Challenges and successes in non-operative management of high-grade malignant bowel obstruction
Introduction
Malignant bowel obstruction (MBO) is associated with significant morbidity and mortality. A retrospective analysis of all U.S. hospitalizations for MBO revealed that overall in-hospital mortality rate was between 21.4% [2010] and 24.5% [2006] for these patients (1). MBO often represents a terminal event for patients with metastatic cancer, irrespective of operative or non-operative management. Operative management has the advantage of resolving bowel obstruction, but portends a higher mortality compared to conservative management; with conservative management, there is the risk of recurrence despite lower mortality (1).
Conservative management generally involves bowel rest, decompression via nasogastric tube (NGT) or sometimes venting gastrostomy, intravenous (IV) hydration and nutrition, and analgesia (2,3). Corticosteroids are useful given their anti-inflammatory and antiemetic properties and somatostatin analogs like octreotide counteract vasoactive intestinal peptide thereby decreasing intraluminal intestinal secretions. (2) Additionally, proton pump inhibitors, histamine-2 blockers, and anticholinergics (such as scopolamine and glycopyrrolate) can decrease smooth muscle spasms and acid secretion. Nevertheless, despite various options, consideration of goals of care, availability of medications and routes of administration, and individual risks and benefits must be considered. The palliative approach to MBO is aimed at minimizing obstructive symptoms of pain, nausea and vomiting, but also determining how to manage symptoms with limited enteral access. Eliciting patient preferences for gustatory pleasure with eating is also an important consideration.
Case presentation
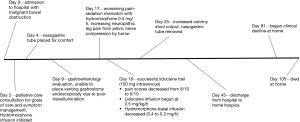
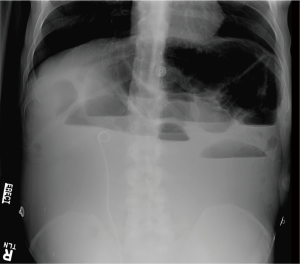
At age 43, Mr. A was diagnosed with stage III rectal carcinoma, and was treated with neoadjuvant radiation and capcitabine; subsequently with abdominoperineal resection and colostomy. Pathology showed perineural involvement and positive lymph nodes, so he received adjuvant chemotherapy with FOLFOX (5-fluorouracil and oxaliplatin) followed by capcitabine. Computerized tomogram (CT) scan showed “no convincing evidence of metastases or recurrence”. From that point, Mr. A was lost to follow-up until 3 years later, when he presented with pelvic pain and urinary retention. He was found to have a new pelvic mass, ureteral stricture, and pulmonary metastases, and planned to resume chemotherapy with capcitabine, oxaliplatin and bevacizumab. Unfortunately, he presented to the emergency room with no colostomy output for 2 weeks (Figure 1; Day 0), and imaging revealed high-grade MBO with three transition points suspected (see Figure 2).
The patient was cachectic, debilitated, and had new hydronephrosis, so it was decided that the surgical management of MBO likely outweighed benefits, and Mr. A was managed conservatively. One week into his hospitalization, he voiced a strong desire to eat to enjoy the pleasure of taste. He was aware of his poor prognosis, and in this context, despite risks and benefits of eating; he made the decision to eat. NGT decompression was not a long-term solution, so conversation points emphasized shared-decision making with post-hospital planning. Mr. A did receive IV fluids, which were later stopped due to third-spacing. The option of venting gastrostomy via radiology was explored, but due to overlying fixed bowel, this was not technically feasible. Meanwhile, the patient experienced worsening pain. An attempt at venting gastrostomy via esophagogastroduodenoscopy was unsuccessful, as despite NGT “decompression”, the stomach and visualized small bowel were full of food of varying age and no transillumination was feasible. The gastroenterologist was astonished the patient was not vomiting profusely in the setting.
Despite initial control of abdominal pain with IV hydromorphone, Mr. A subsequently developed worsening lower extremity neuropathic pain due to suspected nerve compression by the pelvic mass and previous neurotoxic chemotherapy. Attempts at adjusting hydromorphone were limited by sedation. The patient declared sedation was unacceptable at that time, as he wanted to remain awake and engaged in life.
With MBO, enteral medications such as gabapentinoids or tricyclic antidepressants, were not feasible. IV methadone was considered but was not available. Mr. A was not deemed to be a candidate for neuroaxial analgesia given limited prognosis. He was receiving parenteral dexamethasone 4 mg twice daily as well as octreotide 50 mcg three times daily. Given his desire to be awake and limited enteral options, we considered lidocaine infusion. Lidocaine can be an effective adjuvant for neuropathic pain via sodium channel blockade, and has been reported to have a favorable risk-benefit profile. A 100-mg lidocaine challenge over 30 minutes resulted in a decrease pain score from 8/10 to 5/10, so continuous infusion at 0.5 mg/kg/h as per the protocol of Ferrini and Paice (4) was begun. Lidocaine was effective in pain control, but the patient became more drowsy with opioid potentiation, so hydromorphone dose was decreased.
