Breakthrough expected in translational research of targeted therapy for small cell lung cancer
Small cell lung cancer (SCLC) accounted for 12-15% of the 213,380 lung cancer cases and 160,390 deaths newly reported in the U.S. in 2010 (1). SCLC is a highly aggressive disease characterized by rapid multiplication, high growth fractions, early dissemination and metastasis, and high sensitivity to first-line chemotherapy and radiotherapy. About 60-70% of the patients are not diagnosed until extensive-stage disease (ED) (2). Platinum-based chemotherapy has been the mainstream treatment for almost three decades, while radiation therapy is only applicable for patients with limited-stage disease (LD). Despite a remission rate (RR) of up to 80% with first-line therapy, the median overall survival (OS) is only 12 to 20 months and 7 to 11 months for patients with LD and ED, respectively. Regardless of severity, most patients will eventually experience disease recurrence after chemotherapy or resistance to chemotherapy, and only 6-12% of LD patients and 2% of ED patients can survive up to 5 years (3). A very poor prognosis is expected in the case of relapse or progression within three months of first-line treatment. Patients experiencing recurrence beyond three months after initial therapy are still sensitive to subsequent treatment, and thus considered to potentially benefit from second-line chemotherapy (4). Therefore, new therapies have to be developed for improving the prognosis of those patients. Although different treatment strategies have been employed, including optimized order of administration, maintenance treatment and addition of a third cytotoxic drug, little success has been reported. On the other hand, new targeted drugs have offered several prospects in clinical studies, and breakthroughs can be expected in some of the translational studies. This study reviews the findings of latest clinical trials on the application of targeted agents in treating small cell lung cancer.
Overview of recent translational research of small-cell lung cancer (SCLC)
Ongoing translational research has focused on the following targeted drugs: angiogenesis inhibitors, matrix metalloproteinase (MMP) inhibitors, mTOR inhibitors, c-kit inhibitors, Bcl-2 antagonists, topoisomerase inhibitors, and so on (Table 1).
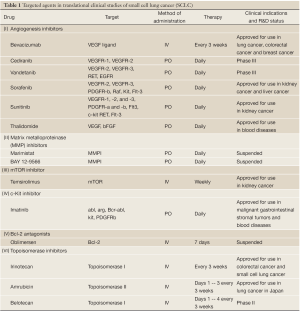
Full table
Targeted drugs for small cell lung cancer (SCLC) (see for the phase II and phase II clinical trials)
Angiogenesis inhibitors
Angiogenesis, which covers from the emergence to maturity of blood vessels, is a process through each stage of malignancies, and is also involved in distant metastasis. Measurement of the angiogenesis microvessel count (MVC) is an important factor for predicting the risk of tumor metastasis and survival in non-small cell lung cancer (NSCLC). The average MVC is higher in small cell lung cancer than that of NSCLC, while a high MVC and high VEGF expression are indicative of poor prognosis in the former (5).
