for CR and PR noted in Table 2. While some studies contained 10–20 patients (7,16,32,42), others featured a study population in excess of 300 patients (8,9,35,37). PR ranged from 14% (6) to 62% (33), CR from 4% (42) to 39% (45) and OR from 24% (6) to 81% (5,7) (Table 2).
Full table
ITT analysis
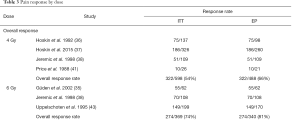
10 Gy had the highest overall OR of 81%. 6, 8 and 4 Gy had 74%, 60% and 54% OR rates respectively. CR was also highest for 10 Gy at 37%. 6 Gy seconded at 30% while 8 and 4 Gy had 22% and 21% respectively. The highest PR rate was 6 Gy (44%), followed by 10 Gy (43%), 8 Gy (38%) and 4 Gy (32%) (Table 3).
Full table
EP analysis
10 Gy registered the highest overall OR of 84%. 6, 8 and 4 Gy had 81%, 72% and 66% rates respectively. CR was also highest for 10 Gy at 39%. 6 Gy had 33%, while 8 and 4 Gy had 27% and 26% respectively. 6 Gy had the highest PR rate (48%), with 10, 4 and 8 Gy reported at 45%, 40% and 38% (Table 3).
Adverse events
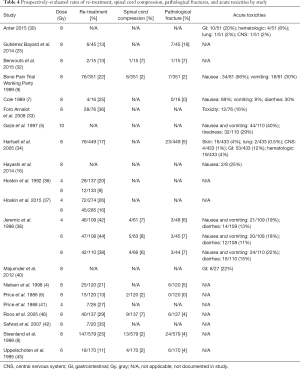
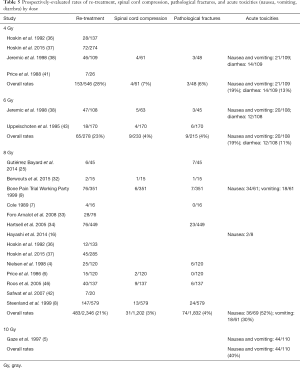
Sixteen studies (4,6-9,25,32-34,36-38,41-43,46) reported the incidence of re-treatment, 7 on the occurrence of spinal cord compression (6,8,9,32,38,43,46), 11 on the frequency of pathological fracture (4,6-9,25,32,35,38,43,46) and 9 on acute toxicities (5,7,9,16,30,33,35,38,39). Re-treatment varied from 9% (36) to 44% (38), while spinal cord compression and pathological fracture spanned 2% (6,8,9) to 8% (38) and 0% (6,7) to 16% (25), respectively. Acute toxicities, when specified, were reported as hematologic (30,35), lung (30,35), central nervous system (CNS) (30,35,39), gastrointestinal (GI) (30,35), nausea (5,7,9,16,38), vomiting (5,7,9,38), diarrhea (7,38) and fatigue/tiredness (5) (Table 4).
Full table
When analyzed by dosage, 4 Gy had the highest incident of re-treatment (28%), followed by 6 Gy (23%) and 8 Gy (21%). Similarly, 4 Gy had the highest incidence of spinal cord compression and pathological fracture (7% and 6%, respectively) compared to 6 Gy (4% for both) and 8 Gy (3% and 4%, respectively). Nausea and vomiting were reported together in the 4, 6 and 10 Gy setting, with the higher dose of 10 Gy reporting the most incidence at 40%. Nausea and vomiting were separately reported in the 8 Gy setting at 52% and 30%, respectively. Diarrhea occurred more frequently in the 4 Gy (13%) than 6 Gy (11%) (Table 5). However, the information of the radiation area was not detailed enough in the publications to allow further analysis of the gastro-intestinal side effects.