Over the next several days, the patient was awake with adequate pain control. Unexpectedly, the patient began having intermittent colostomy output and slow clinical improvement of his MBO symptoms. This was despite repeat CT scan confirming ongoing high-grade MBO which actually looked radiographically worse. The patient felt well enough to want to try to leave the hospital, and that became his main objective—planning to return to the hospital when symptom worsened, as he did not wish to die at home. On hospital day 43, the patient was discharged home with hospice while continuing the lidocaine infusion at 0.9 mg/kg/h, as well as parenteral octreotide, hydromorphone and dexamethasone. Despite the initial resistance by infusion providers to supply these medications, our interdisciplinary team was able to provide sufficient evidence to support the plan. Fortunately, the patient had additional coverage through the veterans administration, which allowed this plan to come to fruition outside of hospice’s per diem limits. Despite a plan to return to the hospital when needed, the patient stayed at home with adequate pain and MBO relief and was able to spend quality time with his family, dying 2 months later at home with hospice.
Discussion
Given heterogeneity of patients with MBO, it is critical to consider patient preferences, especially when approaching life’s end. Some patients may wish to limit interventions such as IV fluids and NGT placement; some may be open to more aggressive interventions such as surgery and stenting if indicated. The National Comprehensive Cancer Network guidelines highlight this variability with overall prognosis guiding therapeutic options (2). With Mr. A’s preferences to derive pleasure from eating, and his preference to be alert and engaged, we had to think creatively; opting to treat both neuropathic and visceral pain from MBO, with reduced opioids and concomitant lidocaine infusion. As this was not standard practice locally, challenges existed in administering the medication on our palliative care unit (had not been done up to that point), as well as in persuading home infusion companies to reach beyond individual clinical experience to consider providing this treatment.
Ferrini and Paice’s work (4) was instrumental in moving forward, and subsequent work by Peixoto and Hawley (5) (published shortly after this case), was helpful in persuading pharmacists and home infusion agencies to develop approaches for future patients. With limited per diem reimbursement in home hospice, cost-containment is an important consideration. According to our pharmacists, the average whole cost of lidocaine for infusion is 4 USD per day. Additionally, in appropriate clinical situations, lidocaine can be quite effective. Peixoto and Hawley’s case series (5) demonstrates that lidocaine has been a useful option when other treatments have been unsuccessful, with 49% of patients reporting major pain relief response with generally little harm from the infusion. The most common side effects reported were drowsiness (30.7%), perioral numbness (13.4%), nausea (5.7%), and minor fluctuations of blood pressure (3.8%). Importantly, Peixoto and Hawley emphasize that lidocaine infusion in this manner does not require electrocardiographic monitoring, which tends to be a major barrier to implementing lidocaine protocols.
Another key consideration is how improved pain control and alertness allowed our patient to work on legacy building with family and friends—including making recordings of playing his guitar and being able to go out of the hospital to dine with friends. Despite the high-grade nature of his MBO, this patient inexplicably was able to tolerate reduced oral intake without need for long-term NGT decompression, and he did have return of ostomy output attributed to partial pharmacologic resolution with dexamethasone and octreotide. The dynamic process of care with MBO is often challenging, as patients report vacillating between feelings of doing well and wanting to take more oral intake, but also worrying about when symptoms will reoccur. Fortunately, Mr. A was able to tolerate small amounts of intake, have reasonable symptom management, and was able to spend a quality time on legacy building and life-closure activities.
Conclusions
Despite options for surgical, interventional, and medical approaches to MBO, the palliative approach must involve shared-decision making and interdisciplinary support, which can lead to creative patient-centered solutions during this dynamic disease process. For the patient presented herein, a multi-faceted approach allowed for adequate pain control with less pain-sedation mismatch, improved personal engagement in his care plan, and the ability to honor the patient’s request to take oral feedings for pleasure. Despite plans for readmission for end-of-life care, the patient’s symptoms were so well managed with this multimodal approach that he opted to remain at home during the final weeks of his life.
Acknowledgements
None.
Footnote
Conflicts of Interest: The case was presented during Case Sessions at the 2016 American Academy of Hospice and Palliative Medicine (AAHPM) and the Hospice and Palliative Nurses Association (HPNA) Annual Assembly in Chicago, Illinois on March 11, 2016.
Informed Consent: Informed consent was obtained from the patient’s next of kin (as patient has died) for publication of this case report and any accompanying images.
References
- Alese OB, Kim S, Chen Z, et al. Management patterns and predictors of mortality among US patients with cancer hospitalized for malignant bowel obstruction. Cancer 2015;121:1772-8. [Crossref] [PubMed]
- Levy M, Smith T, Alvarez-Perez A, et al. Palliative Care Version 1.2016. J Natl Compr Canc Netw 2016;14:82-113. [Crossref] [PubMed]
- Mercadante S, Casuccio A, Mangione S. Medical treatment for inoperable malignant bowel obstruction: a qualitative systematic review. J Pain Symptom Manage 2007;33:217-23. [Crossref] [PubMed]
- Ferrini R, Paice JA. How to initiate and monitor infusional lidocaine for severe and/or neuropathic pain. J Support Oncol 2004;2:90-4. [PubMed]
- Peixoto RD, Hawley P. Intravenous lidocaine for cancer pain without electrocardiographic monitoring: a retrospective review. J Palliat Med 2015;18:373-7. [Crossref] [PubMed]