Since angiogenesis inhibitors are free of overlapping toxicity with chemotherapy drugs, they can be added to multiple chemotherapeutic options for treatment of several solid tumors (Table 1). Bevacizumab (Avastin) combined with cytotoxic agents has been approved for the treatment of metastatic colon cancer, breast cancer and non-small cell lung cancer. Three Phase II trials have evaluated bevacizumab in combination with chemotherapy in patients with ED-SCLC. The first study, conducted by ECOG (E3501) in 64 patients with ED-SCLC. Four cycles of bevacizumab 15 mg/kg in combination with standard first-line chemotherapy, DDP 60 mg/m2 d1 and Vp16 120 mg/m2 d1-3 for 21 days, were administered. Treatment with bevacizumab alone was continued in progression-free patients until disease progression or unacceptable toxicity. The primary endpoint was achieved with an overall RR of 64%, PFS 4.7 months; 30% patients had PFS of 6 months. The OS was 10.9 months (Table 2) (6). In the second study (CALGB 30306 trial), 72 treatment-naïve patients were treated with six cycles of bevacizumab (15 each/kg d1, DDP 30 mg/m2 d1, 8 irinotecan 85 mg/m2 d1,8), 21 days per cycle. The overall RR, PFS and OS were similar to the findings of ECOG 3501 trial, at 62%, 7.0 and 10.6 months, respectively (7). In the third trial, 51 patients with ED-SCLC were treated with carboplatin AUC4 d1, irinotecan 60 mg/m2 d1,8,15 and bevacizumab 10 mg/kg, repeated every two weeks. The total response rate (ORR) was 84%, PFS 9.1 months, and OS 12.1 months (8). Common grade 3/4 toxicity events included cytopenias, hypertension, thrombosis, bleeding, proteinuria, diarrhea and fatigue. In another Phase II trial, the sustained effect of bevacizumab in combination with chemotherapy was assessed for LD-SCLC patients. Fifty-seven patients received 61.2 GY radiotherapy plus carboplatin AUC5 d1 and irinotecan 50 mg/m2 d1,8 for four cycles. After combined radiotherapy and chemotherapy, progression-free patients received bevacizumab 10 mg/kg every two weeks for 10 cycles. Despite a RR of 80%, the PFS was slightly longer by the first and second years (at 53% and 54%, respectively) with OS of 71% and 29%, similar to the findings in patients undergoing traditional cisplatin-etoposide chemotherapy and radiothearpy alone.
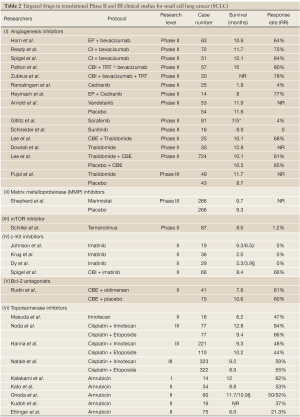
Full table
Cediranib (AZD2171, Recentin) is an effective VEGFR-1/VEGFR-2 inhibitor. It can antagonize c-kit, PDGFR-b and Flt-4 at nanoscale concentrations while selectively sparing other serine/threonine kinases. It has been proved that oral administration of cediranib once daily can inhibit the conduction of VEGF. In a Phase I trial, a select group of 14 patients with SCLC and large cell neuroendocrine carcinoma were treated with cediranib combined with DDP+Vp16, resulting in a RR of 77% and PFS of up to eight months. Grade 3/4 toxicity events included bleeding duodenal ulcer, renal failure and hemoptysis (9).
Vandetanib (AZD6474) is an oral vascular inhibitor against VEGFR-2, VEGFR-3, RET and EGFR/HER1. In a Phase II trial, maintenance therapy with vandetanib 300 mg qd and placebo was delivered to 107 subjects, including 53 patients with ED or LD-SCLC and 54 matched control subjects. The CR or PR rates were close to the result with induction chemotherapy, and no difference was shown in the sustained effect between the treatment and control groups, with PFS of 2.7 and 2.6 months, and OS of 11.9 and 10.6 months, respectively. However, statistical analysis showed that vandetanib yielded longer overall survival in LD-SCLC patients than ED-SCLC patients (HR 0.45; unilateral P=0.07), and less benefit was observed in the female group. Interestingly, such findings of vandetanib were also found in non-small-cell lung cancer (NSCLC). Preclinical research data suggested a possible link with the superimposed inhibitory effects of vandetanib against both EGFR and VEGF pathways, which eliminated the resistance of cells resistant to EGFR inhibitors (10). The cause of its potential benefits to SCLC patients is still unclear. Adverse events in the vandetanib group included mild hemoptysis, severe diarrhea, and mouth pain.
Sorafenib (Bay43-9006), is an oral inhibitor of multiple kinases, which inhibits tumor cell proliferation by suppressing Raf, KIT, Flt-3, VEGFR-2, VEGFR-3 and PDGFR-b. In a SWOG trial, 81 patients with disease progression after first-line platinum-based chemotherapy were treated a 28-day cycle with sorafenib 400 mg alone twice a day. Eighteen of them (22%) discontinued treatment due to drug-related adverse events or side effects. Three patients achieved PR (4%) and 25 patients achieved SD (32%). Platinum-sensitive patients had an OS of seven months, while insensitive patients had an OS of five months. Common grade 3/4 toxicity events included rash, flu-like symptoms and metabolic disorders. Despite a high termination rate (>20%) due to toxicity, SWOG still suggested further research to evaluate the efficacy of the drugs on small cell lung cancer (11).