Full table
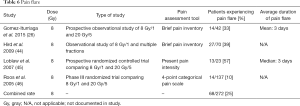
Pain flare documented across four studies (26,44-46) pertained to the 8 Gy dosage. Three different pain assessment tools were used—Brief Pain Inventory (26,44), Present Pain Intensity (45) and a 4-point categorical pain scale (46). Pain flare rates ranged from 10% (46) to 57% (45), with the overall combined rate being 25%. Gomez-Iturriaga et al. noted a mean pain flare duration of 3 days (26), while Loblaw et al. reported a median duration of 3 days (45) (Table 6).
Full table
Discussion
This systematic review contains nine additional studies when compared with that of Dennis et al. (24), and also combined pain response rates reported by studies. Although the combined rates suggest that 10 and 6 Gy may produce superior OR and CR compared to 8 Gy, and 6 Gy may result in better PR than 8 Gy under EP, it is important to note that only a few studies document doses other than 8 Gy. The last study examining 6 Gy was from 2002 (34) and the only study examining 10 Gy was published in 1997 (5). The overall rates for doses other than 8 Gy need to be interpreted with caution especially in non-randomised studies. The three studies that did compare SFRT doses were conducted in 1992 (36), 1998 (38) and recently in 2015 (37). Hoskin et al. compared 4 and 8 Gy in 1992 and 2015, and concluded both times that 8 Gy produced superior pain response rates (36,37). Similarly, Jeremic et al. reported that 8 Gy had better pain response than 6 and 4 Gy SFRT (38). To date, there have been no trials comparing a single 8 Gy versus a single 10 Gy or higher.
There was a wide range of pain response rates in the heavily-studied 8 Gy arm, likely accounted for by the different criteria for pain response set out by each study. While CR generally had the same criteria (no pain following SFRT), the different parameters for PR may have led to different outcomes. Some studies noted PR as any improvement in pain scale (5,43,46), while others required at least a 2-point improvement on their pain scale and variable use of analgesics (41). Cultural influences could also have impacted the reporting of pain, with studies being conducted in different geographical locations (24).
The considerable amount of studies investigating 8 Gy SFRT and its accompanying overall lower rates of re-treatment, spinal cord compression and pathological fracture verifies the safety of administration. This reproducible data sets a standard for future SFRT doses to be compared against (24). 10 Gy has the highest response rates but with increased side effects in this review. Future efforts can be directed to confirm the efficacy of 10 Gy when compared with a single 8 Gy while minimizing the side effects of nausea and vomiting.
Pain flare was only well-documented in the 8 Gy SFRT setting, making it difficult to be compared to other doses. While Kirkbride and Aslanidis did present an abstract regarding pain flare in the 12 Gy SFRT, their results were never published in a paper (47). Although pharmaceutical responses have been examined to manage pain flare (48-50), clinicians should also examine whether there is a dose response with the occurrence of pain flare.
This review was not without limitations. It only included English papers, thereby missing out on other published studies (51,52) that comparably reported OR, CR and PR in a similar setting. Additionally, there was a lack of statistical analysis to determine if certain doses are significantly more efficacious than others. As a result, rankings of response rates based on a few percentage-points should be interpreted with great caution, taking into account heterogeneity of data and also lack of weighting of studies.
8 Gy SFRT was the most commonly administered dose for palliation of bone metastases. While 8 Gy SFRT cannot decisively be determined as the optimal dose for pain relief, studies that did directly compare different doses reported better pain responses for 8 Gy over 4 and 6 Gy (36,39). With extensive data supporting its efficacy and safety, 8 Gy SFRT should be the standard for all future comparable treatments, in an attempt to determine which dose produces the maximum benefit.
Acknowledgements
We thank the generous support of Bratty Family Fund, Michael and Karyn Goldstein Cancer Research Fund, Joey and Mary Furfari Cancer Research Fund, Pulenzas Cancer Research Fund, Joseph and Silvana Melara Cancer Research Fund, and Ofelia Cancer Research Fund.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Conway JL, Yurkowski E, Glazier J, et al. Comparison of patient-reported outcomes with single versus multiple fraction palliative radiotherapy for bone metastasis in a population-based cohort. Radiother Oncol 2016;119:202-7. [Crossref] [PubMed]
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006;12:6243s-6249s. [Crossref] [PubMed]
- van der Linden YM, Lok JJ, Steenland E, et al. Single fraction radiotherapy is efficacious: a further analysis of the Dutch Bone Metastasis Study controlling for the influence of retreatment. Int J Radiat Oncol Biol Phys 2004;59:528-37. [Crossref] [PubMed]
- Nielsen OS, Bentzen SM, Sandberg E, et al. Randomized trial of single dose versus fractionated palliative radiotherapy of bone metastases. Radiother Oncol 1998;47:233-40. [Crossref] [PubMed]
- Gaze MN, Kelly CG, Kerr GR, et al. Pain relief and quality of life following radiotherapy for bone metastases: a randomized trial of two fractionated schedules. Radiother Oncol 1997;45:109-16. [Crossref] [PubMed]
- Price P, Hoskin PJ, Easton D, et al. Prospective randomised trial of single and multifraction radiotherapy schedules in the treatment of painful bony metastases. Radiother Oncol 1986;6:247-55. [Crossref] [PubMed]
- Cole DJ. A randomized trial of a single treatment versus conventional fraction in the palliative radiotherapy of painful bone metastases. Clin Oncol (R Coll Radiol) 1989;1:59-62. [Crossref] [PubMed]
- Steenland E, Leer JW, van Houwelingen H, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol 1999;52:101-9. [Crossref] [PubMed]
- Bone Pain Trial Working Party. 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: randomised comparison with a multifraction schedule over 12 months of patient follow-up. Radiother Oncol 1999;52:111-21. [Crossref] [PubMed]
- Koswig S, Budach V. Remineralization and pain relief in bone metastases after different radiotherapy fractions (10 times 3 Gy vs 1 time 8 Gy). A prospective study. Strahlenther Onkol 1999;175:500-8. [Crossref] [PubMed]
- Tong D, Gillick L, Henderickson FR. The palliation of symptomatic osseous metastases: final results of the Study by the Radiation Therapy Oncolo Gy Group. Cancer 1982;50:893-9. [Crossref] [PubMed]
- Niewald M, Tkocz HJ, Abel U, et al. Rapid course radiation therapy vs more standard treatment: a randomized trial for bone metastases. Int J Radiat Oncol Biol Phys 1996;36:1085-9. [Crossref] [PubMed]
- Blitzer PH. Reanalysis of the RTOG study of the palliation of symptomatic osseous metastases. Cancer 1985;55:1468-72. [Crossref] [PubMed]
- Okawa T, Kita M, Goto M, et al. Randomized prospective clinical study of small, large and twice-a-day fraction radiotherapy for painful bone metastases. Radiother Oncol 1988;13:99-104. [Crossref] [PubMed]
- Rutter CE, Yu JB, Wilson LD, et al. Assessment of national practice for palliative radiation therapy for bone metastases suggests marked underutilization of single-fraction regimens in the United States. Int J Radiat Oncol Biol Phys 2015;91:548-55. [Crossref] [PubMed]
- Hayashi S, Tanaka H, Hoshi H. External beam radiotherapy for painful bone metastases from hepatocellular carcinoma: multiple fractions compared with an 8- Gy single fraction. Nagoya J Med Sci 2014;76:91-9. [PubMed]