Sunitinib (SU11248, Sutent) is a novel multi-targeted small molecule inhibitors against mainly VEGFR-1,2,3, PDGFR-a/b, Flt3 and c-kit, which rearranges receptor encoding during proto-oncogene transfection (ret) and Flt3. CALGB was delivered to patients with ED-SCLC (DDP 80 mg/m2 d1, Vp16 100 mg/m2 d1-3, 21-day cycle; sunitinib 25 mg PO, qd) Two patients discontinued treatment due to neutropenia. This ongoing study is designed to assess the maintenance effect of sunitinib following chemotherapy, which has been otherwise evaluated by two existing trials. Lubiner and his colleagues evaluated maintenance treatment with sunitinib 25 mg (PO qd) following carboplatin and irinotecan chemotherapy in ED-SCLC patients. The resultant TTP was 7.6 months and 91% patients had an OS of 6 months (12). However, those findings remained uncertain due to a small sample size. Schneider and colleagues prescribed 4-6 week medication with sunitinib 50 mg qd to 16 patients with ED-SCLC who had undergone platinum-based chemotherapy. Half of the patients discontinued sunitinib due to drug toxicity. The median PFS was 6.3 months and OS 8.9 months. The study was ended before schedule as it did not achieve the primary endpoint. Grade 3/4 adverse events included thrombocytopenia, fatigue, weakness, hypothyroidism, hand and foot syndrome and hypertension (13).
Thalidomide, an immune response modifier, may also have anti-angiogenic and anti-inflammatory effects by inhibiting tumor-produced VEGF and fibroblast growth factors (bFGF). The prospects of this agent were shown in previous Phase II small-scale studies of small cell lung cancer. Thirty patients with ED-SCLC received maintenance therapy with thalidomide (PO 200 mg qd) after induction chemotherapy in a Phase II clinical trial. The OS was 12.8 months, one-year survival rate 51.7%, and average time of thalidomide treatment 79 days, indicating satisfying tolerability of this drug (14). However, thrombotic events and bone marrow suppression were common during thalidomide treatment. Two large Phase III clinical trials have evaluated the efficacy of thalidomide in combination with chemotherapy treatment for patients with small cell lung cancer. In the first trial, carboplatin AUC5 d1, Vp16 120 mg/m2 iv/gtt, d1, 100 mg PO bid, d2-3 with or without thalidomide were administered. A total of 724 LD or ED-SCLC patients were enrolled. The OS reached 10.1 and 10.5 months in the thalidomide and control groups, respectively, without difference, though thalidomide treatment caused more thrombotic events (19%:10%, hazard ratio 2.13, 95% confidence interval 1.41-3.20). In the other Phase III trial, ED-SCLC patients were treated in 28-day cycles with thalidomide 400 mg qd, epirubicin 40 mg/m2 d1, cisplatin 100 mg/m2 d2 and cyclophosphamide 400 mg/m2 d1-3. Ninety-two patients who had received two cycles of conventional chemotherapy were randomly assigned to receive four-course chemotherapy with or without thalidomide. A certain number of patients terminated thalidomide therapy (33%:19%) due to toxicity. Longer OS was obtained in the thalidomide group compared with the control group (11.7:8.7 months), and patients of the former group had a significantly higher opportunity to receive second-line treatment (67%:46%) (15).
At the ASCO annual meeting this year, Lu Shun from China presented a Phase II multi-center clinical trial he conducted to evaluate Endostar (recombinant human endothelin) combined with chemotherapy as the first-line treatment for ED-SCLC patients. The protocol included six 21-day cycles of Endostar 7.5 mg/m2 iv/gtt, d1-14; carboplatin AUC 5 d1-5, Vp16 60 mg/m2 d1-5. A total of 137 patients were enrolled (Endostar/control group: 68/69), and those who achieved CR, PR and SO were administered with the agent alone for maintenance until disease progression. The primary endpoint was PFS and the secondary endpoint OS and RR. At last, the two groups had similar median PFS (6.2:5.9 months, P=0.163, HR 0.762), median OS were 12.4:12.3 months, P=0.475, and overall RRs were 76.5%:68.1%, P=0.275. The conclusion was that carboplatin in combination with etoposide plus Endostar could neither significantly improve PFS nor prolong OS, though the toxicity was tolerable.
MMP inhibitors
Matrix metalloproteinases (MMPs) comprise a large family of proteolytic enzymes. Their main function is associated with degradation of the outer cell membrane, including the basement membrane. MMPs are involved in the invasion capacity of tumor cells, maintenance of the tumor microenvironment, angiogenesis of the primary tumor lesions and metastasis, as well as other biological processes. Elevated levels of multiple MMPs have been found in small cell lung cancer, including gelatinase A (MMP-2), stromelysin-1 (MMP-3), matrilysin (MMP-7), gelatinase B (MMP-9), membrane type MMP-1 (MT1-MMP; MMP-14) and stromelysin 3, indicating poor survival. Pre-clinical research revealed that MMP inhibitors (MMPI) could limit the growth and regional spread of solid tumors, including lung cancer. Marimistat is a synthetic, orally absorbed MMP inhibitor. A placebo-controlled, randomized, double-blind clinical trial that enrolled 532 previously untreated patients with LD or ED-SCLC patients for maintain tratment with marimistat 10 mg bid for two years concluded that the OS results were similar between the marimistat and placebo groups, at 9.7 and 9.3 months, respectively, while the marimistat group presented worse quality of life. So far, findings of other studies of MMPIs in small cell lung cancer have been disappointing as well (16).
mTOR inhibitor
Temsirolimus (CCI-779) is an mTOR inhibitor. Mammalian target rapamycin (mTOR) is a serine/threonine specific kinase of the PI3K kinase (PIKK) family, and the mTOR signaling pathway is involved in cell growth, survival and angiogenesis. The activation of this pathway can enhance the resistance to chemotherapy drugs in experiments in vitro, while its suppression can lead to increased sensitivity to chemotherapy. In a phase II study, ECOG assessed the maintenance effect of intravenous administration with temsirolimus 25 or 250 mg weekly for 87 patients who had obtained CR, PR or SD after induction chemotherapy. The OS was 8 months and PFS 2.2 months. Slightly longer OS was observed in the 250 mg group compared to the 25 mg group (9.5:6.6 months). Grade 3/4 adverse events included thrombocytopenia, hypophosphatemia, neutropenia, and fatigue. Everolimus, an oral mTOR inhibitor, was used in the second trial (RAD001) for 40 patients with recurrent SCLC after one or two chemotherapy plans. As a result, no grade 4 toxicity was found. RR was 26%, PFS 1.4 months, and OS 5.5 months, indicating low efficacy of the drug as retreatment for small cell lung cancer (17). Hence, there is no evidence that mTOR inhibitors are effective against the condition.
Kit inhibitors
Kit is a glycoprotein and transmembrane receptor tyrosine kinase. High expression of Kit and its ligands and stem cell factor has been found inside the tumor body of small cell lung cancer, which is considered to be of prognostic significance. Imatinib (STI-571) is an oral small molecule that inhibits ABL (abelson kinase), BCR-ABL, ARG (ABL-related gene), c-kit and PDGFR. A preclinical model showed that imatinib inhibited the growth and signal transduction of small cell lung cancer cell lines. Three Phase II trials suggested that single-agent imatinib was essentially ineffective for patients with SCLC. The dosage options included 400 mg bid and 600 mg qd. Two Phase I and a Phase II clinical trials of combined medication did not proceed so well that they were terminated before schedule (18). The reason could include insufficient drug concentration of the inhibitory receptors, bypassed receptor inhibition via downstream passage, lack of activated c-kit mutation in small cell lung cancer or failure of regulatory functionality as the agent acted on mutated receptors only.