- Thirion P, O’Sullivan L, Chayton-Lea A, et al. ICORG 05-03: prospective randomized non-inferiority phase 3 trial comparing two radiation schedules in malignant spinal cord compression not proceeding with surgical decompression. Int J Radiat Oncol 2014;90:1263-4. [Crossref]
- Maranzano E, Trippa F, Casale M, et al. 8 Gy single-dose radiotherapy is effective in metastatic spinal cord compression: results of a phase III randomized multicenter Italian trial. Radiother Oncol 2009;93:174-9. [Crossref] [PubMed]
- Rades D, Lange M, Veninga T, et al. Preliminary results of spinal cord compression recurrence evaluation (score-1) study comparing short-course versus long-course radiotherapy for local control of malignant epidural spinal cord compression. Int J Radiat Oncol Biol Phys 2009;73:228-34. [Crossref] [PubMed]
- Loblaw DA, Mitera G, Ford M, et al. A 2011 updated systematic review and clinical practice guideline for the management of malignant extradural spinal cord compression. Int J Radiat Oncol Biol Phys 2012;84:312-7. [Crossref] [PubMed]
- Lutz S, Berk L, Chang E, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys 2011;79:965-76. [Crossref] [PubMed]
- American Academy of Hospice and Palliative Medicine: Five Things Physicians and Patients Should Question [Internet]. 2012 [cited 2016 Jul 12]. Available online: http://www.choosingwisely.org/wp-content/uploads/2015/01/Choosing-Wisely-Recommendations.pdf
- Oncology: Ten Things Physicians and Patients Should Question [Internet]. 2014 [cited 2016 Jul 12]. Available online: http://www.choosingwiselycanada.org/recommendations/oncology/
- Dennis K, Makhani L, Zeng L, et al. Single fraction conventional external beam radiation therapy for bone metastases: a systematic review of randomized controlled trials. Radiother Oncol 2013;106:5-14. [Crossref] [PubMed]
- Gutiérrez Bayard L, Salas Buzón Mdel C, Angulo Paín E, et al. Radiation therapy for the management of painful bone metastases: Results from a randomized trial. Rep Pract Oncol Radiother 2014;19:405-11. [Crossref] [PubMed]
- Gomez-Iturriaga A, Cacicedo J, Navarro A, et al. Incidence of pain flare following palliative radiotherapy for symptomatic bone metastases: multicenter prospective observational study. BMC Palliat Care 2015;14:48. [Crossref] [PubMed]
- Li KK, Hadi S, Kirou-Mauro A, et al. When should we define the response rates in the treatment in the treatment of bone metastases by palliative radiotherapy? Clin Oncol (R Coll Radiol) 2008;20:83-9. [Crossref] [PubMed]
- Chow E, Hoskin PJ, Wu J, et al. A phase III international randomised trial comparing single with multiple fractions for re-irradiation of painful bone metastases: National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) SC 20. Clin Oncol (R Coll Radiol) 2006;18:125-8. [Crossref] [PubMed]
- Amouzegar-Hashemi F, Behrouzi H, Kazemian A, et al. Single versus multiple fractions of palliative radiotherapy for bone metastases: a randomized clinical trial in Iranian patients. Curr Oncol 2008;15:151. [PubMed]
- Anter AH. Single Fraction versus Multiple Fraction Radiotherapy for treatment of painful bone metastases: a Prospective Study; Mansoura experience. Forum Clin Oncol 2015;6:8-13.
- Badzio A, Senkus-Konefka E, Jereczek-Fossa BA, et al. 20 Gy in five fractions versus 8 Gy in one fraction in palliative radiotherapy of bone metastases. A multicenter randomized study. Nowotwory 2003;53:261-4.