Bcl-2 (B-cell leukemia/lymphoma gene 2) antagonists
Bcl-2 is an important apoptosis regulator that highly expressed in multiple tumors, including small cell lung cancer. The cytotoxicity of many chemotherapeutic drugs is regulated through the bcl-2 apoptotic pathway, and high bcl-2 expression is associated with the resistance to those drugs. Preclinical studies have mostly been focused on the development of agents that directly inhibit the antisense oligonucleotide of Bcl-2 mRNA to promote chemotherapy effects. Oblimersen (G3139) is a synthetic antisense nucleotide that binds to the first six codons of Bcl-2 mRNA. There are two ongoing Phase I trials of this agent for the treatment of small cell lung cancer, but the results are not satisfactory. The one treated chemotherapy-resistant SCLC patients with oblimersen 3 mg/kg/d d1-8 and paclitaxel 150 mg/m2 d6. In the other, chemotherapy-naïve SCLC patients were treated with oblimersen 5 mg/kg/d d1-8, carboplatin AUC6 d6, and etoposide 80 mg/m2, d6,8. In the first trial, objective effectiveness of oblimersen combined with paclitaxel was not observed, while only two patients with high blood oblimersen reached stable disease (19). The RR was 86% and median TTP 5.9 months in the second trial. CALGB conducted a randomized Phase II clinical trial using carboplatin with or without oblimersen, in combination with etoposide, which enrolled 50 patients with ED-SCLC. Although the two groups had similar RR (61%:60%), worse PFS and OS were found in the oblimersen group (6.0:8.6 months vs. 7.6:10.6 months). The author speculated that the patients did not benefit from oblimersen because the drug missed the target site. Despite disappointing findings of the above studies, a new-generation oral BCL-2 antagonist has been developed and entered the stage of clinical study of small cell lung cancer.
Topoisomerase I and II inhibitors
DNA topoisomerases I and II consist the key ribozymes to regulate DNA topology. Topoisomerases I and II can cause DNA breakage and reunion reaction, contributive to the translation and replication of DNA. Etoposide (VP-16) is a semi-synthetic podophyllotoxin that inhibits topoisomerase II and is considered to be combined with cisplatin (DDP) for treatment of ED-SCLC and LD-SCLC. Irinotecan (CPT-11) is a topoisomerase I inhibitor that has been widely used in the treatment of small cell lung cancer, with an effective rate of 47% against relapsed SCLC (20). It is used in combination with cisplatin or carboplatin for previously untreated or relapsed small cell lung cancer. In a Japanese controlled Phase III study (JCOG 9511), 154 previously untreated SCLC patients were assigned to receive 28-day cycles of DDP 60 mg/m2 and irinotecan 60 mg/m2 d1,8,15, or 21-day cycles of DDP 80 mg/m2 and VP16 100 mg/m2 d1-3 (control group). The study was closed before schedule due to significantly higher response rate in the treatment group at 84% vs. 66%, with a median survival time of 12.8 vs. 9.4 months, and 1- and 2-year survival rate of 58% vs. 38% and 19.5% vs. 5.2%, respectively. Better outcomes were recognized in the DDP + irinotecan group compared to the DDP + Vp16 group (P=0.002) (21). However, two trials that enrolled 1,002 patients in North America failed to confirm the results of the Japanese study. Therefore, VP-16-containing programs have been used as the standard first-line treatment in North America, while irinotecan-containing programs were used in Japan instead. For patients undergoing second-line treatment or retreatment, several Phase II trials in Europe and the United States inclined towards the combination of carboplatin and irinotecan, which had a RR of 58-67%, PFS of 5-6 months and OS of 9-10 months.
As a synthetic anthracycline, Amrubicin is a new embedded topoisomerase II inhibitor. Which has 200-fold anti-tumor activities when converted into its active metabolite, amrubicinol, compared to the original form (22). It has been approved for the treatment of non-small cell lung cancer and small cell lung cancer in Japan. After dose escalation, the recommended dose was set at 35-40 mg/m2 d1-3. There are several Phase II trials evaluating the efficacy of amrubicin against refractory or recurrent small cell lung cancer. The efficacy was slightly higher than 50%, PFS 4 months, OS 8-10 months and one-year survival 28%. A large-scale study assigned 60 patients who were sensitive to platinum-based first-line chemotherapy to receive second-line treatment with amrubicin 40 mg/m2 d1-3 (21-day cycles). The overall RR was 52%, and PFS, OS and one-year survival rates were 4.0 months, 11.7 months and 48.2%, respectively (23). These findings seemed better than topotecan, which is usually used as a second-line treatment of small cell lung cancer. Another two Phase II trials evaluated the efficacy in chemotherapy sensitive and resistant patients in North American, yielding results of 40% and 21.3%, respectively (24,25).
The combination of amrubicin and platinum has been put into clinical research. Forty-one previously untreated patients with ED-SCLC were enrolled in Phase I-II trials and treated with 21-day cycles of DDP 60 mg/m2 d1 and amrubicin 40 or 45 mg/m2, d1-3. With similar efficacy to single-agent therapy, the medication was associated with neutropenia/leukopenia as the primary toxic effect. The RR was 87.8%, and median OS 14.1 months (26). In a study of combined use with carboplatin, 21-day cycles of amrubicin 30, 35 or 40 mg/m2 d1-3 and carboplatin AUC 5 were administered. Sixteen treatment-naïve ED-SCLC patients were enrolled. The toxicity included dose-limiting neutrophil toxicity, thrombocytopenia, gastric ulcer, abnormal liver function and febrile neutrophia. The RR was 73% and further research was recommended. Amrubicin 35 mg/m2 was recommended to be used in combined chemotherapy, and the maximum tolerated dose was determined as 100 mg/m2. Although these results suggest that amrubicin is effective either used as a single agent or in combination, its effect is yet to be determined in non-Asian populations. Hence, this drug is not advised for use in the North American and European populations expect for clinical trials.
Belotecan (CKD-602, camptobell) is a potent topoisomerase I inhibitor currently applied in clinical studies of small-cell lung cancer in Japan. A phase II study enrolled 27 patients with previously untreated ED-SCLC for initial treatment or with chemotherapy-sensitive LD-SCLC for retreatment, using belotecan 0.4, 0.5 or 0.6 mg/m2, d1-5 (3-week cycles). The objective overall effective rate was 43%. While patients were more sensitive to initial chemotherapy, 20% of those undergoing retreatment were sensitive to chemotherapy. The median PFS were 4.8 and 3.3 months, and OS 11.9 and 10.5 months, respectively (27). Another phase II study assigned 37 patients with the same conditions as the previous study to 3-week cycles of belotecan 0.5 mg/m2. With similar effective rates, 66% treatment-naïve patients and 29% retreatmnet patients were sensitive to chemotherapy. Based on those promising results, a Phase I study evaluated previously untreated SCLC patients using 21-day cycles of belotecan 0.4-0.6 mg/m2 d1-4 and DDP 60mg/m2 d1. The effective rate was 77%, and PFS and OS 7.5 and 15.9 months, respectively. A belotecan dose of 0.5 mg/m2 was recommended for subsequent Phase II studies (28). A phase II enrolled 30 ED-SCLC patients for first-line therapy with 3-week cycles of DDP 60 mg/m2 d1 and belotecan 0.5 mg/m2 d1-4, yielding satisfying results with RR of 70%, PFS of 6.9 months and OS of 19.2 months (29).
Becatecarin is an antitumor antibiotic that inhibits topoisomerases I, II, and embedded DNA. In an ongoing Phase II clinical trial, 35 chemotherapy-sensitive relapsed SCLC patients are being treated with 21-day cycles of becatecarin 140 mg/m2 d1-5 (30).
Hence, new topoisomerase I and II inhibitors seem to have certain activities against small cell lung cancer under clinical settings, though continued research is still needed, particularly in non-Asian populations. However, whether these new drugs are more active against tumor cells than etoposide or not needs to be determined.
In addition to the translational studies on targeted drugs above, alkylating drugs (e.g., bendamustine), platinum-based drugs (e.g., picoplatin) and anti-metabolic drugs (e.g., pemetrexed) are also being evaluated in clinical studies despite difficult and slow progress.