- Berwouts D, De Wolf K, Lambert B, et al. Biological 18[F]-FDG-PET image-guided dose painting by numbers for painful uncomplicated bone metastases: A 3-arm randomized phase II trial. Radiother Oncol 2015;115:272-8. [Crossref] [PubMed]
- Foro Arnalot P, Fontanals AV, Galcerán JC, et al. Randomized clinical trial with two palliative radiotherapy regimens in painful bone metastases: 30 Gy in 10 fractions compared with 8 Gy in single fraction. Radiother Oncol 2008;89:150-5. [Crossref] [PubMed]
- Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst 2005;97:798-804. [Crossref] [PubMed]
- Güden M, Kurt E, Ulutin C. Six gray single dose radiotherapy in the treatment of metastatic bone pain. Tohoku J Exp Med 2002;197:111-4. [Crossref] [PubMed]
- Hoskin PJ, Price P, Eaton D, et al. A prospective randomised trial of 4 Gy or 8 Gy single doses in the treatment of metastatic bone pain. Radiother Oncol 1992;23:74-8. [Crossref] [PubMed]
- Hoskin P, Rojas A, Fidarova E, et al. IAEA randomized trial of optimal single dose radiotherapy in the treatment of painful bone metastases. Radiother Oncol 2015;116:10-4. [Crossref] [PubMed]
- Jeremic B, Shibamoto Y, Acimovic L, et al. A randomized trial of three single – dose radiation therapy regimens in the treatment of metastatic bone pain. Int J Radiat Oncol Biol Phys 1998;42:161-7. [Crossref] [PubMed]
- Nuzzo M, Macchia G, Torre G, et al. Single fraction radiotherapy (8 Gy) on painful bone metastases with involvement of the adjacent soft tissues. Radiother Oncol 2015;115:S697-8. [Crossref]
- Majumder D, Chatterjee D, Bandyopadhyay A, et al. Single Fraction versus Multiple Fraction Radiotherapy for Palliation of Painful Vertebral Bone Metastases: A Prospective Study. Indian J Palliat Care 2012;18:202-6. [Crossref] [PubMed]
- Price P, Hoskin PJ, Eaton D, et al. Low dose single fraction radiotherapy in the treatment of metastatic bone pain: A pilot study. Radiother Oncol 1988;12:297-300. [Crossref] [PubMed]
- Safwat E, El-Nahas T, Metwally H, et al. Palliative fractionated radiotherapy for bone metastases clinical and biological assessment of single versus multiple fractions. J Egypt Natl Canc Inst 2007;19:21-7. [PubMed]
- Uppelschoten JM, Wanders SF, de Jong JMA. Single-dose radiotherapy (6 Gy): palliation in painful bone metastases. Radiother Oncol 1995;36:198-202. [Crossref] [PubMed]
- Hird A, Chow E, Zhang L, et al. Determing the incidence of pain flare following palliative radiotherapy for symptomatic bone metastases: results from three Canadian cancer centers. Int J Radiat Oncol Biol Phys 2009;75:193-7. [Crossref] [PubMed]
- Loblaw DA, Wu JS, Kirkbride P, et al. Pain flare in patients with bone metastases after palliative radiotherapy--a nested randomized control trial. Support Care Cancer 2007;15:451-5. [Crossref] [PubMed]
- Roos DE, Turner SL, O’Brien PC, et al. Randomized trial of 8 Gy in 1 versus 20 Gy in 5 fractions of radiotherapy for neuropathic pain due to bone metastases (Trans-Tasman Radiation Oncolo Gy Group, TROG 96.05). Radiother Oncol 2005;75:54-63. [Crossref] [PubMed]
- Kirkbride P, Aslanidis J. Single fraction radiation therapy for bone metastases – a pilot study using a dose of 12 Gy. Clin Invest Med 1996;19:S87.
- Chow E, Loblaw A, Harris K, et al. Dexamethasone for the prophylaxis of radiation-induced pain flare after palliative radiotherapy for bone metastases – a pilot study. Support Care Cancer 2007;15:643-7. [Crossref] [PubMed]
- Hird A, Zhang L, Holt T, et al. Dexamethasone for the prophylaxis of radiation-induced pain flare after pallaitive radiotherapy for symptomatic bone metastases: a phase II study. Clin Oncol (R Coll Radiol) 2009;21:329-35. [Crossref] [PubMed]
- Chow E, Meyer R, Ding K, et al. Dexamethasone in the prophylaxis of radiation-induced pain flare after palliative radiotherapy for bone metastases: a double-blind, randomised placebo-controlled, phase 3 trial. Lancet Oncol 2015;16:1463-72. [Crossref] [PubMed]
- Koswig S, Budach V. Remineralization and pain relief in bone metastases after different radiotherapyf ractions (10 times 3 Gy vs 1 time 8 Gy). A prospective study. Strahlenther Onkol 1999;175:500-8. [Crossref] [PubMed]
- Altundag MB. Single (500c Gy, 800c Gy) and multifraction (300 x 10c Gy) radiotherapy schedules in the treatment of painful bone metastases. Turk Hematoloji-Onkoloji Dergisi 2002;12:16-21.