In summary, breakthroughs can be expected in the translational research of targeted therapy for small cell lung cancer. Although the process in SCLC-related translational research is not as smooth as that with non-small cell lung cancer, several prospects are evident: (I) With the support of the anti-angiogenesis theory and basic research, as well as a growing number of relevant trials, encouraging achievements have been made using the combination of bevacizumab chemotherapy/radiotherapy. More and more small-molecule multi-targeted angiogenesis inhibitors are being developed. While endostar, a novel drug developed by China, has drawn certain attention, the old drug thalidomide may also be included in a new treatment option; (II) Despite unsatisfactory results with MMP inhibitors, mTOR inhibitors, Kit inhibitors and Bcl-2 inhibitors, relevant preclinical studies have provided guidance for subsequent research; and (III) New topoisomerase inhibitors amrubicin and belotecan have revealed their prospects in Asia, particularly the combination with platinum, but further research will be needed in the Caucasian population.
In the future, SCLC-related research should focus on: (I) strengthening the multidisciplinary collaboration in systematic studies of possible treatment plans; (II) identifying key targets or drive targets; (III) enlarging the study team to encourage the participation of clinicians and patients; and (IV) looking for the breakthrough point bearing in mind the populational differences regarding non-small cell lung cancer.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300.
- Clark R, Ihde DC. Small-cell lung cancer: treatment progress and prospects. Oncology (Williston Park) 1998;12:647-58; discussion 661-3.
- Jänne PA, Freidlin B, Saxman S, et al. Twenty-five years of clinical research for patients with limited-stage small cell lung carcinoma in North America. Cancer 2002;95:1528-38.
- Ardizzoni A, Hansen H, Dombernowsky P, et al. Topotecan, a new active drug in the second-line treatment of small-cell lung cancer: a phase II study in patients with refractory and sensitive disease. The European Organization for Research and Treatment of Cancer Early Clinical Studies Group and New Drug Development Office, and the Lung Cancer Cooperative Group. J Clin Oncol 1997;15:2090-6.
- Lucchi M, Mussi A, Fontanini G, et al. Small cell lung carcinoma (SCLC): the angiogenic phenomenon. Eur J Cardiothorac Surg 2002;21:1105-10.
- Horn L, Dahlberg SE, Sandler AB, et al. Phase II study of cisplatin plus etoposide and bevacizumab for previously untreated, extensive-stage small-cell lung cancer: Eastern Cooperative Oncology Group Study E3501. J Clin Oncol 2009;27:6006-11.
- Ready N, Dudek AZ, Wang XF, et al. CALGB 30306: a phase II study of cisplatin (C), irinotecan (I) and bevacizumab (B) for untreated extensive stage small cell lung cancer (ES-SCLC). J Clin Oncol 2007;25:abstr 7563.
- Spigel DR, Hainsworth JD, Yardley DA, et al. Phase II trial of irinotecan, carboplatin, and bevacizumab in patients with extensive-stage small cell lung cancer. J Clin Oncol 2007;25:abstr 18130.
- Heymach J, Glisson BS, Doebele RC, et al. Phase I open-label study of cediranib plus etoposide (E) and cisplatin as first-line therapy for patients (pts) with small cell lung cancer (SCLC) or lung neuroendocrine cancer (NEC). J Clin Oncol 2010;28:abstr 7050.
- Tabernero J. The role of VEGF and EGFR inhibition: implications for combining anti-VEGF and anti-EGFR agents. Mol Cancer Res 2007;5:203-20.
- Gitlitz BJ, Glisson BS, Moon J, et al. Sorafenib in patients with platinum (plat) treated extensive stage small cell lung cancer (E-SCLC): a SWOG (S0435) phase II trial. J Clin Oncol 2008;26:abstr 8039.
- Lubiner ET, Spigel DR, Greco FA, et al. Phase II study of irinotecan and carboplatin followed by maintenance sunitinib in the first-line treatment of extensive-stage small cell lung cancer. J Clin Oncol 2010;28:abstr 7049.
- Schneider BJ, Gadgeel SM, Ramnath N, et al. Phase II trial of sunitinib maintenance therapy after platinum-based chemotherapy in patients with extensive-stage small cell lung cancer (ES SCLC). J Clin Oncol 2010;28:abstr e18041.
- Lee SM, James L, Buchler T, et al. Phase II trial of thalidomide with chemotherapy and as maintenance therapy for patients with poor prognosis small-cell lung cancer. Lung Cancer 2008;59:364-8.
- Pujol JL, Breton JL, Gervais R, et al. Phase III double-blind, placebo-controlled study of thalidomide in extensive-disease small-cell lung cancer after response to chemotherapy: an intergroup study FNCLCC cleo04 IFCT 00-01. J Clin Oncol 2007;25:3945-51.
- Shepherd FA, Giaccone G, Seymour L, et al. Prospective, randomized, double-blind, placebo-controlled trial of marimastat after response to first-line chemotherapy in patients with small-cell lung cancer: a trial of the National Cancer Institute of Canada-Clinical Trials Group and the European Organization for Research and Treatment of Cancer. J Clin Oncol 2002;20:4434-9.
- Kotsakis AP, Tarhini A, Petro D, et al. Phase II study of RAD001 (everolimus) in previously treated small cell lung cancer (SCLC). J Clin Oncol 2009;27:abstr 8107.
- Spigel DR, Hainsworth JD, Simons L, et al. Irinotecan, carboplatin, and imatinib in untreated extensive-stage small-cell lung cancer: a phase II trial of the Minnie Pearl Cancer Research Network. J Thorac Oncol 2007;2:854-61.
- Rudin CM, Otterson GA, Mauer AM, et al. A pilot trial of G3139, a bcl-2 antisense oligonucleotide, and paclitaxel in patients with chemorefractory small-cell lung cancer. Ann Oncol 2002;13:539-45.
- Masuda N, Fukuoka M, Kusunoki Y, et al. CPT-11: a new derivative of camptothecin for the treatment of refractory or relapsed small-cell lung cancer. J Clin Oncol 1992;10:1225-9.
- Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 2002;346:85-91.
- Yamaoka T, Hanada M, Ichii S, et al. Cytotoxicity of amrubicin, a novel 9-aminoanthracycline, and its active metabolite amrubicinol on human tumor cells. Jpn J Cancer Res 1998;89:1067-73.
- Onoda S, Masuda N, Seto T, et al. Phase II trial of amrubicin for treatment of refractory or relapsed small-cell lung cancer: Thoracic Oncology Research Group Study 0301. J Clin Oncol 2006;24:5448-53.
- Ettinger DS, Jotte RM, Gupta V, et al. A phase II trail of single agent amrubicin (AMR) in patients with ED-SCLC that is refractory or progressive within 90 days of completion of first-line platinum based chemotheraphy. J Clin Oncol 2008;26:abstr 8041.
- Jotte RM, Conkling PR, Reynolds C, et al. A randomized phase II trial of amrubicin (AMR) vs. topotecan as second-line treatment in extensive-disease small-cell lung cancer (SCLC) sensitive to platinum-based first-line chemotherapy. J Clin Oncol 2008;26:abstr 8040.
- Ohe Y, Negoro S, Matsui K, et al. Phase I--II study of amrubicin and cisplatin in previously untreated patients with extensive stage small cell lung cancer (ED-SCLC) (final report). J Clin Oncol 2004;22:abstr 7206.
- Lee DH, Kim SW, Suh C, et al. Belotecan, new camptothecin analogue, is active in patients with small-cell lung cancer: results of a multicenter early phase II study. Ann Oncol 2008;19:123-7.
- Lee DH, Kim SW, Bae KS, et al. A phase I and pharmacologic study of belotecan in combination with cisplatin in patients with previously untreated extensive-stage disease small cell lung cancer. Clin Cancer Res 2007;13:6182-6.
- Lee DH, Kim SW, Suh C, et al. Multicenter phase 2 study of belotecan, a new camptothecin analog, and cisplatin for chemotherapy-naive patients with extensive-disease small cell lung cancer. Cancer 2010;116:132-6.
- Schwandt A, Mekhail T, Halmos B, et al. Phase-II trial of rebeccamycin analog, a dual topoisomerase-I and -II inhibitor, in relapsed “sensitive” small cell lung cancer. J Thorac Oncol 2012;7:751-4.


